Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver condition characterized by the accumulation of fat in liver cells’ cytoplasm (Bellentani and Marino, 2009), and is considered a manifestation of metabolic syndrome in the liver (Yang and Wang, 2023). In addition to liver-related consequences such as cirrhosis and hepatocellular carcinoma (White et al., 2012), individuals with NAFLD, especially those with nonalcoholic steatohepatitis and fibrosis, are under an elevated risk of cardiovascular disease, cancer, diabetes, and overall death (Kirk et al., 2009; Elias et al., 2010; Haufe et al., 2013; Vilar-Gomez et al., 2015; Ma et al., 2017; Mahfood Haddad et al., 2017; Allen et al., 2018; Allen et al., 2019). Over the past 2 decades, driven by the worldwide obesity epidemic, the global prevalence of NAFLD has significantly increased, rising from 25.3% to 38.0%, leading to a substantial healthcare burden (Younossi et al., 2016; Younossi et al., 2023). Consequently, it is imperative to establish preventive measures to counteract the rapid development of NAFLD.
Previous studies have indicated that exercise is not only associated with a reduced risk of NAFLD but also contributes to its alleviation (Miyake et al., 2015; Orci et al., 2016; Vittorio and Lavine, 2020; Park et al., 2023). However, there is currently no consensus on the mechanisms through which exercise affects NAFLD. A predominant perspective is that the impact of exercise on NAFLD is primarily achieved through weight loss (Zhang et al., 2016; Younossi et al., 2021). A randomized controlled trail (RCT) by Zhang et al. found that both moderate and vigorous exercise were equally effective in reducing intrahepatic triglyceride content; however, this effect appeared to be largely mediated by weight loss (Zhang et al., 2016). Moreover, the America Gastroenterological Association (AGA) also recommends that lifestyle modification through diet and exercise to achieve weight loss is beneficial for all NAFLD patients (Younossi et al., 2021). In contrast, another viewpoint contends that exercise has an independent impact on NAFLD, regardless of weight reduction. A longitudinal study from South Korea demonstrated that moderate to vigorous exercise significantly contributed to a decreased risk of NAFLD, regardless of changes in body weight (Sung et al., 2016). An additional RCT further substantiated these findings, indicating that both moderate and high-intensity exercise effectively reduce NAFLD risk, independently of abdominal adiposity or body mass reduction (Winn et al., 2018). Therefore, we conducted this study to explore whether the impact of exercise on NAFLD is solely mediated by changes in body weight or is also independent of the weight mediation.
Materials and methodsStudy design and subjectsIn 2019, a total of 3263 healthy hospital staff who underwent medical examinations at the affiliated hospital of Southwest Medical University were enrolled in the study, all of whom participated in annual check-ups for three consecutive years (2019-2021). Among these subjects, 1592 individuals were excluded for the following reasons: 544 lacked essential data on weight, ultrasonography, or blood biochemical examinations; 435 were missing questionnaire responses; 263 were categorized as heavy drinkers, defined as those consuming 140 g/week or more; 27 tested positive for serologic markers of hepatitis B or C; and 323 were diagnosed with fatty liver based on ultrasonography in 2019. Ultimately, 1671 initially NAFLD-free subjects comprised this cohort study and were retrospectively observed for the development of NAFLD for 2 years (Figure 1).
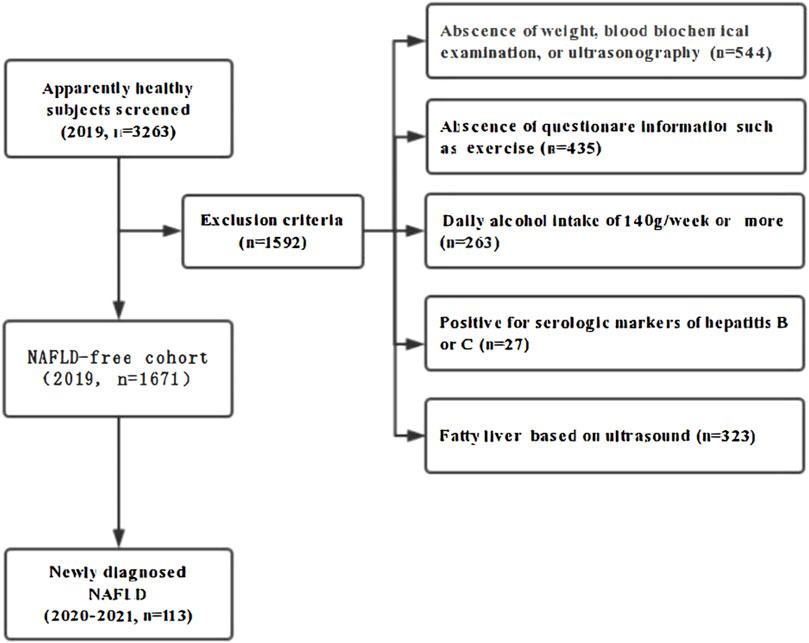
Figure 1. Flow diagram of subjects’ selection. A total of 3,263 adults participated in consecutive annual medical check-ups at the Southwest Medical University Affiliated Hospital. Among these subjects. 1592 individuals were excluded for the following reasons: 544 lacked essential data on weight. Ultrasonography. or blood biochemical examinations: 435 were missing questionnaire responses: 263 were categorized as heavy drinkers. Defined as those consuming 140 g/week or more; 27 tested positive for serologic markers of hepatitis B or C; and 323 were diagnosed with fatty liver based on ultrasonography in 2019. Ultimately. 1671 initially NAFLD-free subjects comprised this cohort study and were retrospectively observed for the development of NAVLD for 2 years.
