Stroke is one of the major diseases with a global burden, with acute ischemic stroke (AIS) being the predominant (1). If ischemic stroke is not treated with timely and effective vascular recanalization, it can lead to neurological damage, decreased quality of life, and even death (2) in patients. Intravascular intervention is one of the main treatment methods for acute ischemic stroke, which can significantly improve the prognosis of AIS patients within 6 h of onset (3). In 2015, five randomized controlled trials showed that mechanical thrombectomy (MT) within 6 h of onset was more effective in improving 90-day neurological function in patients with acute anterior circulation large vessel occlusive stroke (ACLVOS) when compared to drug treatment (4–8). In recent years, some clinical studies have observed the efficacy and safety of MT on AIS patients within 24 h of onset. The results of the 2018 DAWN and DEFUSE 3 studies indicate that MT within 6–24 h of AIS onset can still benefit patients (9, 10). AIS can be classified into atherosclerosis, cardiogenic thromboembolism, and other types based on the mechanism (TOAST classification), among which arterial atherosclerosis is the predominant type. The main pathophysiological mechanism of arterial atherosclerosis is the instability of cerebral artery atherosclerotic plaque, which can form a thrombus to block the blood vessels when the plaque ruptures. Therefore, MT requires the use of dual antiplatelet drugs for therapy. In some guidelines for AIS patients, the use of a combination of aspirin with clopidogrel is recommended for at least 1 month after mechanical thrombectomy (11, 12). However, the pathological basis of atherosclerotic AIS is similar to that of acute coronary syndrome (ACS). After interventional treatment, ACS often requires 1–2 years of dual antiplatelet therapy (DAPT). Considering that prolonged DAPT for AIS after MT may increase the risk of cerebral hemorrhage, the current duration of DAPT is relatively short. In clinical practice, we used DAPT on some AIS patients for 3 months. This study aimed to investigate the efficacy and safety of 3-month DAPT in AIS patients at 3 months after MT through a retrospective study.
Methods Study populationThis study included AIS patients who were hospitalized from May 2018 to March 2023 for retrospective analysis. According to the duration of DAPT after MT, the patients were classified into a 1-month group (1-M group) and a 3-month group (3-M group). The inclusion criteria were as follows: age is ≥ 18 years; the diagnostic criteria for AIS is met (13); the time from onset to admission is ≤ 24 h; and the CT scan at admission showed significant ischemic penumbra and met the imaging screening criteria in the DEFUSE 3 study (10): core infarct volume of <70 mL, ischemic volume/core infarct volume of ≥ 1.8 mL, mismatch volume of ≥ 15 mL; at admission, the National Institute of Health Stroke Scale (NIHSS) score was ≥ 6 points; the patient received regular follow-up after discharge; and the clinical data are complete. The exclusion criteria were as follows: concomitant malignant tumors, renal failure, and heart failure; rheumatic diseases; received coronary artery stent implantation within 1 year prior to the onset of stroke; atrial fibrillation and cardiogenic cerebral embolism; at admission, CT examination showed concurrent cerebral hemorrhage and an Alberta stroke program early CT score (ASPECTS) of <6; loss of follow-up. This study was approved by the Ethics Committee of Bethune International Peace Hospital (approval number: 2021-KY-142), and written informed consent was waived.
Mechanical thrombectomyAfter comprehensive evaluation on admission, the patient was immediately treated with MT. After sufficient local infiltration anesthesia with lidocaine, the Seldinger technique was used to puncture the right femoral artery, and then, an 8F arterial sheath was inserted, followed by aortic arch angiogram and cerebral angiography. After identifying the location of the thrombus, a thrombectomy was conducted. Stent thrombectomy: After passing through the occluded area and confirming the true lumen of the blood vessel, the microwire and microcatheter were inserted into the Solitaire FR stent (Medtronic Ltd, USA), and then, the thrombectomy device was retrieved under negative pressure. Thrombolysis: under the guidance of microwires, the Sofia aspiration catheter (MicroVention Ltd, USA) or ACE aspiration catheter (Penumbra Inc., USA) was inserted until it met the thrombus, and then, negative pressure was maintained and aspiration was performed for 90 s. After removing the thrombus, a repeated cerebral angiography was performed to evaluate the treatment effect and the presence of bleeding. If the residual stenosis rate in the local area was >70% or if a small thrombus escaped from the distal end, measures such as balloon angioplasty, stent implantation, and intra-arterial thrombolysis were used for remediation.
