Thyroid cancer (TC) is the most common malignant tumor of the endocrine system, and its incidence is increasing (1). Over 90% of cases are differentiated thyroid cancer (DTC). While the majority of DTC cases have a favorable prognosis, with a 10-year survival rate of 90% (2), a subset of patients experience recurrence or distant metastases even after surgery, 131I therapy, and thyrotropin (TSH) inhibition therapy (3), and progress to radioactive iodine-refractory differentiated thyroid cancer (RAIR-DTC) (4).
Current therapies for RAIR-DTC, including targeted therapy and external beam radiotherapy, have advantages and disadvantages. Although the targeted drugs sorafenib and lenvatinib are approved for treating RAIR-DTC (5), the impact on overall survival is insignificant (6). There are apparent drug resistance and adverse reactions (5), such as hand-foot syndrome, myelosuppression, hypertension, proteinuria, and diarrhea (7), which make it challenging to meet clinical needs. Molecular targeted drugs, such as mitogen-activated protein kinase (MAPK) inhibitors and phosphatidylinositol 3-kinase (PI3K)/AKT inhibitors, have the potential to redifferentiate RAIR-DTC (8). Although the combination of 131I therapy after redifferentiation therapy showed positive effects in RAIR-DTC, long-term follow-up data are lacking. Issues such as population screening, exploration of individualized treatment, and criteria for evaluating therapeutic response remain to be addressed. External beam radiotherapy cannot continuously affect the complete division cycle of the tumor; it damages the surrounding tissue. In addition, TC cells are hypersensitive to external beam radiotherapy (9), limiting local control’s effectiveness.
After Whitmore (10) successfully implanted 125I seeds to treat prostate cancer in 1972, 125I seed implantation became increasingly popular after the success of TPS in the 1980s. Over the last three decades, due to its effective local control, minimally invasive nature, low complication rates, and good tolerability, 125I seed implantation has been widely utilized in treating malignant tumors such as prostate and lung cancer (11). This study aims to explore the application of 125I seed implantation in treating bone, lymph node, and lung metastases in RAIR-DTC. Further details are provided below.
2 Materials and methods2.1 Clinical dataData from 36 RAIR-DTC patients hospitalized at Jiangxi Cancer Hospital from January 2015 to February 2022 were retrospectively collected. The average age was 57.39 ± 14.76 years, with 14 males and 22 females. A total of 73 lesions were recorded, including four local recurrences and metastases in various parts of the body: 19 in lymph nodes (8 central, three lateral cervical, two supraclavicular, and six mediastinal), 4 in lung (3 in the left lung and 1 in the right lung), 4 in pleura, and 42 in bones (1 in the mandible, 18 in the vertebrae, 4 in the sternum, 6 in the ribs, 2 in the extremities, 11 in the pelvis). Of the bone metastases, 28 had bone destruction and soft tissue formation, while 14 had bone destruction only. The staging of patients is based on the American Joint Committee on Cancer's 8th edition thyroid cancer staging system (12). The Jiangxi Cancer Hospital Ethics Committee approved all treatment plans. In advance of the surgery, patients were informed about their condition, the expected efficacy of 125I seed implantation therapy, alternative treatments such as external beam radiotherapy and chemotherapy, as well as potential side effects and toxic effects. The patient signed an informed consent form after accepting the treatment plan. A total of 1,971 125I seeds were implanted. The median number of particles injected per lesion was 20 (range 4 to 146) with a median radioactivity of 2.59 × 107 Bq (range 1.85 × 107 to 3.33 × 107 Bq). Table 1 provides detailed baseline data on the patients and foci.
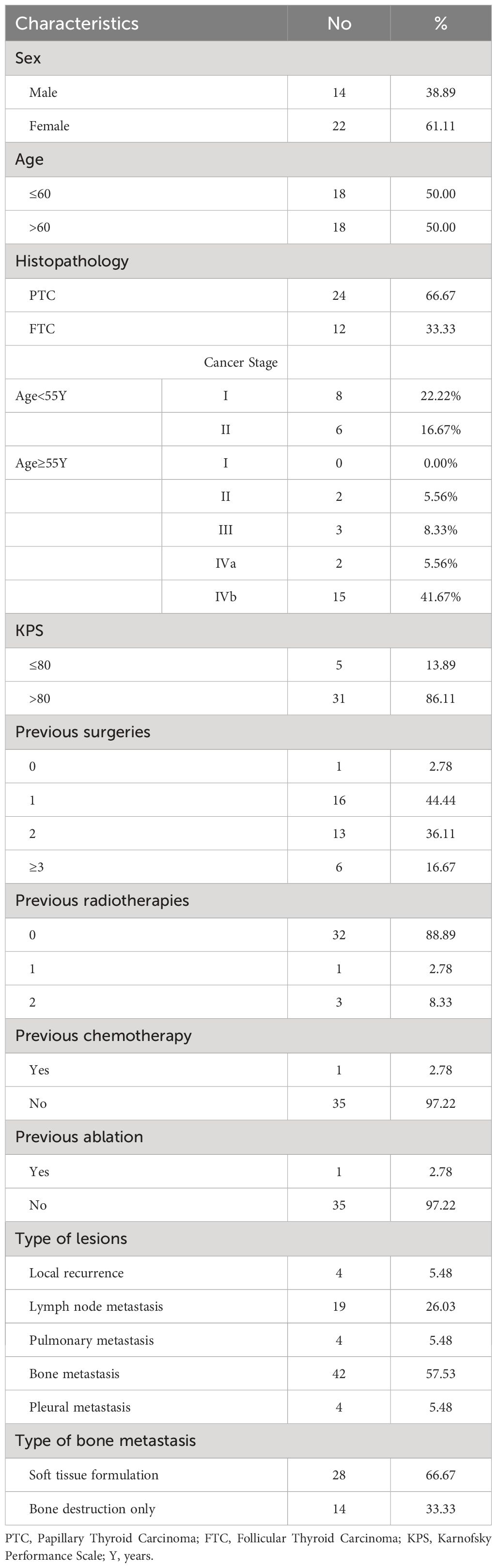
Table 1 Patient characteristics(n=36).