Baseline examinationsBaseline examinations included a health habit inventory, anthropometric measurements, hepatic ultrasonic examination and biochemical measurements.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate were assessed utilizing an automated sphygmomanometer with the individual seated. Height while standing and body weight were gauged with the subject not wearing footwear or outer clothing. Body mass index (BMI, kg/m2) was computed as the weight in kilograms divided by the square of the height in meters. The examinations were administered between 8:00–11:00 a.m. Participants were instructed to fast for at least 12 h prior to the examination and to refrain from exercise the day before. The biochemical parameters measured included levels of albumin (ALB), globulin (GLB), direct bilirubin (DBIL), indirect bilirubin (IBIL), triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), fasting blood glucose (FBG), creatinine (Cr), blood urea nitrogen (BUN), serum uric acid (SUA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and homocysteine (HCY). All biochemical parameters were measured by an automatic biochemical analyzer (BS-2200M).
Assessment of outcomes and definitionsHypertension was defined as either self-reported use of antihypertensive medication or having a systolic blood pressure greater than or equal to 140 mmHg, or a diastolic blood pressure of ≥90 mmHg.
Participants’ exercise status was ascertained through the query: “Have you exercised more than 30 min per week in the past 3 months?” For those who select “yes, ”further questions concerning the sort of exercise were asked in order to gauge its intensity. Low-intensity exercise was characterized by maintaining a heart rate between 100–120 beats per minute, with a slight increase in body warmth, typically exemplified by activities like slow walking. Medium-intensity exercise, on the other hand, involved sustaining a heart rate of 130–150 beats per minute, noticeable sweating, faster breathing, and a moderate sense of exertion, as observed in activities such as brisk walking, jogging, bicycling, or tai chi. High-intensity exercise was described by a heart rate ranging from 160–170 beats per minute, accompanied by sweating and heavy breathing, and was often associated with activities like basketball, soccer or swimming.
Weight change values were computed by subtracting the weight in 2019 from the weight in 2021, and weight maintenance was defined as a weight gain not exceeding 2% of the 2019 weight, which was further adjusted to a weight gain of no more than 1% when conducting sensitivity analysis in the Supplementary Material.
The diagnosis of fatty liver was based on the results of abdominal ultrasonography conducted using a GE LOGIQ E9 sonography machine equipped with a 2.0–5.0 MHz probe. Ultrasound examinations were performed by senior imaging specialists. Hepatic steatosis was diagnosed based on characteristic echo patterns in accordance with conventional criteria. These patterns included evidence of diffuse hyperechogenicity of the liver relative to the kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic structures (Targher et al., 2005). NAFLD was diagnosed by abdominal ultrasonography, and exclusion criteria included excessive alcohol use (greater than 140 g per week) or viral infections (Jian-gao and Chinese Liver Disease, 2010).
Statistical analysesContinuous variables were expressed as means ± standard deviations or medians with upper and lower quartiles, and were compared using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test, depending on the normality of data. Categorical variables were described in terms of the number of cases and composition ratios, and were compared using the chi-square test. Univariate and multivariate logistic regression models were used to analyze the effect of exercise habits on NAFLD risk. Model fitting was performed using the enter method, and confounding variables including age, gender, and blood biochemical parameters were incorporated into the multivariate model. Furthermore, to ascertain whether weight maintenance serves as a mediator, additional analyses were conducted to assess the effect of exercise habits on weight maintenance as well as the impact of weight maintenance on the risk of NAFLD. The indirect effects of the exercise habit on NAFLD risk (via weight maintenance) were calculated using the product method, but results of the difference method are also provided (Cashin et al., 2023). As a sensitivity study, all comparable analyses were re-performed after lowering the weight maintenance criteria from no more than 2%–1%. All statistical analyses were performed using the SPSS software package version 25.0 for Windows. p < 0.05 (2-tailed) was considered as statistically significant.
ResultsBaseline characteristicsAt baseline, the mean age of the 1671 participants was 33.2 years, of which 338 (20.2%) were males. Subjects were categorized into three groups based on their exercise habits: the non-exercise group, the low-intensity exercise group, and the moderate to high-intensity exercise group. Except for the prevalence of hypertension, levels of ALB, DBIL, TG, and FBG, statistically significant differences were observed among the three groups in all other baseline characteristics. Generally, individuals habitually engaging in moderate to high-intensity exercise were older, had higher BMI and WC, lower pulse rate, and elevated biochemical markers compared to those in the non-exercise or low-intensity exercise groups (Table 1).