Postoperative medicationPatients in the 1-M group received 100 mg oral aspirin and 75 mg clopidogrel daily for 1 month after endovascular treatment. The 3-M group received DAPT treatment according to the same protocol, lasting for 3 months. After the completion of DAPT, aspirin was continued for at least 1 year. If patients have diabetes, hypertension, and coronary heart disease, they should be treated according to relevant guidelines. Specifically, both groups of patients received atorvastatin at a daily dose of 20–40 mg.
Follow-upIn this study, all patients underwent regular outpatient follow-up at 1 month, 3 months, and 6 months after surgery. The information of follow-up included the following: postoperative mRS score at 90 days and adverse events, mainly including death, recurrence of cerebral infarction, and symptomatic intracranial hemorrhage (sICH). The follow-up was conducted in an outpatient clinic, by telephone, or through WeChat.
Data collection and outcomesAll data were collected from the electronic medical record system. Baseline data include age, gender, smoking history, alcohol use, disease history, physical examination results, laboratory test results, and imaging results on admission. Information on in-hospital management and all follow-up outcomes was collected. The primary outcome was the mRS score at 90 days. The mRS score was assessed by physical examination in an outpatient clinic 90 days after onset. Secondary outcomes included a good prognosis (mRS score of 0–2) 90 days after the endovascular intervention, mortality, recurrence of cerebral infarction, Barthel's index, Montreal Cognitive Assessment (MoCA) score, and incidence of sICH at the 6-month follow-up. The prespecified adverse event was sICH defined by the National Institute of Neurological Disorders and Stroke (NINDS) criteria.
