Department of Plant Breeding & Molecular Genetics, Institute of Biosciences & Technology, MGM
University, Chhatrapati Sambhajinagar 431003, Maharashtra State India.
Corresponding author email:aashwinn9@gmail.com
Article Publishing HistoryReceived:
Accepted After Revision:
ABSTRACT:In this comprehensive study, the synergetic impact of sodium azide and EMS mutagenic treatments on ‘Parbhani Kranti’ okra seeds and plants were rigorously examined. The study employed okra seeds in a CRD, implementing five treatments (T0 to T4) with varied concentrations of Sodium Azide and EMS. The primary goal was to assess genetic purity, covering morphological parameters, DUS tests; Physiological observations included chlorophyll, RW content and molecular analysis by using SSR Marker. Morphological parameters, including shoot, root, seedling length, and leaf area were adversely affected, with T4 showing the most stunted growth.Flowering delay and altered fruit characteristics were also observed, correlating with mutagen concentrations. The findings of this research reveal that seeds subjected to mutagenic treatments exhibited significantly reduced germination rates when compared to the control.
The most substantial reduction, at 70%, were observed in seeds treated (T4) and was detrimentally influenced by these mutagenic interventions. The increase chlorophyll (mean 1.47) and RWC (mean 47.06) complex plant responses to mutagenic elements. Potential adaptive or tolerance mechanisms, including increased chlorophyll production and water retention, may be activated to mitigate stress. Genetic purity assessments exposed variations in DNA content and quality across different treatments. Among the two SSR marker differentiated allelic size of 260 and 300 bp. The allelic sizes are specific to the seed and pollen of the hybrid. These findings hold implications for developing novel okra cultivars and ensuring genetic purity in hybrid seed production, contributing to agricultural sustainability.
KEYWORDS:Chemical Mutagen, Mutation Breeding, Okra, Polymerase Chain Reaction, SSR Primers
Download this article as: Copy the following to cite this article:Dhole M. S, Kshirsagar A. B, Shinde A. A, More K. N. Synergetic impact of Sodium azide and ethyl methane sulfonate treatment on SSR marker-Based Assessment of Okra Seedling Genetic Purity. Biosc.Biotech.Res.Comm. 2023;16(4).
Copy the following to cite this URL:Dhole M.S, Kshirsagar A.B, Shinde A.A, More K.N. Synergetic impact of Sodium azide and ethyl methane sulfonate treatment on SSR marker-Based Assessment of Okra Seedling Genetic Purity. Biosc.Biotech.Res.Comm. 2023;16(4). Available from: <a href=”http://surl.li/peglf“>http://surl.li/peglf</a>
INTRODUCTION
Okra (Abelmoschus esculentus Moench) is a warm-season annual herbaceous vegetable crop from the Malvaceae family, believed to originate in South Africa. It holds significant economic importance due to its widespread consumption as a cooked vegetable (Mishra et al., 2015). Characterized by its self-pollinating nature, it grows as a shrub, reaching 1 to 2 meters in height, with large leaves (20 to 40 cm in length, exhibiting 3-7 lobes) and round, large, grey seeds (Akinyele and Osekita, 2006).
It thrives during the summer months, dependent on specific climatic factors like light, temperature, and water availability. An average rainfall of 665 mm is crucial for its productive cycle, though germination can be challenging (Tiwari et al., 1998), (More et al., 2023).
Beyond its agricultural significance, okra is a rich source of essential vitamins and minerals, including carbohydrates, iron, potassium, and magnesium. Okra seeds are notable for their high protein content (15%-26%) and edible oil content exceeding 14%. The entire okra plant, including its mucilaginous fruit, is edible and can be consumed fresh, cooked, added to salads or soups, or preserved. The seeds are also rich in unsaturated fatty acids, water, proteins, carbohydrates, and fiber (Kumar et al., 2013). Additionally, the seeds contain high levels of polysaccharides, contributing to their medicinal properties, and are even used to create a coffee substitute known as “chocolate de los negros” (Mishra et al., 2015).