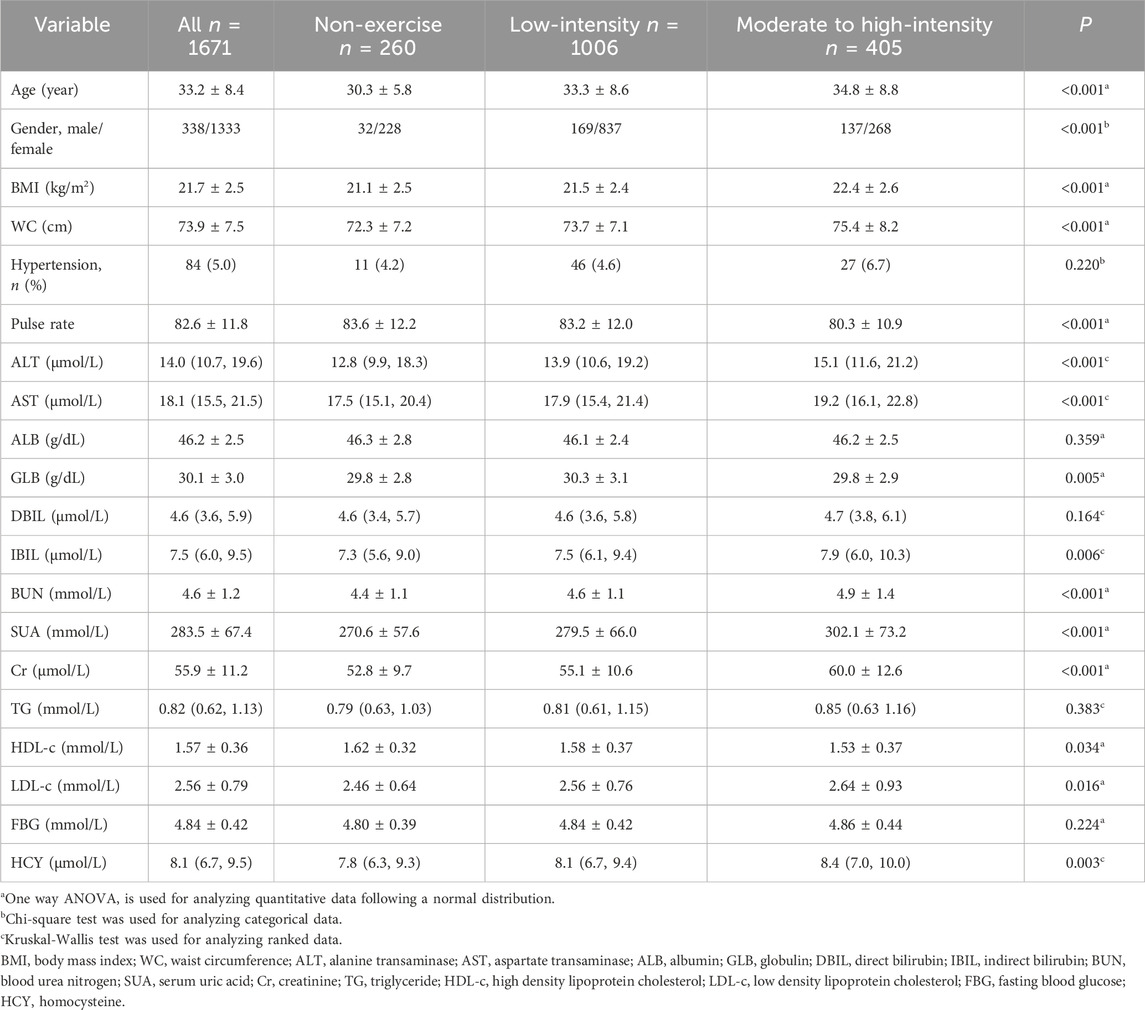
Table 1. Baseline characteristics of populations with different exercise habits.
Exercise and weight maintenanceIndividuals with a regular habit of moderate to high-intensity exercise experienced an average weight reduction of 0.16% over a 2-year period, whereas those who never engaged in exercise and those who participated in low-intensity exercise observed weight gains of 1.78% and 1.58%, respectively, with statistically significant differences. The proportions of individuals who experienced a weight increase of less than 2% over the 2-year period were 52.3% for non-exercisers, 56.8% for low-intensity exercisers, and 66.6% for moderate to high-intensity exercisers. A clear increasing trend in weight maintenance rates with exercise intensity was observed (P for trend <0.001). The likelihood of successful weight maintenance for moderate to high-intensity exercisers was 1.82 times higher compared to non-exercisers in crude model (model 1). After adjusting for confounders, this value decreased to 1.56 but remained statistically significant. Furthermore, an overall increasing trend of control rates with higher exercise intensity was also observed (Table 2). The impact of moderate to high-intensity exercise on weight maintenance remained consistent after changing the threshold from 2% to not exceeding 1% of the baseline body weight (Supplementary Table S3).
