Jaswinder Gill, Karl J. Crossen, Christopher Blauth, Faraz Kerendi, Saumil R. Oza, Anthony R. Magnano, Mark A. Mostovych, Michael E. Halkos, David Tschopp, Jose Osorio, Paul Tabereaux, William Boedefeld, Kenneth Civello, Syed Ahsan, John Yap, Sreedhar Billakanty, Steve Duff, Otto Costantini, Eric Espinal, Andy Kiser, Christian Shults, David Pederson, James Garrison, David M. Gilligan, Michael G. Link, Marcin Kowalski, Christopher Stees, Jason S. Sperling, Israel Jacobowitz, Felix Yang, Yisachar J. Greenberg, David B. De Lurgio
AbstractBackground: CONVERGE was a prospective, multicenter, randomized controlled trial that evaluated the safety of Hybrid Atrial Fibrillation Convergent (HC) and compared its effectiveness to endocardial catheter ablation (CA) for the treatment of persistent atrial fibrillation (PersAF) and longstanding PersAF (LSPAF). In 2020, we reported that CONVERGE met its primary safety and effectiveness endpoints. The primary objective of the present study is to report CONVERGE trial results for quality of life (QOL) and Class I/III anti-arrhythmic drug (AAD) utilization following HC.Methods: Eligible patients had drug-refractory symptomatic PersAF or LSPAF and a left atrium diameter ≤6.0 cm. Enrolled patients were randomized 2:1 to receive HC or CA. Atrial Fibrillation Severity Scale (AFSS) and the 36-Item Short Form Health Survey (SF-36) were assessed at baseline and 12 months; statistical comparison was performed using paired t-tests. AAD utilization at baseline through 12 and 18 months post-procedure was evaluated; statistical comparison was performed using McNemar’s tests.Results: A total of 153 patients were treated with either HC (n=102) or CA (n=51). Of the 102 HC patients, 38 had LSPAF. AFSS and SF-36 Mental and Physical Component scores were significantly improved at 12 months versus baseline with HC overall and for the subset of LSPAF patients treated with either HC or CA. The proportion of HC patients (n=102) who used Class I /III AADs at 12 and 18 months was significantly less (33.3% and 36.3%, respectively) than baseline (84.3%; P<0.001). In LSPAF patients who underwent HC (n=38), AADs use was 29.0% through 18 months follow-up versus 71.1% at baseline (P<0.001).
Cover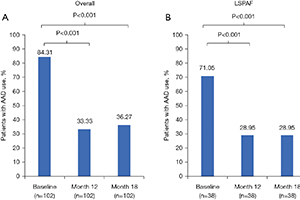
留言 (0)