Okra is cultivated globally, covering approximately 1.117 million hectares and yielding 8.706 million tonnes, with an average productivity of 7.8 tonnes per hectare. India is the largest contributor to okra production, accounting for 72% of the world’s output. States like Gujarat, Maharashtra, Andhra Pradesh, and others are primary regions for okra cultivation (Gandhi, 2014). India and Nigeria are leading producers, while minor producers include Pakistan, Ghana, Egypt, and more.
Okra serves as a profitable crop for many developing countries, generating income and employment opportunities, and is predominantly exported to the United States (Adiger et al., 2011). Mutation breeding accelerates genetic variations and the emergence of new species by exposing seeds to chemicals, radiation, or enzymes. Mutation breeding is swifter than traditional methods and introduces both qualitative and quantitative variations (Gupta, 2019).
Physical mutagens involve electromagnetic and particle radiation, while chemical mutagens include alkylating agents and azides. Chemical mutagens are milder and easier to apply, inducing both loss-of-function and gain-of-function mutations, such as herbicide tolerance (Oladosu, et al., 2015). In the future, DNA profiling may be employed for genotype characterization, providing valuable data for plant breeding. Molecular markers, especially Simple Sequence Repeats (SSR), are pivotal in genetic assessment, as they can detect genetic variations at the DNA level, facilitating the evaluation of genetic purity (Gandhi et al., 2014).
Mutagenic treatments, including sodium azide and EMS, induce genetic variations in plants. By subjecting okra seeds to these treatments, a higher mutation rate is achieved, leading to a broader spectrum of genetic variations (Pharmawati et.al, 2018). In combination with SSR markers, this approach allows for precise identification of genetic variations in okra seedlings, facilitating the selection of genetically pure lines and adherence to DUS guidelines (Jamshed et al., 2016).
These findings have significant implications for the development of novel okra cultivars and the crucial task of safeguarding genetic purity in hybrid seed production.
MATERIAL AND METHODS
The experimental site for this study was the Institute of Biosciences and Technology in Chhatrapati Sambhajinagar. Okra seeds obtained from VNKV, Parbhani, were used as the plant material. The research design employed was a Completely Randomized Design (CRD). The primary focus of the study was ‘Parbhani Kranti,’ a variety of Abelmoschus esculentus known for its resistance to yellow vein mosaic viruses. The research involved five treatments labeled as T0, T1, T2, T3, and T4, with each treatment having different concentrations of Sodium Azide and EMS. The treatments were as follows: T0 (Control), T1 (0.10 + 0.05 mM), T2 (0.20 + 0.10 mM), T3 (0.30 + 0.15 mM), and T4 (0.40 + 0.20 mM).
The primary goal of this research was to evaluate genetic purity and related characteristics in ‘Parbhani Kranti’ okra seedlings. The study encompassed both morphological and physiological observations. Morphological observations included monitoring various parameters such as seed weight, germination percentage (calculated as Germination % = (Number of germinated seeds / Total tested seeds) x 100), seedling vigor (determined by shoot-root length difference x Germination %), and measurements of shoot and root length at 10, 20, and 30 days post-germination.
The length of seedlings and leaf area was also recorded. Additionally, survival percentage was calculated based on the comparison of surviving plants to the initial number of plants, and a Distinctness, Uniformity, and Stability (DUS) test was conducted on 30 plants divided into three replications. In terms of physiological observations, the study involved the estimation of chlorophyll content using the Arnon Method with a spectrophotometer. Although it was mentioned that relative water content was measured, the specific method used was not provided.The study also included DNA isolation, which was performed using a Plant Genomic DNA Mini-preparation kit.
This process involved grinding in liquid nitrogen, buffer addition, heating, centrifugation, RNAse A treatment to remove RNA, ethanol precipitation, and air drying. DNA quality was assessed through 0.8% agarose gel electrophoresis, and DNA quantification was carried out using a spectrophotometer and SoftMax Software. Purity was assessed using the L1/L2 ratio. SSR Marker screening was performed via PCR amplification with 18 microsatellite markers linked to okra traits, while correlation detection examined the relationships between SSR banding patterns and fruit length, strength, and yield quality. The PCR program included denaturation, annealing, extension steps, and a final extension at 72°C.