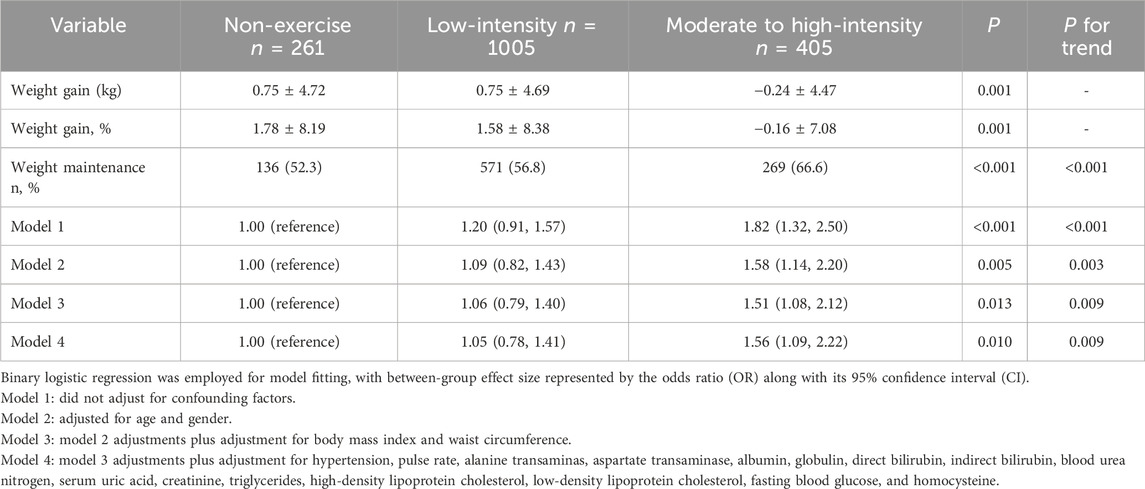
Table 2. Effect of exercise on weight change and weight maintenance.
Weight maintenance decreases the NAFLD riskBetween 2019 and 2021, individuals who experienced a weight gain of no more than 2% (weight maintenance group) had a NAFLD incidence rate of 5.6%, significantly lower than the incidence of 8.4% observed in those who gained more than 2% of their weight (weight gain group) (p = 0.029). The risk of NAFLD in weight maintenance group was only 65.5% (95% CI: 44.7%–96.0%) of that in the weight gain group. After controlling for confounding factors, the weight maintenance group exhibited a NAFLD risk of only 34.9% (95% CI: 21.8%–56.0%) compared to the weight gain group (Table 3). After altering the weight maintenance criteria to not exceeding 1% of the baseline weight, its effectiveness in reducing the risk of NAFLD remained robust (Supplementary Table S4).
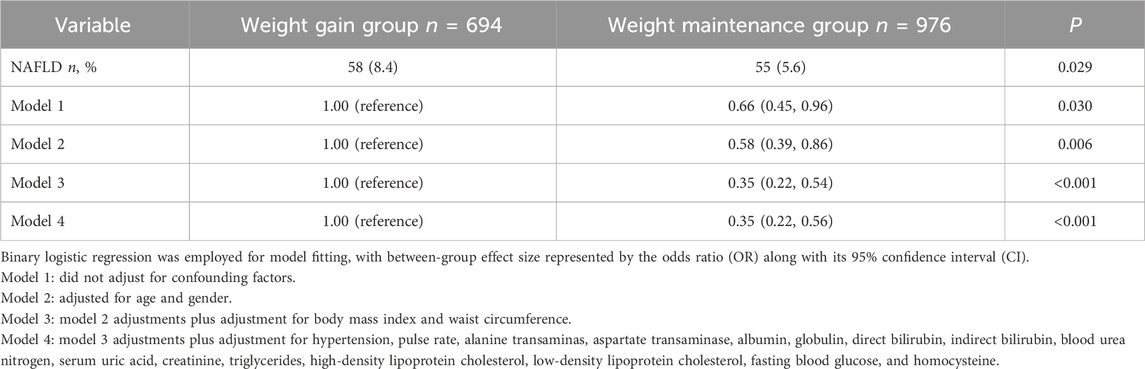
Table 3. Weight maintenance and the risk of NAFLD.
Exercise decreases the risk of NAFLDThe incidence rates of NAFLD within a 2-year period for individuals who never exercised, engaged in low-intensity exercise, and participated in moderate to high-intensity exercise were 6.9%, 7.7% and 4.4%, respectively. Univariate analysis revealed no statistically significant differences among the three groups. However, in several multivariable models (models 2–4) adjusted for different numbers of confounding factors, both non-exercise group and low-intensity group exhibited significantly higher risks of developing NAFLD compared to the moderate to high-intensity group. In Model 4, these risks were 2.42-fold and 2.79-fold higher, respectively. Furthermore, after additional adjustment for weight maintenance, the low-intensity group still had a higher NAFLD risk compared to the moderate to high-intensity group (2.43-fold), while no statistically significant difference was observed between non-exercise group and moderate to high-intensity group. Across all multivariable models, it was consistently observed that as exercise intensity increased, there was a decreasing trend in NAFLD risk (Table 4). After modifying the weight maintenance criteria to no more than 1%, the analysis results remained consistent (Supplementary Table S5).
