As a serious infectious disease, tuberculosis (TB) is mainly caused by Mycobacterium tuberculosis (MTB) infection and is a leading cause of death from a single infectious disease alongside COVID-19. The latest report from the World Health Organization (WHO) showed that there were approximately 10.6 million new cases of TB globally and approximately 0.78 million new TB cases in China in 2021 (1). After infection with MTB, this pathogen is usually not eliminated immediately and might exist in macrophages over a long period due to its ability to evade the host immune system (2). Therefore, the development of TB was affected by the interaction of multiple factors. A previous study found that about a quarter of the global population was infected with MTB, but only 10% of infected people would eventually develop active TB (3). Combined with other studies, it has been found that certain host factors, including genetic factors, malnutrition, and HIV infection, could significantly influence the susceptibility to MTB infection through regulating the host immune status (4, 5).
In recent years, genome-wide association studies and transcriptomic association studies have identified a number of variant loci that affected pulmonary TB (PTB) susceptibility, while the genetic factors that affected the susceptibility to PTB were not fully understood (6). Notably, genetic variations in several immune-related genes were shown to affect the degree of host resistance to MTB infection, disease severity, and drug resistance (7, 8). Previous studies found that polymorphisms in interleukin (IL)-13, IL-13RA1, IL-13RA2, and IL-23R genes were involved in the genetic background of PTB and some clinical features in a Chinese Han population (9, 10). Therefore, it was of great significance to further clarify the effects of immune-related gene genetic variations in PTB.
Studies showed that the differentiation of naive T cells into Thl lymphocytes, which was very important for a successful anti-TB immune response, required the presence of the IL-12 family cytokines (IL-27, IL-35, and IL-12) (11). During MTB infection, IL-27 could promote Th1 cell differentiation by STAT1 phosphorylation against MTB (12). In addition, IL-27 signaling was indispensable for the activation of Tr1 cells and induction of IL-10 expression through STAT3 phosphorylation. It also inhibited immune response to MTB infection and increased disease severity (12). IL-35 consisted of the IL-12 subunit α chain (IL-12A/P35) and EBI3, and expressed in many immune cells (13). Kong et al. demonstrated that serum IL-35 level was increased in patients with active PTB (14), and another study found that the mRNA expression levels of two subunits (p35 and Ebi3) of IL-35 by circulating B cells were also increased in active PTB (15). Therefore, these inflammatory cytokines played essential roles in the pathogenesis of PTB.
The significance of genetic variations in IL-27 and IL-35 genes has been reported in many diseases (16, 17). However, there were few studies on IL-27 gene variation in PTB, and no study on the gene variation of IL-35 in PTB was performed. Candidate genetic association studies remained a useful way to assess the association of gene variation with host immune response to MTB stimulation. Hence, we performed this case–control study to evaluate the possible association between IL-27 and IL-35 gene polymorphism, expression levels, and PTB susceptibility in a Chinese Han population.
Materials and methodsPatients with PTB and controlsIn this study, we enrolled patients with PTB from the Anhui Chest Hospital and collected normal controls from a health examination center. Subjects consisted of unrelated Chinese Han individuals. Patients with multiple clinical manifestations, such as the detection of acid-fast bacilli in sputum smear samples and the results of MTB cultures and chest radiograph, were confirmed to have PTB by an experienced senior physician according to the Diagnosis for PTB (WS 288-2017) and the Classification of TB (WS 196-2017) from the Health Industry Standard of the People’s Republic of China. Patients with PTB who are HIV-positive and who have hepatitis virus infection, cancer, autoimmune diseases, and other infectious diseases were excluded from this study. At the same time, the control group was required to be healthy volunteers with normal chest imaging examination and with no history of TB, infectious diseases, lung disease, and bacterial or viral infection.
Our study was approved by the Ethics Committee of the Anhui Chest Hospital, and the written informed consent of every subject was obtained. Then, peripheral blood (3–5 mL) of subjects was collected for extracting DNA with the Flexi Gene-DNA Kit (Qiagen, Valencia, CA). Meanwhile, we obtained the clinical characteristics, such as drug resistance, fever, pulmonary infection, drug-induced liver injury (DILI), and sputum smear, from the hospital medical record system.
SNP selection and genotypingWe searched Ensembl genome browser 85 and the CHBS_1000g database to obtain genetic variations of the IL-27 and IL-35 genes and then chose the TagSNPs using the Haploview 4.0 software (Cambridge, MA, USA). The candidate TagSNPs were selected based on three criteria: (1) minor allele frequency (MAF) > 0.05 in a Chinese Han Beijing population; (2) an r2 cutoff of 0.8 for linkage disequilibrium; and (3) the flanking 2.0-kb regions of IL-27 and IL-35 genes. Ultimately, nine tagSNPs were selected in this study, namely, three tagSNPs in the IL-27 gene (rs181206, rs153109, and rs17855750) and six tagSNPs in the IL-35 gene (for IL-12A: rs2243123, rs2243135, and rs568408; for EBI3: rs428253, rs4740, and rs9807813).
Subsequently, this study genotyped all single-nucleotide polymorphisms (SNPs) in IL-27 and IL-35 genes by the SNPscan technique, with technical support from the Center for Genetic and Genomic Analysis, Genesky Biotechnologies Inc. (Shanghai).
Enzyme-linked immunosorbent assayPlasma was extracted from peripheral blood (at 3,000 rpm for 10 min at room temperature) and stored at −80°C until processed. The expression levels of IL-27 and IL-35 were determined using an enzyme-linked immunosorbent assay (ELISA) kit (ELK1572 and 4360164, and ELK2745 and 4362411, ELK Biotechnology) according to standard experimental procedures. The IL-27 and IL-35 levels were expressed as nanograms per milliliter.
Statistical analysisThe genotype frequencies of all SNPs among the control group were evaluated for Hardy–Weinberg equilibrium (HWE) by chi-square (χ2). Genotype and allele frequencies between patients with PTB and controls were assessed with logistic regression.