2.1.1 Critical eligibility criteria includedDTC was confirmed by pathology, with local recurrence or distant metastasis of residual thyroid cancer and conforming to any of the following RAIR-DTC criteria: (1) no uptake of 131I in the initial 131I treatment; (2) loss of iodine uptake capacity in previously functional iodine-avid lesions; (3) disease progression after 131I therapy, including gradual enlargement of the lesion and continuously increasing levels of serum thyroglobulin (Tg).
2.1.2 Critical exclusion criteria were as followsPatients with severe physical diseases, such as severe dysfunction of the cardiac, pulmonary, hepatic, or renal systems, poor compliance, inability to tolerate 125I seed implantation, severe acute infectious or chronic infection with acute exacerbation, severe coagulopathy that may lead to serious complications like infection or bleeding, pregnant or lactating women affecting fetal and infant growth and development, patients with cachexia or expected survival of ≤3 months and patients with positive serum anti-thyroglobulin antibodies (TgAb) that may impact the assessment of treatment efficacy.
2.2 MaterialsTianjin Said Biopharmaceutical Co., Ltd. supplies 125I seeds, specifically seed model 6711-99. Physicists verify the quality of seeds by conducting quality control and sampling at least 10% of the seeds (100% if there are fewer than five seeds). They ensure that the seed radioactivity is within a ±5% deviation to meet the required standards for seed quality. Beijing Atomic Hi-Tech Co., Ltd. provided the seed implantation device, and the 18G seed implantation needle was provided by Hakko International Trading (SHANGHAI) Co., Ltd. The CT scan was performed using a SPECT/CT Symbia T2 from Siemens Healthineers, Germany, with a slice thickness of 5 mm, a slice pitch of 2 mm, a current of 48 mA, and a voltage of 130 kV. The KL-SIRPS-3D model from Beijing Astro Technology Ltd. was used as the treatment planning system.
2.3 MethodAll patients received 125I brachytherapy treatment and follow-up care at the Department of Nuclear Medicine within Jiangxi Provincial Cancer Hospital. The preoperative evaluation process includes assessments of coagulation function, liver and kidney function, cardiopulmonary function, and local CT scans. A collaborative effort between physicians and physicists was undertaken to establish a treatment regimen with a prescription dose ranging from 80 to 120 Gy. For individuals who have undergone external beam radiotherapy within five months, a dose of 80 Gy is recommended. For individuals with a history of external beam radiotherapy lasting more than five months or those who have received a cumulative dose of 131I therapy exceeding 2.22*1010 Bq, a dose of 100 Gy is recommended. Patients who did not fall into these specific categories were prescribed 120 Gy. There was a difference in the activity level among 125I particles implanted at different locations. The activity level was 1.85 x 107 Bq for near-surface lesions, 3.33 x 10^7 Bq for bone metastases, and 2.22-2.96 x 107 Bq for other metastatic sites. The CT-guided 125I seed implantation procedure was conducted following the prescribed treatment regimen. Subsequent evaluations were scheduled at 1-, 3-, and 5-months post-surgery, focusing on lesion dimensions, serum Tg levels, pain levels, and adverse reactions. Lesion size was determined using CT imaging, with a total of 73 RAIR-DTC metastases from various locations included in the study. Measurements were conducted for 59 metastases, excluding 14 lesions that solely impacted bone tissue without associated soft-tissue hyperplasia. This rendered the assessment of treatment efficacy based on tumor size alterations unfeasible. Lymph nodes were assessed based on their shortest diameter (Figure 1), while other target lesions were evaluated based on their longest diameter (Figure 2). Each measurable lesion underwent an average measurement derived from a minimum of three readings, with all measurements conducted by the same individual to minimize measurement discrepancies.