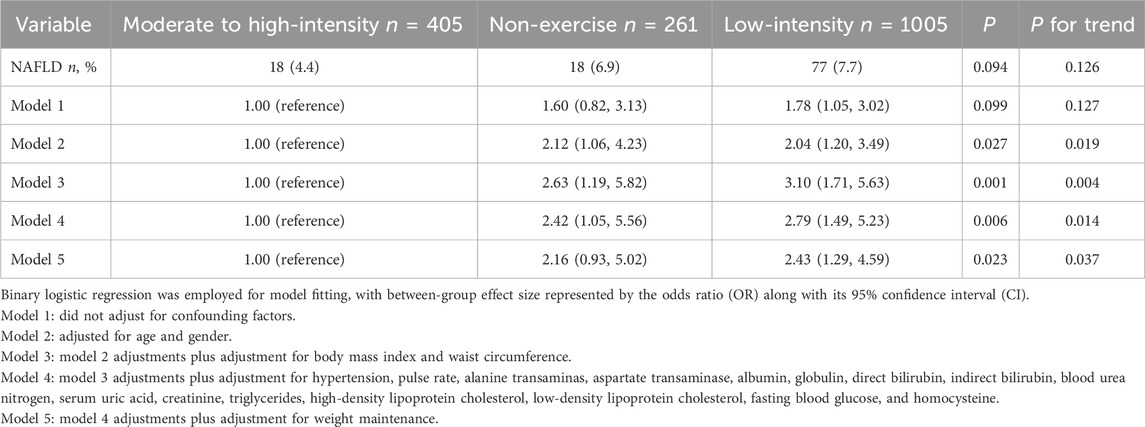
Table 4. The effect of exercise on the risk of NAFLD.
Association between exercise, weight maintenance and NAFLD riskSummarizing the relationship among exercise habits, weight maintenance, and NAFLD risk, as depicted in Figure 2, with weight maintenance serving as a mediator. Exercise contributed to weight maintenance (0.236, p = 0.009), weight maintenance led to a reduction in NAFLD risk (−1.011, p < 0.001), and exercise had a direct impact on lowering NAFLD risk (−0.404, p = 0.037). Consequently, employing the product method, the indirect effect of exercise habits on NAFLD risk through weight maintenance was calculated as −0.239 (0.236 −1.011), accounting for 37.1% of the total effect. Thus, exercise habit had a 62.9% direct impact on NAFLD risk. In contrast, utilizing the difference method, the indirect effect was found to constitute 12.9% of the total effect (Supplementary Table S2). When exercise habits were incorporated into the model as dummy variables, with moderate to high-intensity exercise serving as the reference, similar results were obtained (Supplementary Tables S1, S2; Supplementary Figure S1). In the sensitivity analysis with adjusted weight maintenance criteria, consistent results were obtained. The direct effect of exercise and the indirect effect through weight maintenance were 67.7% and 32.3%, respectively. (Supplementary Tables S6, S7; Supplementary Figure S2).
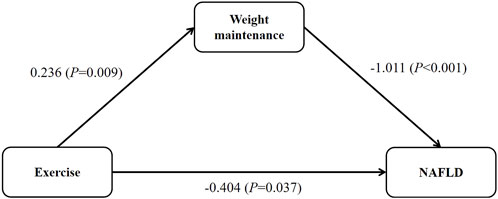
Figure 2. The relationship between exercise, weight maintenance and NAFLD.
DiscussionThe relationship between exercise, weight change, and NAFLD has been extensively investigated, yet results remain inconclusive. This study reveals that individuals engage in moderate to high-intensity exercise, despite being older or with higher BMI, waist circumference, and various biochemical markers, exhibit a significantly lower risk of developing NAFLD within a 2-year period. Exercise can effectively reduce the risk of NAFLD both indirectly, through its impact on weight maintenance, and directly, independent of weight maintenance. Notably, the indirect effect accounts for only approximately 37% of the overall impact of exercise, which is less significant than the direct effect of exercise. Through mediation analysis, our study offers a fresh perspective for a better understanding of the role of exercise in improving NAFLD.
This study identified an intriguing phenomenon: individuals with a habit of moderate to high-intensity exercise at baseline exhibited poorer metabolic profiles, such as higher BMI, TG levels, and lower HDL levels, among others. This could be attributed to the fact that individuals in the moderate to high-intensity exercise group tended to be older. On the other hand, their inclination towards moderate to high-intensity exercise might also be influenced by their less healthy metabolic profiles. However, despite having worse metabolic conditions, individuals engaging in moderate to high-intensity exercise demonstrated a lower risk of developing NAFLD. Through multivariable logistic regression analysis, we adjusted for confounding factors at baseline, including age, BMI, and various metabolic indicators, and found that moderate to high-intensity exercise was associated with a reduced risk of NAFLD. This underscores the potential preventive role of regular exercise habits in NAFLD.