The relationship between IL-27 and IL-35 gene polymorphisms and PTB susceptibility was shown as odds ratio (OR) and 95% confidence interval (95% CI). The association between each SNP with clinical manifestations was evaluated using χ2. Two genetic models (dominant model and negative model) were applied to analyze the association between SNPs and PTB risk, and the SHEsis software was used for haplotype analysis. The IL-27 and IL-35 expression levels were shown as medians [interquartile range (IQR)], and the comparisons of IL-27 and IL-35 expression levels were analyzed by Mann–Whitney U test and Kruskal–Wallis H test. Statistical analysis was performed using SPSS 23.0, and p-value less than 0.05 was regarded as statistically significant.
ResultsDemographic data and clinical manifestations of study subjectsA total of 998 study subjects were included in this study, including 497 patients with PTB and 501 controls. Among patients with PTB, the average age of 313 male and 184 female patients was 44.72 ± 18.03 years. Among controls, the average age of 300 male and 201 female patients was 44.42 ± 3.51 years. Distribution differences regarding sex and age between these two groups were not statistically significant. The distribution of several major clinical manifestations in patients with PTB is shown in Table 1.
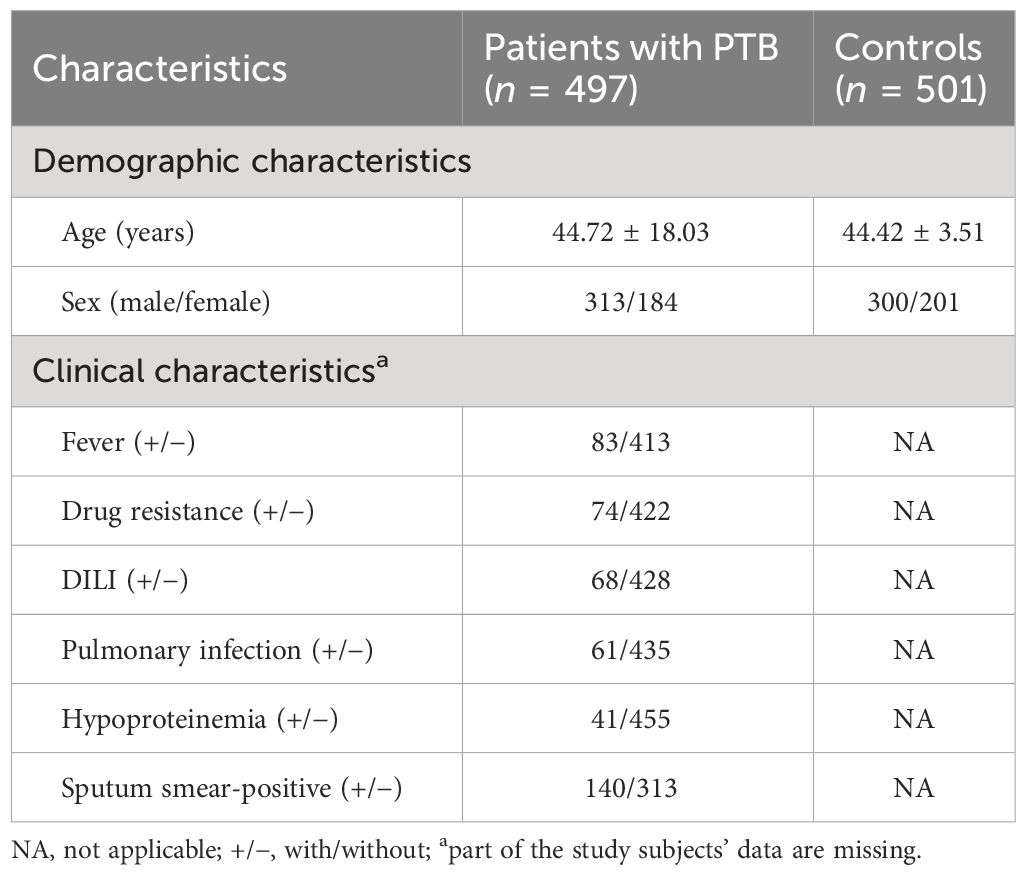
Table 1 The main demographic and clinical characteristics of patients with PTB and controls.
Association of SNPs in IL-27 and IL-35 genes with PTB susceptibilityThe allele and genotype frequencies of nine SNPs in IL-27 and IL-35 genes are presented in Table 2. Apart from the rs181206 variant, the genotype frequencies of other SNPs in the control group were consistent with genetic equilibrium via the HWE test (p > 0.05). Then, the rs181206 variant was not included for analysis.
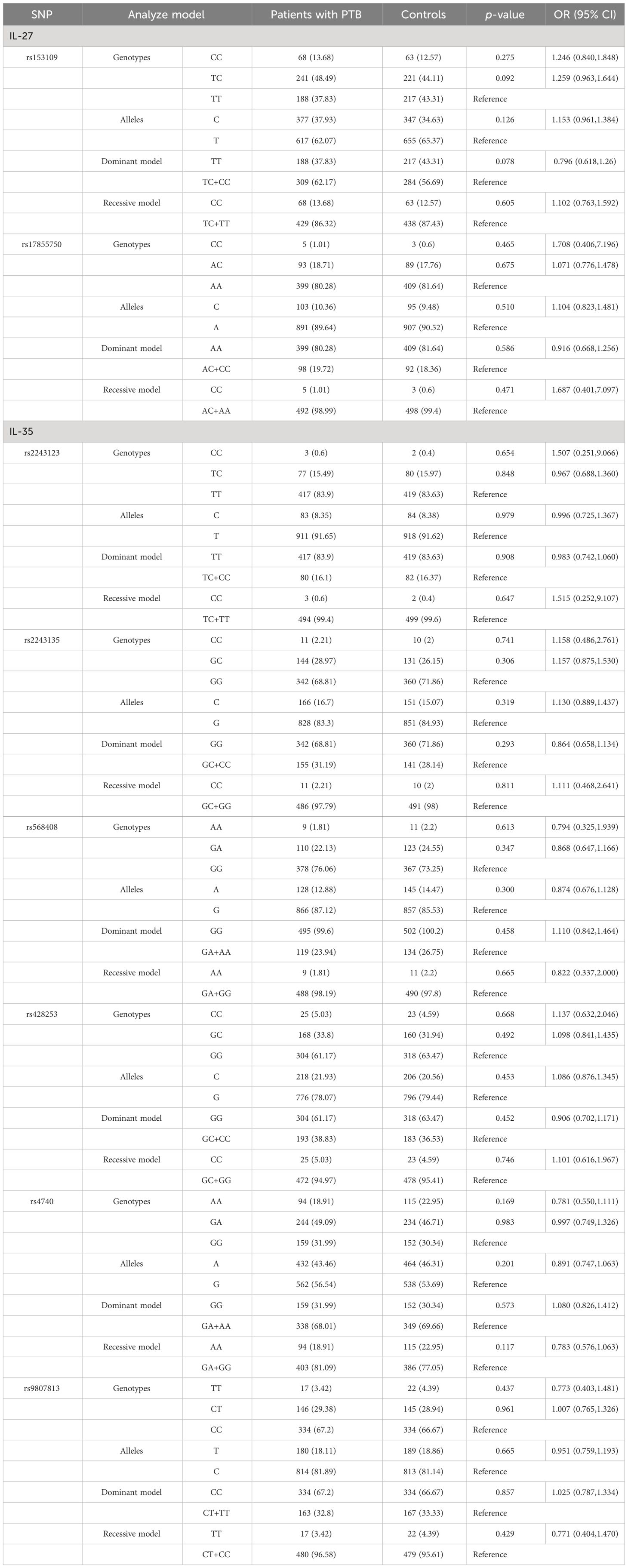
Table 2 IL-27 and IL-35 genes’ genotype and allele frequencies among patients with PTB and controls.