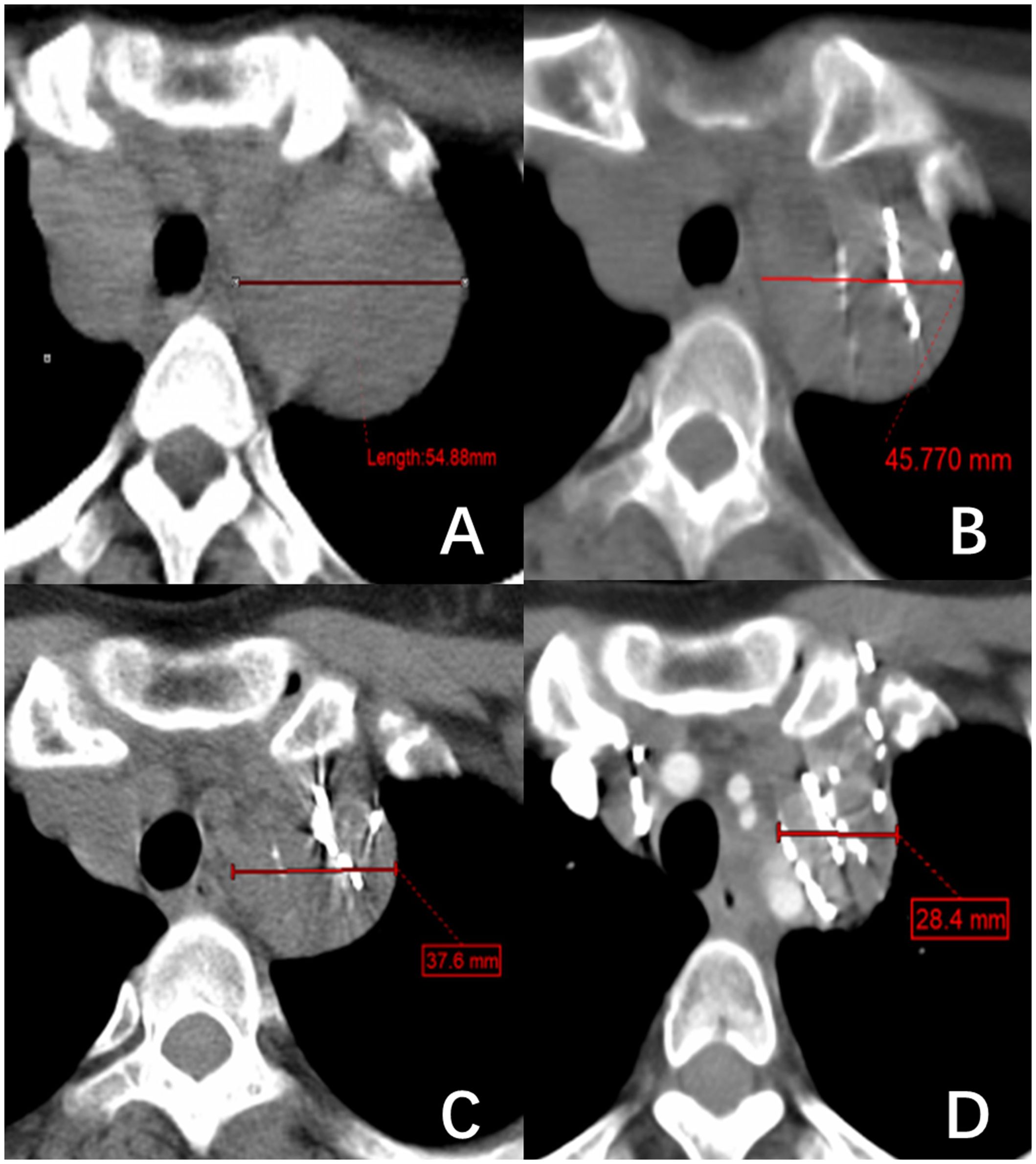
Figure 1 A right superior mediastinal lymph node metastasis of thyroid follicular papillary carcinoma. (A–D) show the changes in lymph node short diameter before 125I brachytherapy (54.88 mm) and after treatment at one month (45.77 mm), three months (37.6 mm), and five months (28.4 mm), respectively.
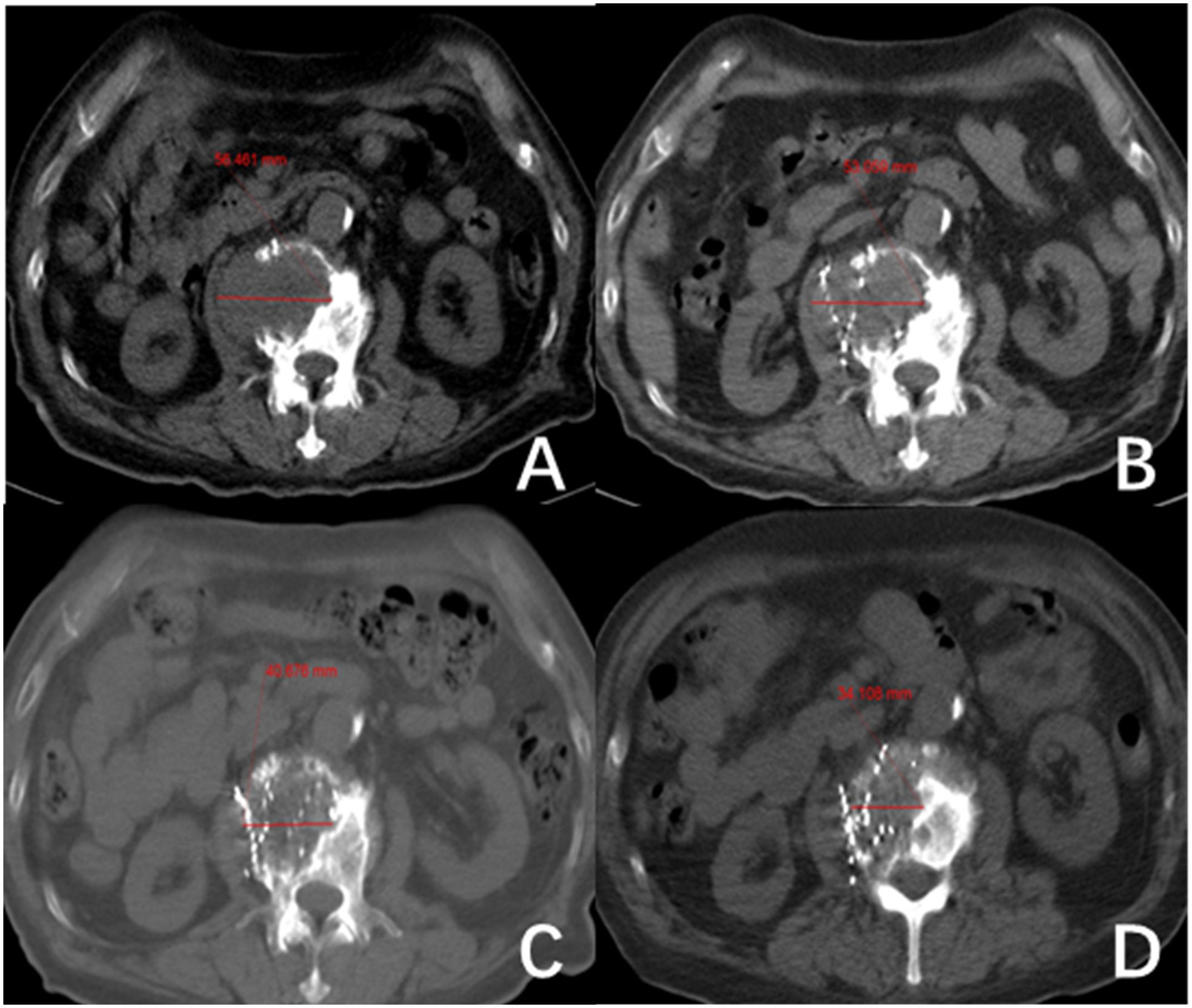
Figure 2 The second lumbar vertebra shows metastasis from follicular thyroid carcinoma (FTC). (A–D) show the changes in length and diameter of paraspinal soft tissue metastasis before 125I seed implantation (56.46 mm) and after treatment at one month (53.1 mm), three months (40.68 mm) and five months (34.11 mm), respectively.