As weight loss is often linked to a decrease in liver fat, offering the potential to reverse disease progression (Elias et al., 2010; Vilar-Gomez et al., 2015), a plethora of studies have emphasized the role of weight reduction in improving NAFLD (Hannah and Harrison, 2016; Katsagoni et al., 2018; Younossi et al., 2021). In the meanwhile, exercise, like food restriction, is frequently recognized as an approach of weight reduction or weight management (Younossi et al., 2021). However, shedding excess weight can be a formidable task, particularly for seemingly healthy individuals. Our present study indicated that apart from its impact on weight, exercise by itself considerably decreases the risk of NAFLD. This is supported by the results of a cohort study with 233,676 participants which found that regardless of alterations in body weight, weekly exercise with a frequency of 1–2 or more times was associated with a decreased risk of NAFLD in the general population (Sung et al., 2016). In addition, another study on individuals after NAFLD remission suggested that, in addition to weight gain, lack of exercise was also a risk factor for NAFLD relapse (Nakanishi et al., 2021). This corroborates the notion that exercise can affect essential metabolic and inflammatory mechanisms that are linked to the development and progression of NAFLD (Richter and Ruderman, 2009). By increasing peroxisome proliferator-activated receptors and adiponectin levels, exercise has the potential to ameliorate hepatic steatosis, leading to improved insulin resistance and enhanced lipolysis (Petridou et al., 2007; Guo et al., 2015). Insights from another study may help us better understand the mechanisms by which exercise affects NAFLD (Colosimo et al., 2023). It highlighted that both metabolic flexibility and insulin resistance play crucial roles in the development of NAFLD through distinct mechanisms. Regular physical activity promotes metabolic flexibility by increasing energy expenditure and facilitating efficient switching between different energy substrates, thereby aiding in the prevention and alleviation of NAFLD. In contrast, weight loss is effective in improving insulin sensitivity but has no effect on metabolic flexibility. To conclude, it can be affirmed that exercise exerts an independent effect on NAFLD risk.
Despite numerous studies examining the impact of exercise and weight changes on NAFLD, few have analyzed the direct effect of exercise on NAFLD as well as its indirect effects through weight changes. In this study, we observed that exercise demonstrates both direct and indirect effects on the development of NAFLD in the general population, with the direct impact being predominant (62.9% vs. 37.1%). These findings underscore the increasing significance of exercise in the general population, advocating exercise without an excessive focus on weight maintenance may also effectively prevent the onset of NAFLD.
Furthermore, our study suggests that only moderate to high-intensity exercise effectively reduces NAFLD risk, whether mediated through weight maintenance or acting independently. Preceding this, several studies have explored the connection between the intensity of exercise and NAFLD. For instance, Kistler et al. reported in a cross-sectional study that NAFLD patients engaging in vigorous physical activity had a lower prevalence of biopsy-confirmed hepatic fibrosis compared to those involved in moderate physical activity, highlighting the role of exercise intensity (Kistler et al., 2011). Similarly, a meta-analysis (Katsagoni et al., 2017) concluded that high-intensity interval exercise training may be more effective in reducing liver steatosis than exercise training of low-to-moderate intensity. In line with these findings, AGA recommends NAFLD patients to engage in 150–300 min of moderate-intensity or 75–150 min of high-intensity exercise per week (Hashida et al., 2017; Younossi et al., 2021). Up to this point, RCT studies investigating the relationship between exercise and NAFLD have predominantly targeted patients and obese individuals. Further randomized controlled research is warranted to establish exercise recommendations for the general population.
This study has several limitations that deserve attention. Firstly, the diagnosis of NAFLD relied on liver ultrasound tests, which, although commonly used, are operator-dependent and have lower sensitivity. In our study, among 1671 individuals without NAFLD at baseline, 113 developed NAFLD within 2 years, indicating an annual incidence rate of approximately 3.4%. This rate is lower than that reported in another cohort study using magnetic resonance imaging diagnosis (median follow-up time of 4.6 years, cumulative incidence rate of 26.5%, and annual incidence rate of approximately 5.8%) (Chen et al., 2023). This discrepancy may be attributed to the lower sensitivity of ultrasound. However, it's noteworthy that our study participants were significantly younger (33.2 vs. 61.6 years), which could potentially confound this observation. Secondly, it was conducted as a retrospective cohort study, utilizing medical examination data and relying on exercise information obtained through self-reported surveys. This approach, while practical for large-scale studies, may have compromised its evidential robustness when compared to RCTs. Thirdly, participants’ exercise habits may change over time, potentially confounding the study results. However, evidence shows that exercise behavior is moderately to highly stable throughout the lifespan, particularly among adults (van der Zee et al., 2019). Furthermore, our follow-up period lasted only 2 years. As a result, it might be argued that this impact is to some extent acceptable. Fourthly, the relatively modest sample size in this study might have led to insufficient statistical power, increasing the chances of non-significant findings in between-group comparisons. Notably, while the study indicated that individuals engaged in moderate to high-intensity exercise had a reduced risk of NAFLD in comparison to those involved in low-intensity exercise, it is important to acknowledge that this difference did not attain statistical significance when compared to the non-exercise group. This could be attributed to the limited sample size. Finally, due to practical constraints, dietary information was not collected in the study, precluding an assessment of its impact.
ConclusionIn the general population, regular moderate to high-intensity exercise independently reduces the risk of NAFLD, with its benefits outweigh those derived from weight maintenance. Therefore, rather than focusing specifically on weight maintenance, promoting routine moderate to high-intensity exercise may be a more beneficial approach for preventing NAFLD.