Our results found no statistically significant difference in allele and genotype frequencies of IL-27 gene rs153109 and rs17855750 polymorphisms among patients with PTB and controls. Similarly, the distribution difference in alleles and genotypes of rs2243123, rs2243135, rs568408, rs428253, rs4740, and rs9807813 variants in the IL-35 gene was not statistically significant (Table 2). Furthermore, two genetic models (dominant model and recessive model) were applied to assess the relationship between the above SNPs with PTB susceptibility, while the results showed no significant association (Table 2).
Haplotype analysisIL-35 consisted of an IL-12 subunit α chain (IL-12A/P35) and EBI3, and the polymorphisms in the IL-35 gene were respectively from IL-12A (rs2243123, rs2243135, and rs568408) and EBI3 (rs428253, rs4740, and rs9807813). Hence, we respectively used the SHEsis software to construct the haplotype of IL-27 gene rs153109–rs17855750, IL-35 gene rs2243123–rs2243135–rs568408, and IL-35 gene rs428253–rs4740–rs9807813, and the main haplotypes with a frequency of more than 3% are shown in Table 3. We suggested that the GAC haplotype (representing rs428253–rs4740–rs9807813) of the IL-35 gene was a protective factor against PTB susceptibility (p = 0.036, OR = 0.751). However, other haplotypes showed no statistical association with PTB susceptibility.
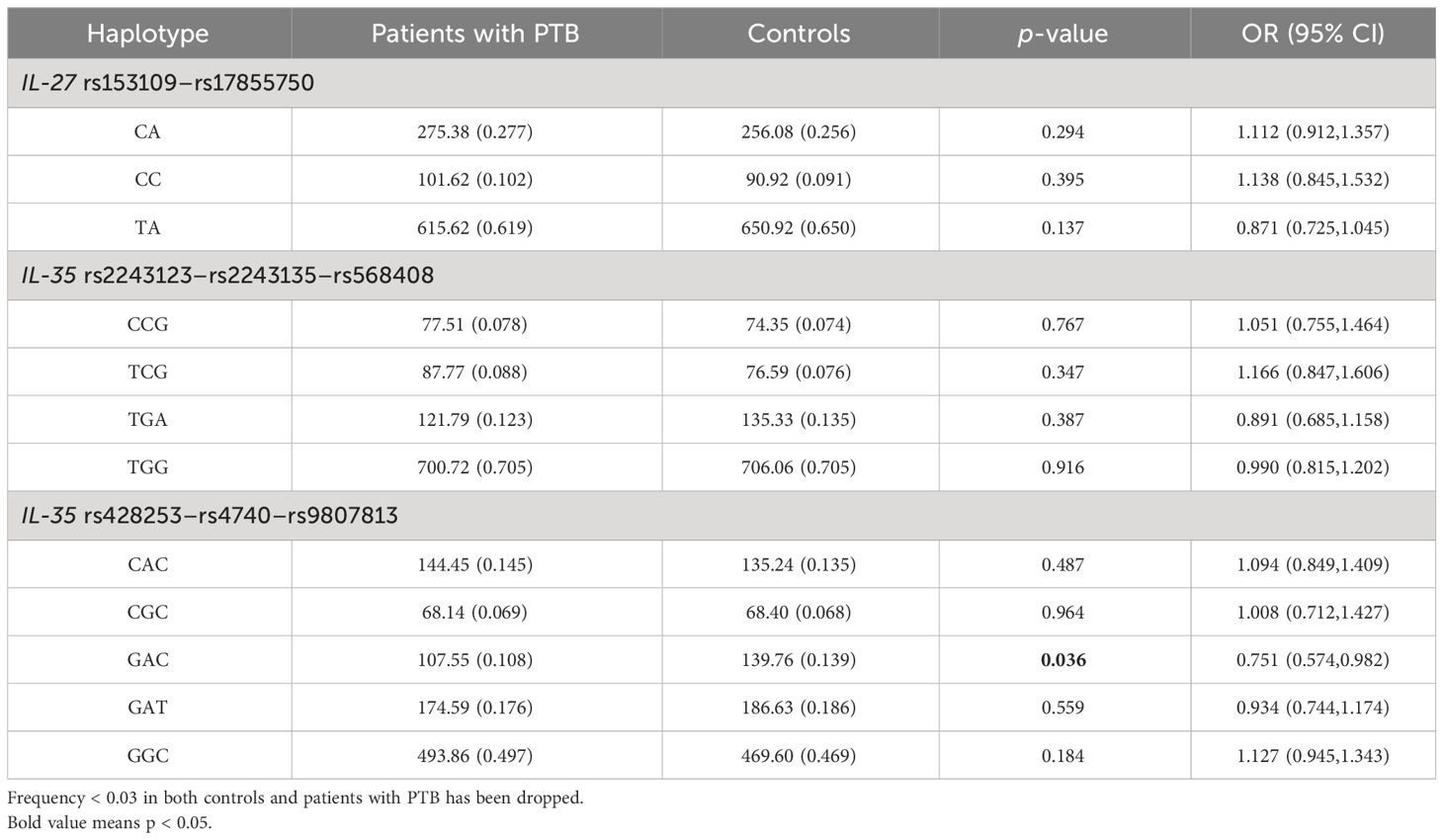
Table 3 Haplotype analysis of IL-27 and IL-35 genes in patients with PTB.