Statistical analysisStatistical Package for the Social Sciences (SPSS, version 24.0, USA) was used to analyze data. Continuous data with normal distribution were presented as mean ± standard deviation and compared between two groups using a two-sample independent t test. Category data were presented as numbers (percentages) and compared between two groups using the chi-square or Fisher's exact test. A two-sided p < 0.05 indicates a statistical difference.
Results Baseline characteristicsAccording to the inclusion and exclusion criteria, a total of 147 patients with AIS were included in the final analysis. Among these patients, there were 108 (73.5%) patients with hypertension, 44 (29.9%) with type 2 diabetes, and 99 (67.3%) with hyperlipidemia. Seventy-eight patients received 1-month DAPT with 100 mg aspirin daily and 75 mg clopidogrel daily, and the remaining 69 patients were treated with DAPT for 3 months. A comparison between the 1-M group and the 3-M group showed no statistical difference regarding baseline characteristics (P > 0.05, Table 1).
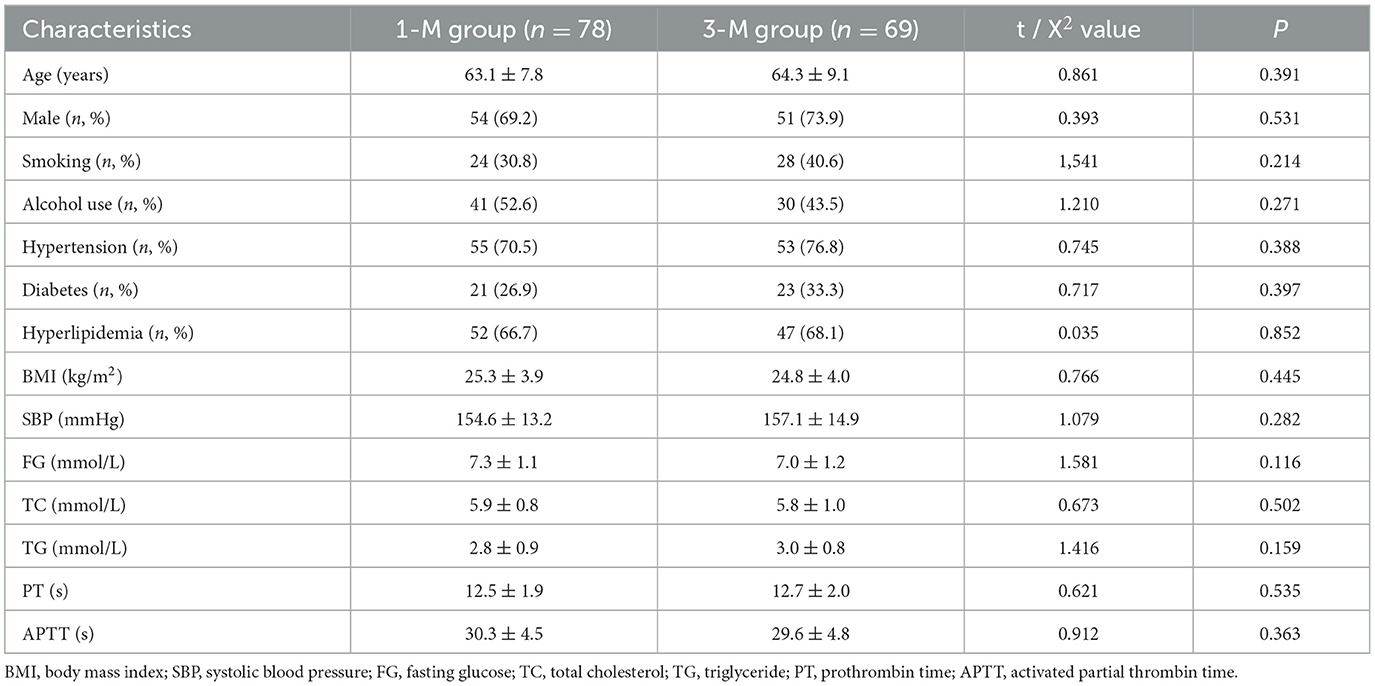
Table 1. Baseline characteristics.
Neurological characteristicsThe time from onset to initiation of MT in patients is 3–14 h, with an average of 6.1 ± 1.5 h. A comparison of neurological characteristics between the 1-M group and the 3-M group also found no statistically significant difference (all P > 0.05, Table 2).
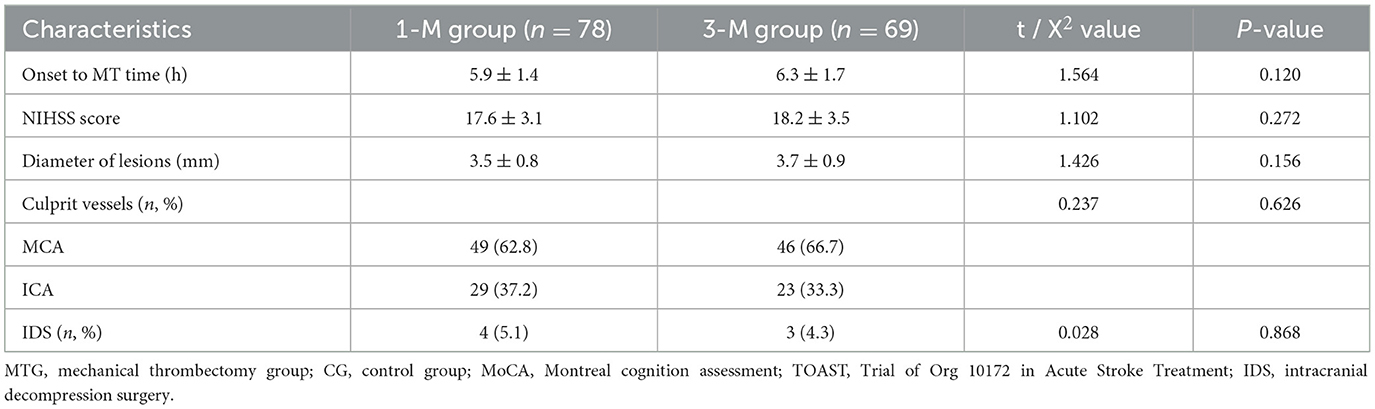
Table 2. Neurological characteristics.