The experimental data were analyzed using statistical methods, with the experiment conducted under a Completely Randomized Design (CRD) (Panse and Sukhmate, et al. 1968). This study represents a comprehensive investigation into the genetic purity and related traits of ‘Parbhani Kranti’ okra seedlings, encompassing both morphological and physiological aspects.
RESULTS AND DISCUSSION
The study aimed to evaluate the Synergetic impact s of chemical mutagens (Sodium azide and EMS) on the morphological, physiological, and molecular characteristics of Okra genotypes. The outcomes of the study are summarized as follows. One-factor ANOVA was used to analyze the morphological and molecular parameters of Okra seedlings at 30 days after sowing (DAS). The data for various treatments are presented in (Table 1). Germination percentage is a crucial parameter for assessing mutagenic Synergetic impact s on plants. It serves as a reliable indicator of mutagen impact on seed viability.
The control group (T0) exhibited the highest germination percentage at 97%. Germination percentages decreased as the dose of mutagen increased. The lowest germination percentage was observed in T4 (0.40 + 0.20) treated with sodium azide + EMS, with a germination rate of 70%. Treatment groups T1, T2, T3, and T4 had germination percentages of 80%, 78%, 73%, and 70%, respectively are shown in (figure 1). The decline in germination can be attributed to the inhibitory Synergetic impact s of mutagens on essential physiological processes, including enzymatic activity, hormonal balance, and mitotic activity crucial for seed germination.
The data indicates a linear relationship between mutagen dose (sodium azide + EMS) and reduced seed germination. These findings demonstrate that increased mutagen concentrations negatively affect seed germination, highlighting the importance of carefully controlling mutagenic treatments for crop improvement. In the next sections of the discussion, the results from other morphological, physiological, and molecular parameters were discussed, providing a comprehensive understanding of the impact of chemical mutagens on Okra genotypes (Table 1).
Table 1. Synergistic Impact of Sodium Azide and EMS on Morphological Traits of Okra
Treatment Morphological Parameters of Okra Seedling (30 Days) Germination % Seedling length (cm) Seed vigour index Leaf area(cm2) Survival % T0 97 26 25.22 50.5 96 T1 80.33 23 21 46 78 T2 78.66 20.66 16.25 44 75 T3 73.33 19 13.93 42 70 T4 70.33 18.33 12.89 34 67 Mean 79.93 21.39 17.86 43.3 77.2 SE 7.39 7.13 7.29 7.22 7.43 CD (0.05) 11.04 2.77 2.65 4.32 10.2These findings demonstrate that increased mutagen concentrations negatively affect seed germination, highlighting the importance of carefully controlling mutagenic treatments for crop improvement. In the next sections of the discussion, the results from other morphological, physiological, and molecular parameters were discussed, providing a comprehensive understanding of the impact of chemical mutagens on Okra genotypes. In this study, the morphological characteristics of the “Parbhani Kranti” okra variety were examined after subjecting it to treatments involving Sodium azide + EMS. Observations were recorded 30 days after germination (Table 1).
The treated plants showed a decrease in shoot length compared to the control. The most significant reduction was observed in seeds treated with T4 (0.40+0.20) of sodium azide and EMS, with a shoot length of 11 cm compared to the control’s 15 cm. Similarly, root length decreased slightly in treated plants, with the lowest root length in seeds treated with T4. The seedling length was affected by the treatment, with a decrease as the treatment concentrations increased are shown in (figure 2) (Table 1).
Leaf area was also reduced in treated plants, and, interestingly, a higher treatment concentration led to both reduced leaf area and an increase in the number of leaves. These results indicate that Sodium azide + EMS treatment has a significant impact on the morphological characteristics of the okra variety, with reduced shoot length, root length, seedling length, and leaf area compared to the control. This suggests that the mutagenic treatment affected the plant’s growth and developments are shown in (figure 3) (Table 1).
Figure 1: Germinated seeds.

Figure 2: Seedling length.

Figure 3: Leaf area.