Association of IL-27 and IL-35 gene polymorphisms and clinical manifestations among patients with PTBThis study also examined whether polymorphisms in IL-27 and IL-35 genes affected the clinical manifestations of PTB, and the results are summarized in Table 4. In the IL-27 gene, the increased frequency of the rs17855750 GG genotype was found in patients with PTB with fever when compared to the patients without fever (p = 0.001). For the IL-35 gene, the rs568408 GA genotype frequency in patients with PTB with DILI was significantly lower than that in the patients without DILI (p = 0.006). In addition, the decreased frequencies of the rs428253 GC genotype, rs4740 AA genotype, and A allele were significantly related to hypoproteinemia in patients with PTB (p = 0.026, p = 0.008, and p = 0.007).
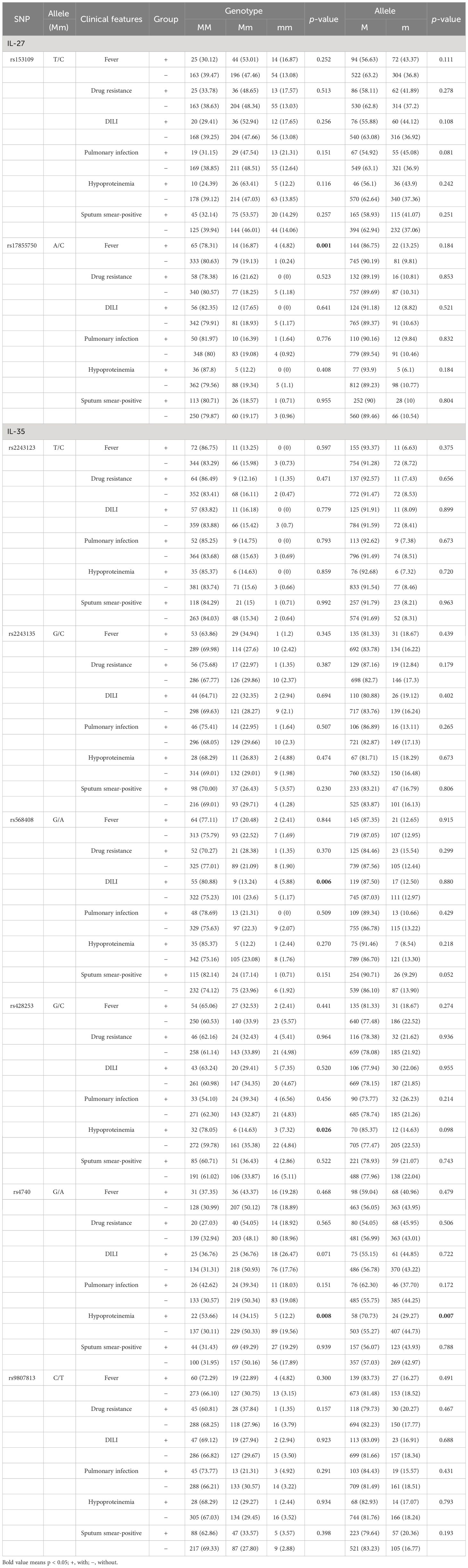
Table 4 IL-27 and IL-35 gene polymorphisms and the clinical manifestations in patients with PTB.
Association of IL-27 and IL-35 expression levels with patients with PTBWe then examined the IL-27 and IL-35 plasma levels in 84 patients with PTB (namely, 59 male and 25 female patients; average age: 50.73 ± 19.24 years) and 84 controls (namely, 57 male and 27 female patients; average age: 49.35 ± 6.12 years). The IL-27 plasma level of patients with PTB and controls were 5.217 (4.791, 5.731) ng/mL and 1.923 (1.748, 2.233) ng/mL, respectively. The IL-35 plasma level of patients with PTB and controls were 2.788 (2.349, 3.244) ng/mL and 2.852 (2.579, 3.017) ng/mL, respectively. We found that when compared to the control group, the plasma level of IL-27 was abnormally elevated in patients with PTB (p < 0.001), while the plasma level of IL-35 was not significantly different (p = 0.827) (Figure 1).