Follow-up outcomesThe follow-up results (Table 3) showed that there were a total of 61 patients in both groups with a 90-day mRS of 0–2, and the average mRS of all patients at 90 days was 3.3 ± 0.9. There was no statistically significant difference in the mRS between the two groups of patients 90 days after surgery (P > 0.05). There was no statistically significant difference in the mortality rate and incidence of sICH between the two groups of patients during the 6-month follow-up period (P > 0.05), but the AIS recurrence rate in the 3-M group was lower than that in the 1-M group (P < 0.05). Three cases of sICH occurred in patients with poor blood pressure control, and the sICH was located at the internal capsule artery. We also observed that patients in the 3-M group had higher improvement in Barthel scores and MoCA improvement at 6 months compared to the 1-M group but did not show statistically significant differences (P > 0.05).
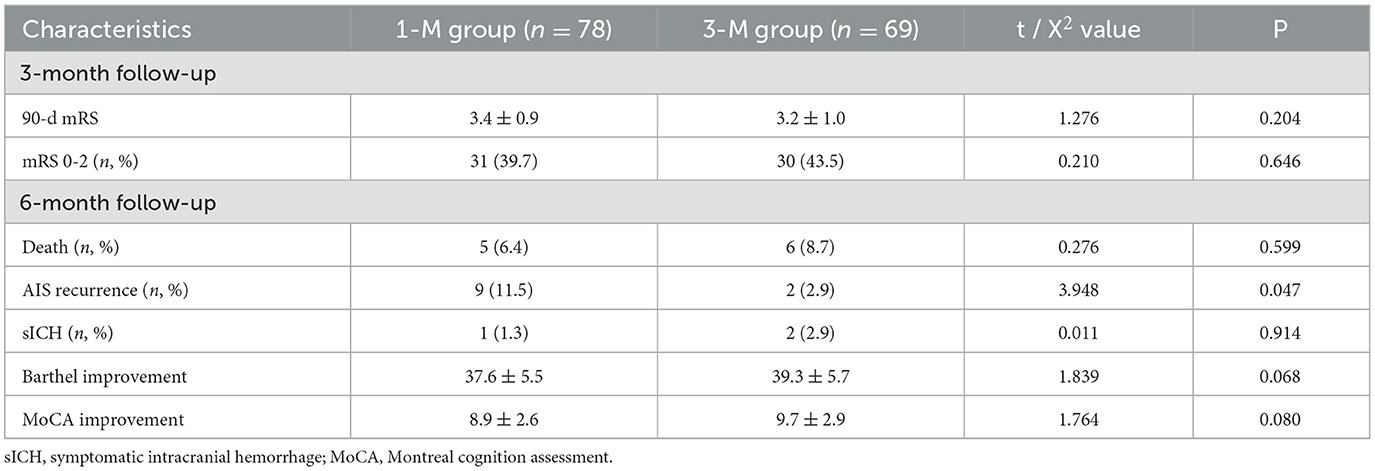
Table 3. Comparison of follow-up results between the two groups of patients.
DiscussionThe preliminary results of this study indicate that 3-month DAPT after MT can significantly reduce the risk of AIS recurrence in AIS patients while not increasing the risk of death and sICH. Our study also found that 3-month DAPT showed a trend toward further improvement in patients' daily living conditions and cognitive abilities.
For AIS patients with non-cardiogenic embolism, the mechanism of culprit vessel occlusion is similar to that of acute myocardial infarction, which is the formation of a thrombus caused by the rupture of an unstable atherosclerotic plaque, leading to vascular occlusion. These AIS patients are often known to have hypertension, diabetes, and hyperlipidemia. In this study, patients with hypertension, diabetes, and hyperlipidemia accounted for 73.5%, 29.9%, and 67.3%, respectively. Moreover, this study excluded AIS caused by cardiogenic thrombus, so the pathogenesis of AIS patients in this study may be atherosclerosis. In the treatment of unstable atherosclerotic plaque, antiplatelet drugs are mainly used to prevent thrombosis, while intensive statin therapy is used to stabilize plaque (14, 15). In this study, patients received long-term treatment with aspirin (100 mg daily) and atorvastatin. The difference was that patients in the 3-M group received clopidogrel for 3 months after surgery, while patients in the 1-M group only used it for 1 month.