Response Evaluation Criteria in Solid Tumors (RECIST 1.1) (13) were used to assess treatment response. Local Effective (LE) was defined as a 30% or greater reduction in the longest diameter of the targeted lesion. Progressive Disease (PD) was defined as a 20% or greater increase in the longest diameter of the targeted lesion, while Stable Disease (SD) was defined as being in between. The Local Efficacy Rate (LER) and Local Control Rate (LCR) were calculated by recording the number of lesions classified as LE, SD, and PD at months 1, 3, and 5. (LER = LE/total number of lesions × 100%; LCR = (LE + SD)/total number of lesions × 100%).
Use the Visual Analogue Scale to record patients’ pain scores. The scale ranges from 0 to 10 points, with pain increasing incrementally. 0 indicates no pain, 1-3 indicate mild pain that is still tolerable and does not interfere with sleep or normal life, 4-6 indicate moderate pain that interferes with sleep and requires analgesic medication to alleviate it, and 7-10 show intense and intolerable pain that seriously interferes with sleep and diet and requires strong analgesic drugs.
Postoperative adverse reactions, including infection, bleeding, pneumothorax, bone marrow suppression, and seed displacement, were recorded. Radiation injury was graded according to the Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) toxicity criteria (14).
2.4 Statistical analysisThe data were analyzed using IBM SPSS 26.0. The count data (n%) and skewed measures [Md (Q1, Q3)] are presented. Group comparisons were conducted using the paired Wilcoxon rank-sum test, with statistical significance set at P < 0.05.
3 ResultsThe diameters of the tumor lengths were 27.45 (12.30, 39.98mm), 20.07 (11.98, 37.58mm) and 20.39 (10.56, 33.20mm) at months 1, 3, and 5 after 125I seed implantation, respectively. These values were significantly reduced compared to the initial diameter of 32.67 (17.70, 45.72mm) before treatment (Z= -6.227, -6.272, -6.189; all P < 0.001). Two consecutive post-treatment results were more minor and statistically significant than the previous results (P < 0.001). At one month postoperatively, there were 14 cases of LE, 44 cases of SD, and 1 case of PD. At three months postoperatively, there were 23 cases of LE, 34 cases of SD, and 2 cases of PD. At five months postoperatively, there were 31 cases of LE, 25 cases of SD, and 3 cases of PD. The LER at 1, 3, and 5 months postoperatively was 23.73% (14/59), 38.98% (23/59), and 52.54% (31/59), respectively. The LCR was 98.31% (58/59) at one month, 96.61% (57/59) at three months, and 94.92% (56/59) at five months postoperatively. The interquartile range includes the data [Md(Q1, Q3)]. Please refer to Table 2 for further details.
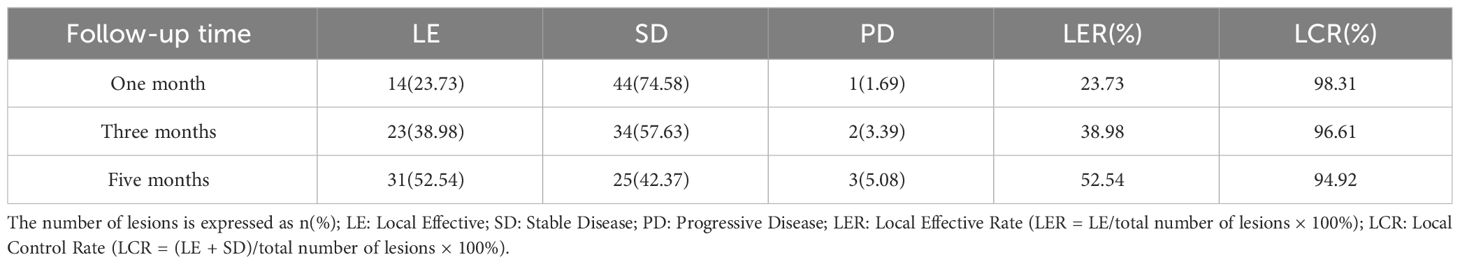
Table 2 The efficacy of radioactive 125I seed implantation.