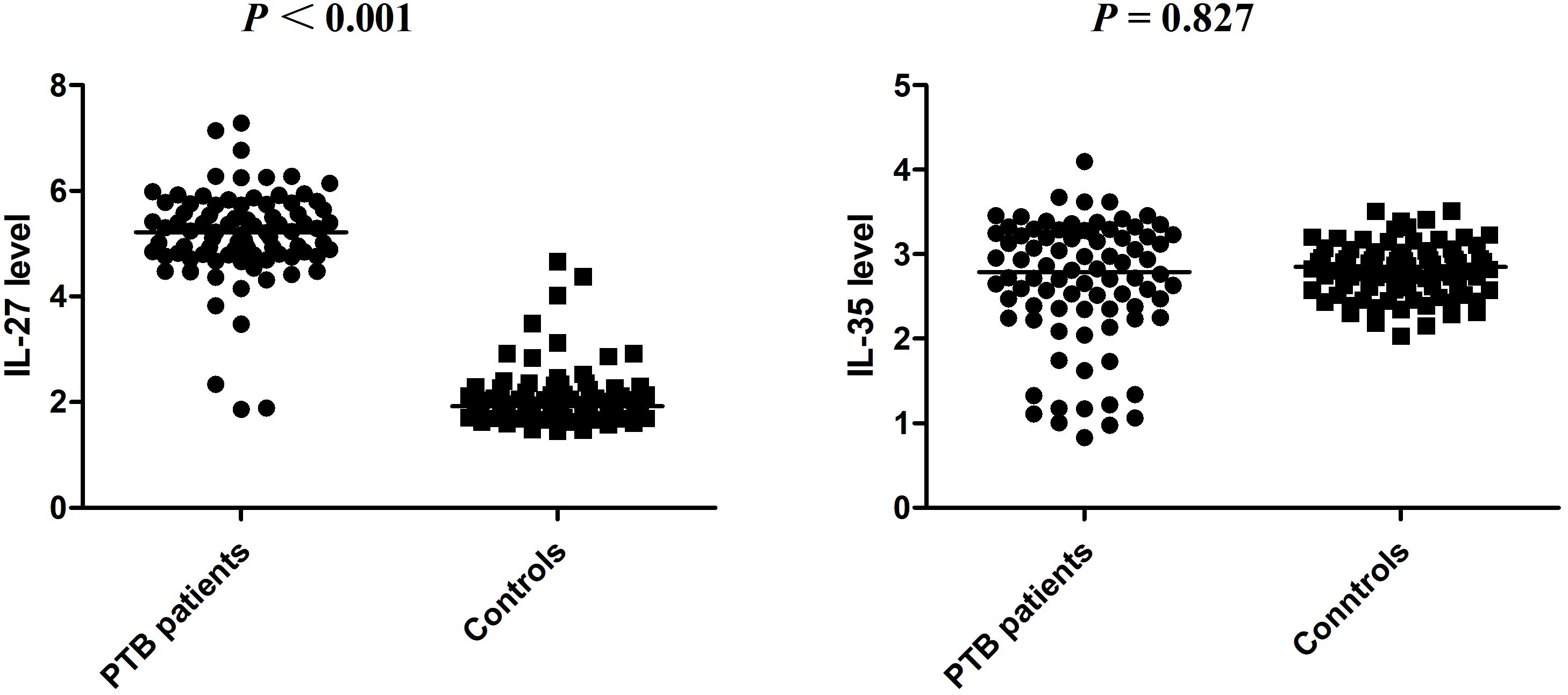
Figure 1 The expression levels of IL-27 and IL-35 in patients with PTB and normal controls.
The possible associations between IL-27 and IL-35 expression levels and several clinical features were also assessed among patients with PTB. The results found that the IL-27 and IL-35 expression levels were not associated with fever, drug resistance, DILI pulmonary infection, hypoproteinemia, and sputum smear-positive in patients with PTB (Table 5).
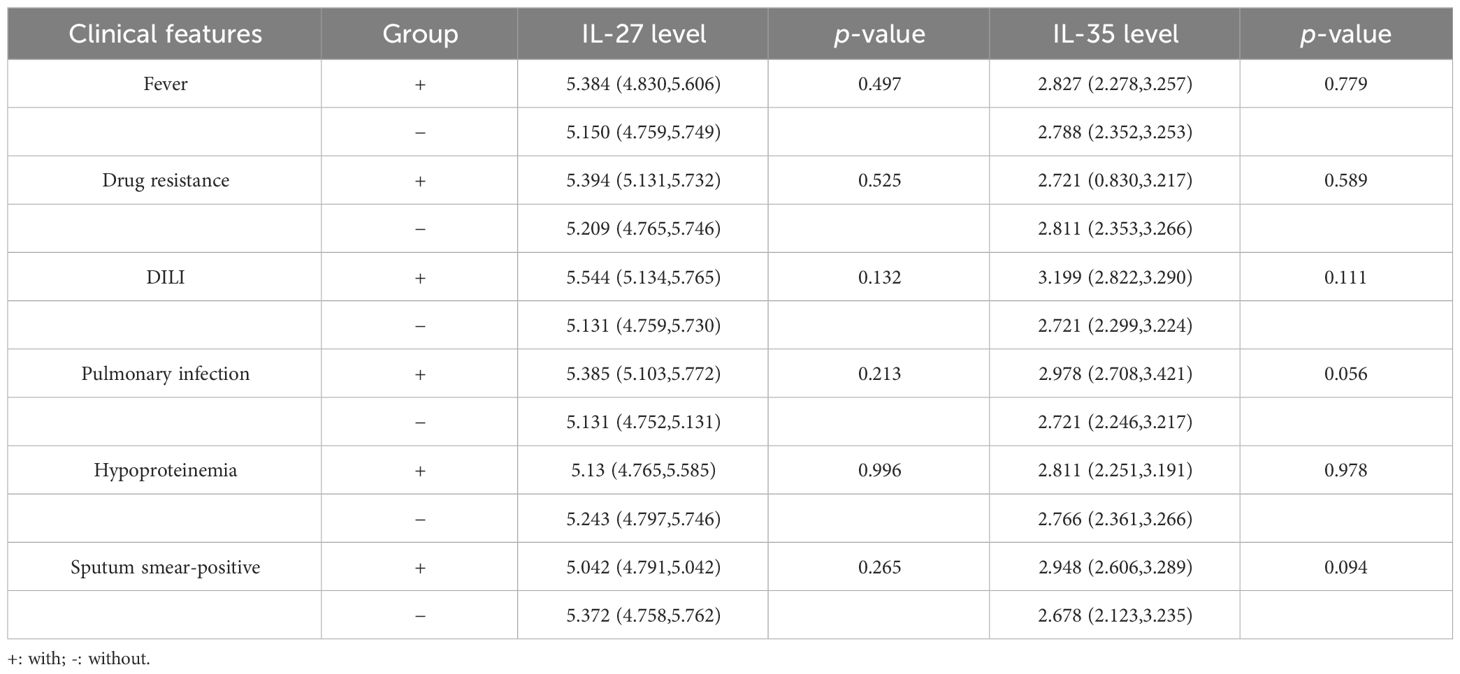
Table 5 The IL-27 and IL-35 levels and the main clinical manifestations among patients with PTB.