Antiplatelet therapy is one of the main treatment measures for patients with acute ischemic stroke. In the CHANCE study, Wang et al. compared the efficacy and safety of DAPT with those of aspirin alone in the treatment of acute minor stroke or transient ischemic attack in a randomized controlled trial. The results showed that, compared with aspirin alone, DAPT significantly reduced the risk of stroke within 90 days after the attack, while it did not increase the risk of cerebral hemorrhage (16). Since Wang et al.'s (16) study, the era of DAPT treatment for ischemic stroke began. At present, for patients with ischemic stroke who receive endovascular treatment and DAPT treatment, existing guidelines recommend that patients with extracranial arch artery lesions receive DAPT for at least 30 days after surgery, and then, they were switched to aspirin alone. For patients with intracranial arterial lesions, it is recommended to switch to aspirin alone after 3–6 months of DAPT (17, 18). If a stent is implanted, the recommended duration of DAPT is 1–3 months, followed by long-term aspirin treatment (19). There is currently insufficient evidence to support clear recommendations from guidelines or consensus on the duration of DAPT use for AIS patients undergoing MT after surgery.
The antiplatelet mechanisms of aspirin and clopidogrel are different, and the combination of the two drugs can have a stronger effect than when using a single drug. Administering DAPT during the acute phase of AIS can effectively prevent thrombus formation at the target lesion site after MT. After a certain period of comprehensive treatment, including antihypertensives, hypoglycemics, and statin drugs, the blood vessels at the target lesion site can be repaired and endothelial function can be restored. However, the exact duration required for endothelial function recovery is currently unclear. Previous studies have focused more on the use of antiplatelet drugs in patients with cerebrovascular disease after stent placement. In the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) study, patients received DAPT with 75 mg/day of clopidogrel and 325 mg/day of aspirin for 3 months. At an average follow-up of 11.9 months, safety evaluations indicated that the incidence of sICH in the stent implantation group compared to that in the intensified drug group was 3.6% (8 cases) vs. 0.4% (1 case) (20–22). Hassan et al. (23), in a previous study, observed the incidence of intracranial and systemic bleeding events, recurrent ischemic stroke, and death in patients treated with dual antiplatelet therapy for more than 1 month. In the study, DAPT was administrated in 110 patients following endovascular treatment with a median duration of 3 months. During the study period, there were two bleeding events (one intracranial and one gastrointestinal), one ischemic stroke, and no deaths (23). It should be noted that, in some studies in Europe and America, the dosage of aspirin is significantly higher than that in Chinese patients. In clinical practice in China, except for the load dose of 300 mg given during acute AIS intervention surgery, the long-term dose after endovascular intervention is 100 mg per day (12). Therefore, when Chinese patients use DAPT, the daily dosage of aspirin is significantly lower than that in European and American patients, so the risk of cerebral hemorrhage may be lower than that in European and American patients. Three patients in this study developed sICH during a 6-month follow-up period, which may be related to uncontrolled hypertension.
Our research findings also suggest that, compared to 1-month DAPT, 3-month DAPT has a trend toward further improvement in patients' daily living and cognitive abilities, which was suggested by slight improvement in Barthel index and MoCA scores. The relatively small sample size of the present study and short follow-up duration might be the reasons for no statistical significance. This finding may be related to the more effective preventive effect of longer DAPT on ischemic stroke, especially in the prevention of minor ischemic stroke or transient ischemic attack. However, currently, there is a lack of evidence to support it.
There are some limitations in this study. First, this study is a single-center study with a small sample size. Second, this study is a retrospective study, and some patients were excluded due to incomplete data, which may lead to case selection bias. Third, there is insufficient information on the retrospective data to assess the presence of asymptomatic mild cerebral hemorrhage. In the future, multicenter, large-sample randomized controlled studies with long-term follow-up are needed to further evaluate the efficacy and safety of 3-month DAPT in AIS patients undergoing mechanical thrombectomy.
ConclusionThe results of this study indicate that a 3-month DAPT can safely and effectively reduce the risk of IS in AIS patients undergoing mechanical thrombectomy during a 6-month follow-up period.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by the Ethics Committee of Bethune International Peace Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective nature of the study.