The serum Tg levels of 36 patients were measured before and after 125I seed implantation. The levels were measured in the 1st, 3rd, and 5th postoperative months. Specifically, the serum Tg levels were 249.45 (79.39, 4718.75) ng/ml before treatment, 193.40 (44.53, 2829.00) ng/ml in the 1st month, 192.10 (25.58, 1758.00) ng/ml in the 3rd month, and 136.25 (16.57, 1553.25) ng/ml in the 5th month. The results showed that the serum Tg level decreased significantly after treatment (Z = -3.881, -4.587, -4.823, all P < 0.001). Furthermore, serum Tg levels had returned to normal or were approaching normal in some patients. Two consecutive post-treatment results were more minor and statistically significant than the previous ones (Z = -3.661, -3.931, and P < 0.001). Please refer to Table 3 for further details.
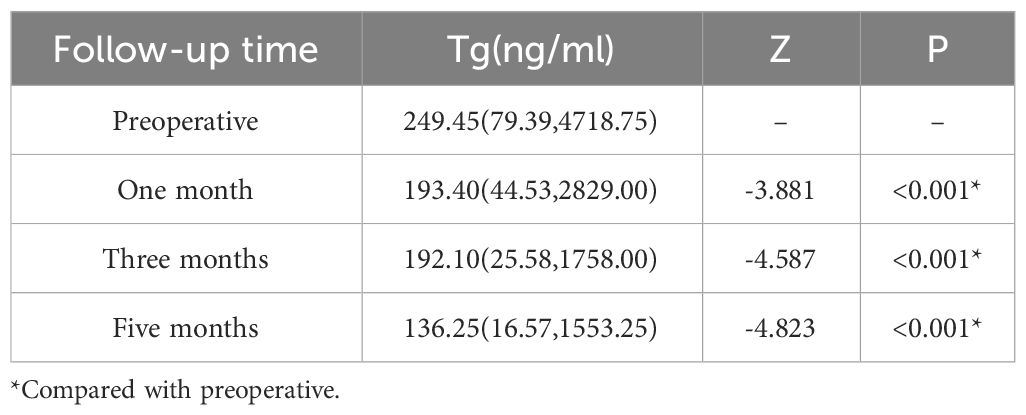
Table 3 The serum Tg level is associated with radioactive 125I seed implantation.
The pain scores of 36 patients were recorded at months 1, 3, and 5 after treatment. The scores were 3.00 (2.25, 4.00), 2.00 (2.00, 3.00), and 2.00 (1.00, 3.00), respectively. These scores were significantly lower than the score of 5.00 (4.00, 6.00) recorded before treatment (Z = -5.339, -5.330, -5.278; all P < 0.001). Statistically significant differences were observed between month one and month 3 (Z = -4.914, P < 0.001) and between month three and month 5 (Z = -4.104, P < 0.001). Please refer to Table 4 for more information on this.
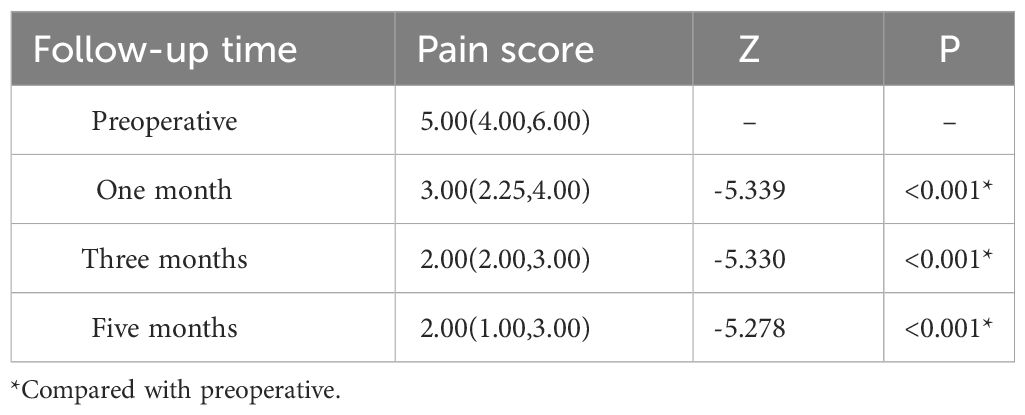
Table 4 Pain score related to radioactive 125I seed implantation.
All 36 patients remained infection-free following 125I seed implantation. Two cases of radiation injury were reported: one case of Grade I radiation dermatitis, which gradually improved after treatment with anti-inflammatory drugs, and one case of Grade I radiation pneumonitis, which resolved without specific treatment. Puncture-related complications occurred in two cases. One minor bleeding was controlled by applying local pressure to stop it. In the other case, a small pneumothorax was detected by CT after a few days and resolved spontaneously. Mild myelosuppression was observed in three cases, which was managed with leukocyte-boosting medications and later determined. Five instances of seed displacement were reported, with one involving the left ventricle, one affecting the left upper lung, and three affecting the chest cavity. Radiological toxicity did not occur in these cases, affecting adjacent vital organs. Further details are provided in Table 5.