Association of IL-27 and IL-35 expression levels with their genotype frequencies in patients with PTBWe also investigated the relationship between IL-27 and IL-35 expression levels and their respective genotypes in 50 patients with PTB. However, no statistically significant association was found in this study (Table 6).
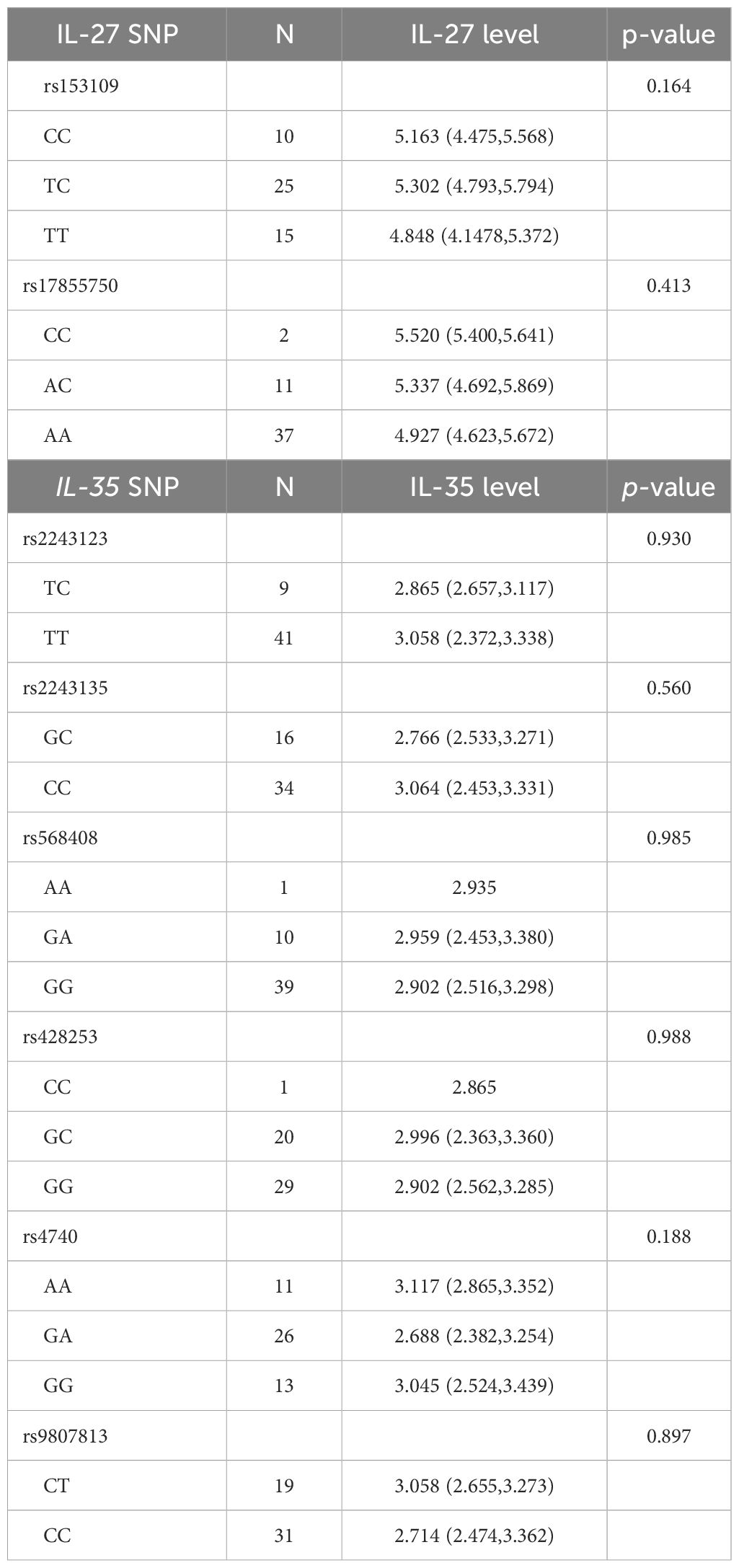
Table 6 Association between IL-27 and IL-35 levels and their genotype frequencies among patients with PTB.
DiscussionThe host immune response was an important factor affecting the development of PTB. The innate and adaptive immune systems of the body were consecutively activated after MTB infection, and immune cells released a large number of cytokines to exert anti-inflammatory effects (18, 19). Abundant studies have shown that IL-27 and IL-35 produced by multiple immune cells were essential for host defense against MTB infection. For example, the expression level of IL-27 in the sputum and plasma of patients with PTB was higher when compared to controls (20), and the serum IL-35 level in patients with PTB was also increased (14). To further assess the roles of IL27 and IL-35 genes in the pathogenesis of PTB, we focused on the analysis of the influence of IL27 and IL-35 gene polymorphisms on the susceptibility to PTB and several major clinical manifestations.
In the process of MTB infection, IL-27 cooperating with IL-12 activated Th1 cells against MTB infection through the JAK/STAT1 pathway, and IL-27 could also induce the activation of Tr1 cells by the JAK/STAT3 pathway, thereby inhibiting the host immune response (12). In addition to serum, the IL-27 concentration in sputum was confirmed to be positively associated with mycobacterial load (20). In addition, IL-27 was considered to be a potential biomarker for the diagnosis of PTB and TB pleurisy by comparing its expression level in pleural effusion and bronchoalveolar lavage fluid (21, 22). Notably, the polymorphism in the IL-27 gene could regulate host innate and adaptive immune response and was associated with infectious diseases, autoimmune diseases, and caner (23–25). For example, the rs17855750 variant was considered to be a risk factor for papillary thyroid cancer in the Chinese population (23), and a protective factor for ulcerative colitis in the Mexican population (24), while rs153109 might contribute to virus clearance in hepatitis C virus (HCV) infection (25). However, our present study revealed no association between rs153109 and rs17855750 polymorphism in the IL-27 gene and susceptibility to PTB. This was consistent with the study by Oh et al., and they suggested that the rs17855750, rs181206, rs153109, and rs181207 variants within the IL-27 gene were unrelated to PTB risk in a Korean population (26). In contrast, rs17855750 polymorphism was found to be significantly related to PTB risk (18). The differences in these studies might be due to factors such as different sample sizes, ethnic differences, host–pathogen interactions, and gene–environment interactions. However, these differences also reminded us that IL-27 gene polymorphism might be involved in PTB susceptibility, while more replication studies were still needed.