Data availability statementThe raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital at Southwest Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsCY: Software, Writing–original draft, Writing–review and editing. PY: Software, Writing–original draft, Writing–review and editing. JD: Investigation, Writing–review and editing. YL: Investigation, Writing–review and editing. XJ: Supervision, Writing–review and editing. BZ: Funding acquisition, Supervision, Writing–review and editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (2022YFC3600600 and 2022YFC3600604), the National Natural Science Foundation of China (U22A20359, 81874283, 81673255), the Recruitment Program for Young Professionals of China, the Promotion Plan for Basic Medical Sciences and the Development Plan for Cutting-Edge Disciplines, Sichuan University, and other Projects from West China School of Public Health and West China Fourth Hospital, Sichuan University. The funder had no role in study design, data collection, analysis, interpretation, writing of the report, or the decision for submission.
AcknowledgmentsWe wish to thank the dedicated team of professionals at the Health Management Centre of the Affiliated Hospital of Southwest Medical University for their assistance during the execution of this research project.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1359476/full#supplementary-material
AbbreviationsNAFLD, nonalcoholic fatty liver disease; RCT, Randomized Controlled Trail; AGA, America Gastroenterological Association; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; ALT, alanine transaminase; AST, aspartate transaminase; ALB, albumin; GLB, globulin; DBIL, direct bilirubin; IBIL, indirect bilirubin; BUN, blood urea nitrogen; SUA, serum uric acid; Cr, creatinine; TG, triglyceride; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; FBG, fasting blood glucose; HCY, homocysteine; ANOVA, one-way analysis of variance; OR, odds ratio; CI, confidence interval.
ReferencesAllen A. M., Hicks S. B., Mara K. C., Larson J. J., Therneau T. M. (2019). The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - a longitudinal cohort study. J. Hepatol. 71 (6), 1229–1236. doi:10.1016/j.jhep.2019.08.018
PubMed Abstract | CrossRef Full Text | Google Scholar
Allen A. M., Therneau T. M., Larson J. J., Coward A., Somers V. K., Kamath P. S. (2018). Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology 67 (5), 1726–1736. doi:10.1002/hep.29546
PubMed Abstract | CrossRef Full Text | Google Scholar
Cashin A. G., McAuley J. H., VanderWeele T. J., Lee H. (2023). Understanding how health interventions or exposures produce their effects using mediation analysis. BMJ 382, e071757. doi:10.1136/bmj-2022-071757
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen H., Liu Y., Liu D., Liang Y., Zhu Z., Dong K., et al. (2023). Sex- and age-specific associations between abdominal fat and non-alcoholic fatty liver disease: a prospective cohort study. J. Mol. Cell Biol. 30, mjad069. doi:10.1093/jmcb/mjad069
CrossRef Full Text | Google Scholar
Colosimo S., Mitra S. K., Chaudhury T., Marchesini G. (2023). Insulin resistance and metabolic flexibility as drivers of liver and cardiac disease in T2DM. Diabetes Res. Clin. Pract. 206, 111016. doi:10.1016/j.diabres.2023.111016
PubMed Abstract | CrossRef Full Text | Google Scholar
Elias M. C., Parise E. R., de Carvalho L., Szejnfeld D., Netto J. P. (2010). Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease. Nutrition 26 (11-12), 1094–1099. doi:10.1016/j.nut.2009.09.001
PubMed Abstract | CrossRef Full Text | Google Scholar
Guo R., Liong E. C., So K. F., Fung M. L., Tipoe G. L. (2015). Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 14 (2), 139–144. doi:10.1016/s1499-3872(15)60355-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Hannah W. N., Harrison S. A. (2016). Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clin. Liver Dis. 20 (2), 339–350. doi:10.1016/j.cld.2015.10.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Hashida R., Kawaguchi T., Bekki M., Omoto M., Matsuse H., Nago T., et al. (2017). Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J. Hepatol. 66 (1), 142–152. doi:10.1016/j.jhep.2016.08.023
PubMed Abstract | CrossRef Full Text | Google Scholar
Haufe S., Haas V., Utz W., Birkenfeld A. L., Jeran S., Bohnke J., et al. (2013). Long-lasting improvements in liver fat and metabolism despite body weight regain after dietary weight loss. Diabetes Care 36 (11), 3786–3792. doi:10.2337/dc13-0102
PubMed Abstract | CrossRef Full Text | Google Scholar
Jian-gao F., Chinese Liver Disease A. (2010). Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Gan Zang Bing Za Zhi 18 (3), 163–166.