Author contributionsLL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. ZL: Data curation, Formal analysis, Investigation, Writing – original draft. CL: Data curation, Formal analysis, Investigation, Writing – original draft. BC: Data curation, Resources, Software, Validation, Writing – original draft. QJ: Investigation, Methodology, Writing – original draft.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
PubMed Abstract | Crossref Full Text | Google Scholar
3. Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. (2020) 48:1654–63. doi: 10.1097/CCM.0000000000004597
Crossref Full Text | Google Scholar
4. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMx140064
PubMed Abstract | Crossref Full Text | Google Scholar
5. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
PubMed Abstract | Crossref Full Text | Google Scholar
6. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
PubMed Abstract | Crossref Full Text | Google Scholar
7. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
PubMed Abstract | Crossref Full Text | Google Scholar
8. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
PubMed Abstract | Crossref Full Text | Google Scholar
9. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
PubMed Abstract | Crossref Full Text | Google Scholar
10. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
PubMed Abstract | Crossref Full Text | Google Scholar
11. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European stroke organisation (ESO)- European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. (2019) 11:535–8. doi: 10.1136/neurintsurg-2018-014568
PubMed Abstract | Crossref Full Text | Google Scholar
12. Chinese Chinese Society of Neurology Chinese Stroke Society Neurovascular Neurovascular Intervention Group of Chinese Society of Neurology. Chinese guidelines for the endovascular treatment of acute ischemic stroke 2022. Chin J Neurol. (2022) 55:565–80. doi: 10.3760/cma.j.cn113694-20220225-00137
Crossref Full Text | Google Scholar
13. Chinese Chinese Society of Neurology Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. (2018) 51:666–82. doi: 10.3760/cma.j.issn.1006-7876.2018.09.004
Crossref Full Text | Google Scholar
14. Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American heart association and American college of cardiology foundation. Circulation. (2011) 124:2458–73. doi: 10.1161/CIR.0b013e318235eb4d
PubMed Abstract | Crossref Full Text | Google Scholar
15. Fleg JL, Forman DE, Berra K, Bittner V, Blumenthal JA, Chen MA, et al. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. (2013) 128:2422–46. doi: 10.1161/01.cir.0000436752.99896.22
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. (2013) 369:11–9. doi: 10.1056/NEJMoa1215340
PubMed Abstract | Crossref Full Text | Google Scholar
17. Chinese Chinese Association of Preventive Medicine Professional Professional Committee of Stroke Prevention and Control Interventional Group. Chinese expert consensus on antiplatelet strategies for interventional treatment of ischemic cerebrovascular disease. Natl Med J China. (2015) 95:803–9. doi: 10.3760/cma.j.issn.0376-2491.2015.11.002
Crossref Full Text | Google Scholar
18. Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, et al. Editor's choice - management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the european society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55:3–81. doi: 10.1016/j.ejvs.2018.03.023
PubMed Abstract | Crossref Full Text | Google Scholar
19. Expert writing group of the Severe Cerebrovascular Disease Branch of the Chinese Stroke Society. Chinese experts consensus on postoperative monitoring and management of intravascular therapy for acute ischemic stroke. Natl Med J China. (2017) 97:162–72. doi: 10.3760/cma.j.issn.0376-2491.2017.03.002
Crossref Full Text | Google Scholar
20. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
PubMed Abstract | Crossref Full Text | Google Scholar
21. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. (2014) 383:333–41. doi: 10.1016/S0140-6736(13)62038-3
PubMed Abstract | Crossref Full Text | Google Scholar
22. Derdeyn CP, Fiorella D, Lynn MJ, Turan TN, Cotsonis GA, Lane BF, et al. Nonprocedural symptomatic infarction and in-stent restenosis after intracranial angioplasty and stenting in the SAMMPRIS trial (stenting and aggressive medical management for the prevention of recurrent stroke in intracranial stenosis). Stroke. (2017) 48:1501–6. doi: 10.1161/STROKEAHA.116.014537
PubMed Abstract | Crossref Full Text | Google Scholar
23. Hassan AE, Zacharatos H, Vazquez G, Rodriguez GJ, Suri MF, Tummala RP, et al. Low risk of intracranial and systemic hemorrhages in patients on dual antiplatelet treatment beyond 1 month following neuroendovascular angioplasty and/or stent placement. J Neuroimaging. (2012) 22:67–73. doi: 10.1111/j.1552-6569.2010.00520.x
留言 (0)