As an important anti-inflammatory cytokine, IL-35 was produced by B cells, regulatory T cells, monocytes, and dendritic cells, and played key roles in many immune-related diseases (27). The involvement of IL-35 in MTB infection had attracted more attention; for example, serum IL-35 level was significantly increased in patients with TB pleural effusion when compared to those with malignant pleural effusion (28). The study by Jiang et al. found that the expression level of IL-35 in peripheral blood mononuclear cells and Treg cells of patients with PTB was significantly increased compared to that in normal controls, and the increased IL-35 level in Treg cells could enhance their inhibitory effect among MTB infection (29). Further analysis showed that IL-35 inhibition resulted in a decrease in the number of Treg cells and an increase in the number of Th1 cells (29). This implied that IL-35 was closely involved in the pathogenesis of PTB; hence, it was very necessary to explore the relationship between IL-35 gene variation and PTB genetic susceptibility. Previous studies found that the IL-35 rs4740 variant showed a significant relationship with a decreased risk for PTB (30), while the rs568408 variant was not related to PTB risk (31). Nevertheless, our study found no significant association between rs2243123, rs2243135, rs568408, rs428253, rs4740, and rs9807813 variants in the IL-35 gene and PTB susceptibility. Interestingly, our study found that the GAC haplotype (rs428253–rs4740–rs9807813) in the IL-35 gene was associated with PTB susceptibility and was a protective factor affecting PTB development, which indicated that IL-35 gene variation had an important role in PTB susceptibility. This study also analyzed whether the IL-35 gene variant affected PTB susceptibility by altering IL-35 expression level. The results showed that there was no significant difference in IL-35 plasma level between patients with PTB and controls, and the above six SNPs had no significant effect on IL-35 plasma level in patients with PTB. Combined with the above results, we speculated that IL-35 gene variation might influence PTB susceptibility, but not through a single SNP, which still needed to be verified by further studies.
Some clinical characteristics of patients with PTB would have an important impact on the treatment process and prognosis of this disease, and these clinical manifestations were also affected by certain gene variations. Zhang et al. found that rs7041 and rs3733359 variants in the GC gene were statistically associated with pulmonary infection and fever in patients with PTB (32). Our previous study demonstrated that the IL-13RA1 rs2495636 polymorphism was related to pulmonary infection and drug resistance, and rs3795175 and rs638376 variants in IL-13RA2 were associated with drug resistance among patients with PTB (10). Analogously, this study revealed a significant association between the IL-27 rs17855750 GG genotype and fever. Moreover, IL-35 rs568408 and rs428253 variants were also reported as key factors in the occurrence of DILI and hypoproteinemia in patients with PTB. The above results indicated that IL-27 and IL-35 gene variations were significantly associated with some clinical manifestations of patients with PTB and might play a role in the development of this disease, which had positive effects on the selection of appropriate treatment regimens for patients with PTB.
Several limitations of this research should be noted. First, this study was a case–control study, and there were some biases in revealing causality. Second, this study did not exclude the potential influence of environmental factors, and the gene–environment interaction was also not established. Third, the sample size of this study might not be considered adequate, which could influence the power of our results.
In summary, our results firstly demonstrated that the GAC haplotype for rs428253, rs4740, and rs9807813 in the IL-35 gene was a protective factor for PTB susceptibility in a Chinese Han population, while IL-27 gene polymorphism was not involved in the susceptibility to PTB. Moreover, IL-27 and IL-35 gene variations might contribute to several clinical manifestations of patients with PTB, such as fever, DILI, and hypoproteinemia.
However, further studies should be conducted to assess the precise function of IL-27 and IL-35 gene variation in MTB infection.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementThis study was approved by the Ethical Committee of Anhui Chest Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsLG: Investigation, Methodology, Writing – original draft. YX: Investigation, Methodology, Writing – original draft. YL: Investigation, Writing – review & editing. PH: Investigation, Writing – review & editing. SL: Formal analysis, Writing – review & editing. YX: Investigation, Writing – review & editing. H-MW: Methodology, Project administration, Writing – review & editing. QH: Data curation, Formal analysis, Methodology, Writing – review & editing. HW: Methodology, Project administration, Writing – original draft.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the grant from Anhui Province Overseas Training Program for Outstanding Youth in Universities (gxfx2017012).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Carabalí-Isajar ML, Rodríguez-Bejarano OH, Amado T, Patarroyo MA, Izquierdo MA, Lutz JR, et al. Clinical manifestations and immune response to tuberculosis. World J Microbiol Biotechnol. (2023) 39:206. doi: 10.1007/s11274-023-03636-x
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Grifoni A, Alonzi T, Alter G, Noonan DM, Landay AL, Albini A, et al. Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis. Front Immunol. (2023) 14:1146704. doi: 10.3389/fimmu.2023.1146704
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Henao MI, Montes C, París SC, García LF. Cytokine gene polymorphisms in Colombian patients with different clinical presentations of tuberculosis. Tuberculosis (Edinb). (2006) 86:11–9. doi: 10.1016/j.tube.2005.03.001
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Sallakci N, Coskun M, Berber Z, Gürkan F, Kocamaz H, Uysal G, et al. Interferon-gamma gene+874T-A polymorphism is associated with tuberculosis and gamma interferon response. Tuberculosis (Edinb). (2007) 87:225–30. doi: 10.1016/j.tube.2006.10.002
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Li HM, Wang LJ, Huang Q, Pan HF, Zhang TP. Exploring the association between Th17 pathway gene polymorphisms and pulmonary tuberculosis. Front Immunol. (2022) 13:994247. doi: 10.3389/fimmu.2022.994247
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Li HM, Tang F, Huang Q, Pan HF, Zhang TP. Investigation on probable association between IL-13, IL-13RA1, and IL-13RA2 genes polymorphism and pulmonary tuberculosis. J Inflammation Res. (2022) 15:4527–36. doi: 10.2147/JIR.S374714
CrossRef Full Text | Google Scholar
11. Urazova OI, Churina EG, Hasanova RR, Novitskiy VV, Poletika VS. Association between polymorphisms of cytokine genes and secretion of IL-12p70, IL-18, and IL-27 by dendritic cells in patients with pulmonary tuberculosis. Tuberculosis (Edinb). (2019) 115:56–62. doi: 10.1016/j.tube.2019.02.003
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Abdalla AE, Li Q, Xie L, Xie J. Biology of IL-27 and its role in the host immunity against Mycobacterium tuberculosis. Int J Biol Sci. (2015) 11:168–75. doi: 10.7150/ijbs.10464
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Xie Q, Xu WD, Pan M, Lan YY, Liu XY, Su LC, et al. Association of IL-35 expression and gene polymorphisms in rheumatoid arthritis. Int Immunopharmacol. (2021) 90:107231. doi: 10.1016/j.intimp.2020.107231
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Kong B, Liu GB, Zhang JA, Fu XX, Xiang WY, Gao YC, et al. Elevated serum IL-35 and increased expression of IL-35-p35 or -EBI3 in CD4(+)CD25(+) T cells in patients with active tuberculosis. Am J Transl Res. (2016) 8:623–33.