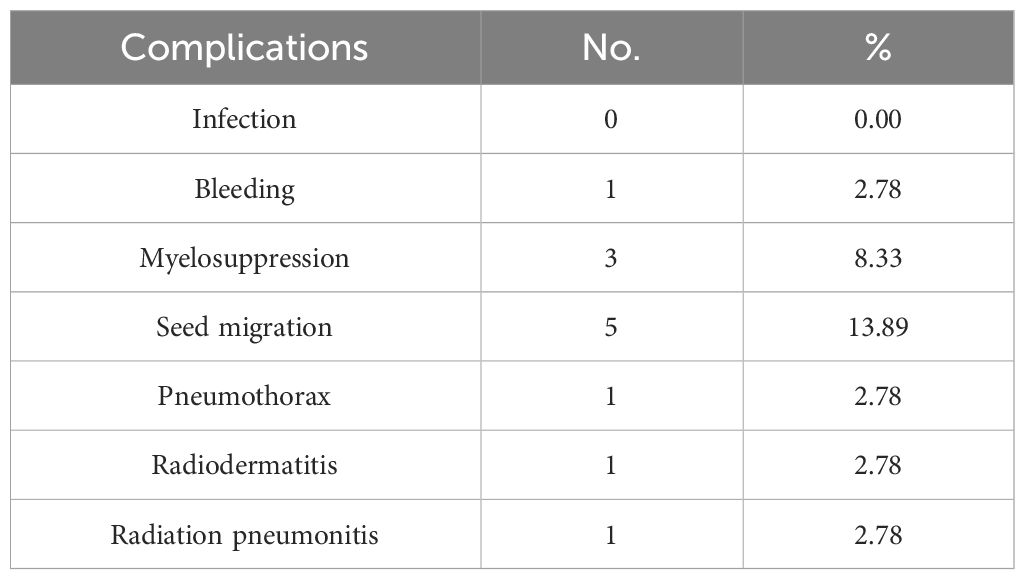
Table 5 Complications related to radioactive 125I seed implantation (n=36).
4 DiscussionThe available studies on the clinical benefit of 125I seed implantation for treating RAIR-DTC are limited, and most studies only focus on single types of metastatic cancer, such as lymph nodes or bone metastases (15, 16). This study aims to comprehensively evaluate the clinical improvement of 125I seed implantation for RAIR-DTC across a wide range of metastatic sites, including locally recurrent foci in the thyroid surgical bed, metastatic lymph nodes in the neck and mediastinum, lung metastases, bone metastases, and pleural metastases.
This study included a total of 73 RAIR-DTC metastases from various sites. However, 14 lesions that only destroyed bone without soft tissue hyperplasia were excluded because their efficacy could not be evaluated based on tumor size changes. The LER of the remaining 59 metastases was 23.73%, 38.98%, and 52.54% at postoperative months 1, 3, and 5, respectively. The LCR was 98.31%, 96.61%, and 94.92% at postoperative months 1, 3, and 5, respectively. The study’s results align with Chen et al.’s (15) report of a 95% LCR for RAIR-DTC bone metastases in 9 cases. Additionally, the results are superior to Chen et al.’s (16) report of over a 90% shrinkage rate of metastatic lymph nodes in the neck of RAIR-DTC through ultrasound-guided 125I seed implantation. The data show that 125I seed implantation effectively reduces the tumor size and achieves local tumor control (refer to Figures 1, 2). The physical properties of 125I seeds, such as their low energy (27~35 keV) and long half-life (59.5 days), strongly correlate with these results. Due to its low energy and long half-life, the radiation emitted by the 125I seeds implanted in the tumor causes minimal damage to surrounding normal tissues while delivering a lethal dose of radiation to the tumor cells. Liyuan Zhang (17) reports that small doses of radiation can stimulate the immune system to suppress tumor growth. Due to the necessity of considering the tolerated dose of surrounding normal tissues, external beam radiotherapy cannot administer a radical dose to tumors. Furthermore, 125I particles emit rays continuously over an extended period, impacting the entire cycle of tumor cell division for an extended duration. In contrast, conventional external beam radiotherapy involves multiple split irradiations, which make tumor cells in the interphase of division insensitive to the rays. This is the primary reason why 125I seeds can effectively treat cases that have failed conventional external beam radiotherapy, chemotherapy, and 131I therapy. Proton and heavy ion radiotherapy can induce double-strand breaks in the chromosomes of tumor cells (18), resulting in a loss of their ability to increase. Due to its unique Bragg peak effect, this effect is achieved with minimal damage to surrounding normal tissues. However, proton and heavy ion therapy are costly and require highly qualified personnel. It is mainly used in clinical research and is still being developed for routine tumor treatment.
The study identified three progressive lesions that may have been caused by the presence of poorly differentiated tumor tissue. These cells have a short doubling time, and the initial dose rate of 125I seed is low (approximately 0.07-0.09 Gy/h) (17), which may result in tumor progression before the 125I seed has had a chance to take effect. 125I seeds can treat tumors by slowly releasing radiation and gradually increasing the cumulative dose in the tumor cells. In this study, most RAIR-DTC tumor cells were relatively inactive and slow-growing. Thus, although the LCR at 1 and 3 months postoperatively were low and most lesions showed SD, the treatment’s effectiveness became more apparent as the cumulative tumor dose increased, consistent with a progressive increase in LCR and LER over time.
Serum Tg is a crucial indicator of postoperative relapse and metastasis in patients with DTC (19). A decrease in serum Tg levels may indicate the effectiveness of DTC treatment if a patient tests negative for TgAb. The study found that the serum Tg levels of the patients decreased after treatment compared to before treatment (both P < 0.001). Additionally, the serum Tg levels of some patients had returned to normal. These findings suggest that 125I seeds can effectively inhibit and kill RAIR-DTC tumor cells.