PubMed Abstract | Google Scholar
Katsagoni C. N., Georgoulis M., Papatheodoridis G. V., Panagiotakos D. B., Kontogianni M. D. (2017). Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism 68, 119–132. doi:10.1016/j.metabol.2016.12.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Katsagoni C. N., Papatheodoridis G. V., Ioannidou P., Deutsch M., Alexopoulou A., Papadopoulos N., et al. (2018). Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: a randomised controlled clinical trial. Br. J. Nutr. 120 (2), 164–175. doi:10.1017/S000711451800137X
PubMed Abstract | CrossRef Full Text | Google Scholar
Kirk E., Reeds D. N., Finck B. N., Mayurranjan S. M., Patterson B. W., Klein S., et al. (2009). Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 136 (5), 1552–1560. doi:10.1053/j.gastro.2009.01.048
PubMed Abstract | CrossRef Full Text | Google Scholar
Kistler K. D., Brunt E. M., Clark J. M., Diehl A. M., Sallis J. F., Schwimmer J. B., et al. (2011). Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am. J. Gastroenterol. 106 (3), 460–469. doi:10.1038/ajg.2010.488
PubMed Abstract | CrossRef Full Text | Google Scholar
Ma J., Hwang S. J., Pedley A., Massaro J. M., Hoffmann U., Chung R. T., et al. (2017). Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J. Hepatol. 66 (2), 390–397. doi:10.1016/j.jhep.2016.09.022
PubMed Abstract | CrossRef Full Text | Google Scholar
Mahfood Haddad T., Hamdeh S., Kanmanthareddy A., Alla V. M. (2017). Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: a systematic review and meta-analysis. Diabetes Metab. Syndr. 11 (1), S209–S216. doi:10.1016/j.dsx.2016.12.033
PubMed Abstract | CrossRef Full Text | Google Scholar
Miyake T., Kumagi T., Hirooka M., Furukawa S., Kawasaki K., Koizumi M., et al. (2015). Significance of exercise in nonalcoholic fatty liver disease in men: a community-based large cross-sectional study. J. Gastroenterol. 50 (2), 230–237. doi:10.1007/s00535-014-0959-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Nakanishi N., Hashimoto Y., Okamura T., Ohbora A., Kojima T., Hamaguchi M., et al. (2021). A weight regain of 1.5 kg or more and lack of exercise are associated with nonalcoholic fatty liver disease recurrence in men. Sci. Rep. 11 (1), 19992. doi:10.1038/s41598-021-99036-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Orci L. A., Gariani K., Oldani G., Delaune V., Morel P., Toso C. (2016). Exercise-based interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin. Gastroenterol. Hepatol. 14 (10), 1398–1411. doi:10.1016/j.cgh.2016.04.036
PubMed Abstract | CrossRef Full Text | Google Scholar
Park Y., Sinn D. H., Kim K., Gwak G. Y. (2023). Associations of physical activity domains and muscle strength exercise with non-alcoholic fatty liver disease: a nation-wide cohort study. Sci. Rep. 13 (1), 4724. doi:10.1038/s41598-023-31686-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Petridou A., Tsalouhidou S., Tsalis G., Schulz T., Michna H., Mougios V. (2007). Long-term exercise increases the DNA binding activity of peroxisome proliferator-activated receptor gamma in rat adipose tissue. Metabolism 56 (8), 1029–1036. doi:10.1016/j.metabol.2007.03.011
PubMed Abstract | CrossRef Full Text | Google Scholar
Sung K. C., Ryu S., Lee J. Y., Kim J. Y., Wild S. H., Byrne C. D. (2016). Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 65 (4), 791–797. doi:10.1016/j.jhep.2016.05.026
PubMed Abstract | CrossRef Full Text | Google Scholar
Targher G., Bertolini L., Poli F., Rodella S., Scala L., Tessari R., et al. (2005). Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54 (12), 3541–3546. doi:10.2337/diabetes.54.12.3541
PubMed Abstract | CrossRef Full Text | Google Scholar
van der Zee M. D., van der Mee D., Bartels M., de Geus E. J. C. (2019). Tracking of voluntary exercise behaviour over the lifespan. Int. J. Behav. Nutr. Phys. Act. 16 (1), 17. doi:10.1186/s12966-019-0779-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., et al. (2015). Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149 (2), 367–378. doi:10.1053/j.gastro.2015.04.005
PubMed Abstract | CrossRef Full Text | Google Scholar
White D. L., Kanwal F., El-Serag H. B. (2012). Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 10 (12), 1342–1359. doi:10.1016/j.cgh.2012.10.001
PubMed Abstract | CrossRef Full Text | Google Scholar
Winn N. C., Liu Y., Rector R. S., Parks E. J., Ibdah J. A., Kanaley J. A. (2018). Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - a randomized trial. Metabolism 78, 128–140. doi:10.1016/j.metabol.2017.08.012
PubMed Abstract | CrossRef Full Text | Google Scholar
Younossi Z. M., Corey K. E., Lim J. K. (2021). AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology 160 (3), 912–918. doi:10.1053/j.gastro.2020.11.051
PubMed Abstract | CrossRef Full Text | Google Scholar
Younossi Z. M., Golabi P., Paik J. M., Henry A., Van Dongen C., Henry L. (2023). The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77 (4), 1335–1347. doi:10.1097/HEP.0000000000000004
PubMed Abstract | CrossRef Full Text | Google Scholar
Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. (2016). Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64 (1), 73–84. doi:10.1002/hep.28431
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang H. J., He J., Pan L. L., Ma Z. M., Han C. K., Chen C. S., et al. (2016). Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 176 (8), 1074–1082. doi:10.1001/jamainternmed.2016.3202
留言 (0)