PubMed Abstract | Google Scholar
15. Dai YC, Wang WD, Zhang JA, Chen C, Luo HL, Xu H, et al. MTB driven B cells producing IL-35 and secreting high level of IL-10 in the patients with active pulmonary tuberculosis. Mol Immunol. (2019) 112:175–81. doi: 10.1016/j.molimm.2019.05.004
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Feng M, Zhou S, Liu T, Yu Y, Su Q, Li X, et al. Association between interleukin 35 gene single nucleotide polymorphisms and the uveitis immune status in a Chinese Han population. Front Immunol. (2021) 12:758554. doi: 10.3389/fimmu.2021.758554
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Yu W, Yang W. Interlukin-27 rs153109 polymorphism confers the susceptibility and prognosis of aplastic anemia in Chinese population. Int J Lab Hematol. (2022) 44:150–6. doi: 10.1111/ijlh.13700
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Li M, Jiao L, Lyu M, Song J, Bai H, Zhang C, et al. Association of IL27 and STAT3 genetic polymorphism on the susceptibility of tuberculosis in Western Chinese Han population. Infect Genet Evol. (2020) 83:104324. doi: 10.1016/j.meegid.2020.104324
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Shaukat SN, Eugenin E, Nasir F, Khanani R, Kazmi SU. Identification of immune biomarkers in recent active pulmonary tuberculosis. Sci Rep. (2023) 13:11481. doi: 10.1038/s41598-023-38372-7
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Cao J, Zhang L, Li D, Xu F, Huang S, Xiang Y, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10. Chest. (2012) 141:121–30. doi: 10.1378/chest.10-3297
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Lin S, Wang Y, Li Y, Xiao D, Guo J, Ma W, et al. Diagnostic accuracy of interleukin-27 in bronchoalveolar lavage fluids for pulmonary tuberculosis. Infect Drug Resist. (2019) 12:3755–63. doi: 10.2147/IDR
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Nie X, Yuan F, Chen P, Pu Y, Zhu J, Wang Y, et al. Association between IL-27 gene polymorphisms and risk of papillary thyroid carcinoma. biomark Med. (2017) 11:141–9. doi: 10.2217/bmm-2016-0283
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Yamamoto-Furusho JK, Posadas-Sánchez R, Alvarez-León E, Vargas-Alarcón G. Protective role of Interleukin 27 (IL-27) gene polymorphisms in patients with ulcerative colitis. Immunol Lett. (2016) 172:79–83. doi: 10.1016/j.imlet.2016.02.010
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Fawzy MM, Wahid A, Nazmy MH, Hashem M, Waked I, Abdelwahab SF. Association of interleukin-27 rs 153109 single nucleotide polymorphism with spontaneous resolution of hepatitis C virus - genotype 4a infection in Egyptian patients. Asian Pac J Cancer Prev. (2016) 17:2093–7. doi: 10.7314/APJCP.2016.17.4.2093
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Oh JY, Sim JK, Jung WJ, Min KH, Lee EJ, Hur GY, et al. Association between interleukin-27 polymorphisms and pulmonary tuberculosis. Int J Tuberc Lung Dis. (2015) 19:702–8. doi: 10.5588/ijtld.14.0773
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Zhang J, Zhang Y, Wang Q, Li C, Deng H, Si C, et al. Interleukin-35 in immune-related diseases: protection or destruction. Immunology. (2019) 157:13–20. doi: 10.1111/imm.13044
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Jiang H, Cui B, Zhang J. Mycobacterium tuberculosis (MTB) antigen-induced upregulation of interleukin-35 expression in patients with MTB infection: In vitro blockade of the effects of interleukin-35 on T lymphocyte subsets. Pathog Dis. (2021) 79:ftab035. doi: 10.1093/femspd/ftab035
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Naz A, Aslam MA, Khan AUH, Rasul S, Manzoor H, Iqbal R, et al. Genetic polymorphism in association with susceptibility to tuberculosis: a study in a Pakistani population. Braz J Microbiol. (2019) 50:429–34. doi: 10.1007/s42770-019-00048-8
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Wang J, Tang S, Shen H. Association of genetic polymorphisms in the IL12-IFNG pathway with susceptibility to and prognosis of pulmonary tuberculosis in a Chinese population. Eur J Clin Microbiol Infect Dis. (2010) 29:1291–5. doi: 10.1007/s10096-010-0985-0
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Zhang TP, Chen SS, Zhang GY, Shi SJ, Wei L, Li HM. Association of vitamin D pathway genes polymorphisms with pulmonary tuberculosis susceptibility in a Chinese population. Genes Nutr. (2021) 16:6. doi: 10.1186/s12263-021-00687-3
留言 (0)