The progression of RAIR-DTC may cause severe pain and a significant reduction in patients’ quality of life, mainly when metastatic lesions occur in the bone and pleura (20). The results of this study indicate that 125I seed implantation can effectively relieve and reduce patient pain, resulting in significantly lower pain scores post-treatment compared to pre-treatment.
While 125I seed implantation is minimally invasive, it is still considered an invasive treatment. Therefore, it may lead to complications related to puncture, such as bleeding, pain, and pneumothorax. As tumor volume and metastases increase, the number and activity of 125I seeds required for implantation also increase, leading to higher radiation exposure and an increased risk of complications. In addition, adverse reactions related to radiation, such as radioactive damage and necrosis, may be experienced (21). Three patients experienced mild bone marrow suppression after 125I seed implantation. Patient 1 had 146 particles implanted with an activity of 2.22*107 Bq in an extensive mediastinal lymph node metastasis. In contrast, patients 2 and 3 had 57 and 88 seeds planted with an activity of 2.59*107 Bq in bone metastases with soft tissue formation, respectively. A small number of adverse events were reported in this study, including pneumothorax, bleeding, radiation pneumonitis, dermatitis, and mild myelosuppression. These issues were resolved through observation or symptom management, indicating the relative safety of 125I brachytherapy.
5 Conclusions125I seed brachytherapy has demonstrated reasonable local tumor control, effective pain relief, and minimal side effects in RAIR-DTC. However, this study has several limitations compared to similar research efforts. These limitations include a small sample size, the absence of a control group, and insufficient reliability in data analysis. These limitations hinder a comprehensive depiction of the actual scenario in particle therapy. Furthermore, the follow-up period is relatively short, which limits a thorough evaluation of the medium- and long-term effectiveness of 125I brachytherapy (22). This is especially true regarding potential toxic side effects resulting from dose accumulation. The therapy effectiveness evaluation is based solely on changes in tumor dimensions observed through CT scans. However, this approach neglects the consideration of the tumor’s functional metabolism at the treatment site post-implantation (refer to Figure 3) or alterations in other tumor sites induced by treatment that may enhance immune responses (23). It is essential to conduct further comprehensive clinical investigations to explore these aspects.
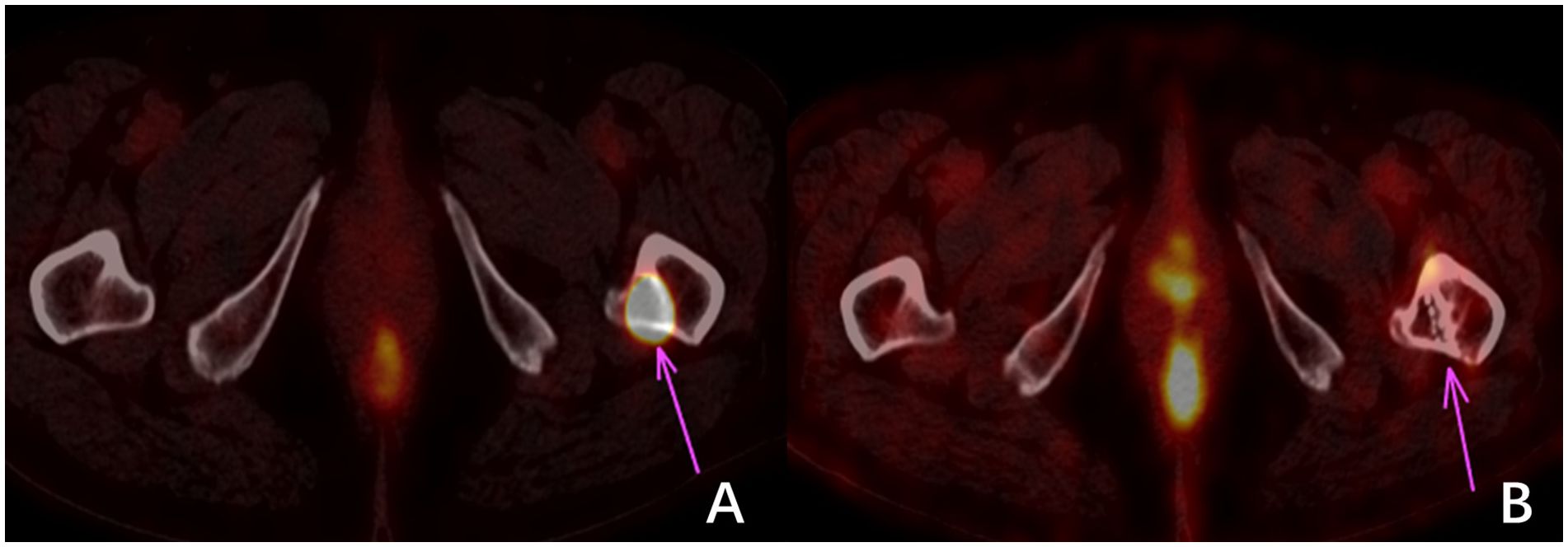
Figure 3 18F-FDG imaging of bone metastasis in the left proximal femur of thyroid carcinoma. (A) shows a high uptake of 18F-FDG in the metastatic focus before treatment. In contrast, (B) shows no significant change in the size of the metastatic focus and bone destruction three months after treatment. However, no 18F-FDG uptake indicates that the tumor cells were inactivated.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Jiangxi Cancer Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsQW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. LT: Project administration, Supervision, Validation, Writing – review & editing. XT: Data curation, Investigation, Visualization, Writing – original draft. WW: Funding acquisition, Project administration, Writing – original draft. YS: Investigation, Writing – original draft. ZW: Investigation, Writing – original draft. MK: Investigation, Writing – review & editing. ZC: Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Chinese Society of Clinical Oncology Guidelines Working Committee. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for differentiated thyroid cancer 2021. J Cancer Control Treat. (2021) 34:1164–200. doi: 10.3969/i.issn.1674-0904.2021.12.013
CrossRef Full Text | Google Scholar
2. Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, et al. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid. (2015) 25:1106–14. doi: 10.1089/thy.2015.0104
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Chinese Society of Nuclear Medicine. Clinical guidelines for 131I therapy of differentiated thyroid cancer. Chin J Nucl Med Mol Imaging. (2021) 41:218–41. doi: 10.3760/cma.j.cn321828-20201113-00412[5
CrossRef Full Text | Google Scholar
5. Teng LS, Xu ZH, Wang WB. Advances in targeted therapy for refractory differentiated thyroid cancer. Tumor Prev Treat Res. (2023) 5:452–7. doi: 10.3971/i.issn.1000-8578.2023.23.0009
CrossRef Full Text | Google Scholar
6. Tang XW, Shi L, Bu T, Wang F. Current status and latest advances in targeted therapy for refractory thyroid cancer. J Immunoassay Clin Chem. (2020) 7:1255–60. doi: 10.11748/bimy.issn.1006-1703.2020.07.035
CrossRef Full Text | Google Scholar
7. Hong CM, Ahn BC. Redifferentiation of radioiodine refractory differentiated thyroid cancer for reapplication of I-131 therapy. Front Endocrinol (Lausanne). (2017) 8:260. doi: 10.3389/fendo.2017.00260
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Huang NS, Wei WJ, Xiang J, Chen JY, Guan Q, Lu ZW, et al. The efficacy and safety of anlotinib in neoadjuvant treatment of locally advanced thyroid cancer: A single-arm phase II clinical trial. Thyroid. (2021) 31:1808–13. doi: 10.1089/thy.2021.0307
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Di X, Zhang H, Liu X, Zhao J, Gao Z, Yu H, et al. A new technique for trans-perirectal iodine-125 seed implantation in prostatic cancer guided by CT and 3D printed template: Two case reports. Front Oncol. (2022) 12:1031970. doi: 10.3389/fonc.2022.1031970
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Li P, Fan J, Zhang K, Wang J, Hu M, Yang S, et al. Interstitial 125I brachytherapy as a salvage treatment for refractory cervical lymph node metastasis of thoracic esophageal squamous cell carcinoma after external irradiation with a CT-guided coplanar template-assisted technique: A retrospective study. Technol Cancer Res Treat. (2022) 21. doi: 10.1177/15330338221103102
CrossRef Full Text | Google Scholar
12. Lamartina L, Grani G, Arvat E, Nervo A, Zatelli MC, Rossi R, et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO#2). Endocr Relat Cancer. (2018) 25:L7–L11. doi: 10.1530/ERC-17-0453
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, et al. [New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1)]. Gan To Kagaku Ryoho. (2009) 36:2495–501. doi: 10.1016/j.ejca.2008.10.026
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. (1995) 31:1341–6. doi: 10.1016/0360-3016(95)00060-C
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Chen Z, Tan L, Su Y, Xie J, Zhou A, Zou W, et al. Clinical application of 125I seed implantation in the treatment of refractory thyroid cancer bone metastasis. Chin J Nucl Med Mol Imaging. (2018) 38:14–6. doi: 10.3760/cma.j.issn.2095-2848.2018.01.004
CrossRef Full Text | Google Scholar
16. Chen W, Luo YK, Zhang Y, Song Q, Tang J. Ultrasound-guided implantation of radioactive 125I seed in radioiodine refractory differentiated thyroid carcinoma. BMC Cancer. (2021) 21:834. doi: 10.1186/s12885-021-08500-5
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Kong Y, Ma Y, Zhao X, Zhang L. Research progress on the sensitization of radiotherapy to the efficacy of PD-1/PD-L1 inhibitors. Chin J Radiat Oncol. (2021) 30(9):984–8. doi: 10.3760/cma.j.cn113030-20201119-00559
CrossRef Full Text | Google Scholar
19. Yuan MX, Zhao HN, Yu ZW, Zhang J. Clinical significance of monitoring the change in TGAb levels when serum Tg values are disturbed in patients with differentiated thyroid cancer. Clin Med Prog. (2022) 6:5236–44. doi: 10.12677/ACM.2022.126758
CrossRef Full Text | Google Scholar
20. Jiao D, Wu G, Ren J, Han X. Radiofrequency ablation versus 125I-seed brachytherapy for painful metastases involving the bone. Oncotarget. (2016) 7:87523–31. doi: 10.18632/oncotarget.11983
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Li Rf, Wang YD, Yan Y, Yang P, Sha F, Li W, et al. Implantation of 125I seeds for the treatment of non-small cell lung cancer: evaluation of short-term effect. J interventional Radiol. (2014) 23(1):65–8. doi: 10.3969/j.issn.1008-794X.2014.01.018
CrossRef Full Text | Google Scholar
22. Su Y, Wang J, Huang L, Xie L, Yu X, Zha J. Clinical efficacy of iodine-125 (125I) seed implantation in patients with iodine-refractory differentiated thyroid cancer. Am J Cancer Res. (2023) 13:4794–802.
PubMed Abstract | Google Scholar
23. Wang C, Sun B, Wang H, Shi H. Research progress in the molecular biological mechanism of 125I seed in the treatment of Malignant tumors. J interventional Radiol. (2023) 32:1263–8. doi: 10.3969/j.issn.1008-794X.2023.12.020
留言 (0)