Table 2. Synergetic impact s on the Number of Flowers and
Number of Fruits in Okra Plants
Duration (Days)
No. of Flowers Flowering to FruitingDuration (Days)
No. of Fruits Fruit Colour Shape Height (cm) Weight (gm) T0 45 10 20 3 Green Straight 8.3 10.03 T1 48 6 23 2 Green Curved 7.2 8.35 T2 50 8 21 2 Green Curved 6.9 7.17 T3 49 9 19 3 Green Straight 5.5 5.09 T4 44 11 19 4 Green Straight 4.8 4.53 Mean 47.2 8.8 20.4 2.8 Green Straight 6.54 7.03 SE 7.39 2.38 7.32 1.43 4.67 5.87 CD (0.05) 11.04 6.89 11.01 5.39 12.02 11.8(Treatment T0 ; Number of seed /fruit 62; T1 Number of seed /fruit 57; T2 Number of seed /fruit 53; T3 Number of seed /fruit 50 ; T4 Number of seed /fruit 51)
The table 2 shows the impact of mutagen treatments on okra plants, specifically in terms of flowering, fruiting, and fruit characteristics.
Plants treated with higher mutagen concentrations flowered earlier and had more flowers. For example, T4 had the highest number of flowers at 11, while T1 had the lowest with 6 flowers.
Higher mutagen concentrations led to more fruits, with T4 producing the most (4 fruits), and the control (T0) having 3 fruits. Fruits from higher concentration treatments (T3 and T4) were shorter and lighter. For example, T4 had the shortest fruits at 4.8 cm and the lowest weight at 4.53 gm. In contrast, the lower concentration (T1) produced longer and heavier fruits. The number of seeds per fruit varied among treatments, with T0 having the most seeds (62), and T3 having the fewest (50) shown in (figure 4) (Table 2). Mutagen concentration affects flowering, fruiting, and fruit characteristics in okra. Higher concentrations lead to more flowers and fruits but result in shorter, lighter fruits with fewer seeds, while lower concentrations produce larger fruits with more seeds.
Figure 4. Synergetic impact of mutation on Fruit Height.

DUS Characteristics: The profile of 36 Okra cultivars was established using a set of morphological characteristics as prescribed in the DUS test guidelines for Okra. These characters were employed to establish distinctiveness, uniformity, and stability of each cultivar, forming the basis for cultivar protection. The Parbhani Kranti cultivar was utilized, and chemical mutagenic treatment was applied. Subsequently, the resultant variety was used for the analysis of DUS characteristics. Out of these 29 DUS characters, the states in which each character was found are presented in (Table 3).
Table 3. Distinguishing Unique Characteristics of Cultivated Plant Varieties (DUS)
Sr.No. Characters States Variety 1 Stem: Colour Green Green 2 Stem: Intensity of green colour Light Light 3 Leaf blade: Depth of lobbing Medium Medium 4 Stem: Number of nodes at first flowering Many (>8) Many (>8) 5 Flowering: Time Late (>45 days) Late (>45 days) 6 Leaf blade: Width (cm) Large (>25) Large (>25) 7 Leaf blade: Serration of margin Strong Strong 8 Leaf blade: Colour between veins Green Green 9 Leaf blade: Intensity of colour between veins Medium Medium 10 Vein: Colour Light Green Light Green 11 Petiole: Length (cm) Medium (20-30) Medium (20-30) 12 Flower: Petal colour Yellow Yellow 13 Flower: Petal base colour Both side Both side 14 Flower: Length (cm) Large (>5) Large (>5) 15 Fruit: Colour Green Green 16 Fruit: Length (cm) at marketable stage Medium Medium 17 Fruit: Diameter Medium (1.0-.5) Medium (1.0-.5) 18 Fruit: Surface between ridges Flat Flat 19 Fruit: Pubescence Medium Medium 20 Fruit: Constriction of basal part Strong Strong 21 Fruit: Shape of apex Acute Acute 22 Fruit: Number of locules <6 <6 23 Plant: Number of branches Many (>4) Many (>4) 24 Stem: Diameter Large (>1.5) Large (>1.5) 25 Plant: Height Tall (>120) Tall (>120) 26 Fruit: Length of Physiologically mature fruit Long (>15) Long (>15) 27 Seed Colour Green Green 28 Seed: Hairiness Present PresentIn the physiological characterization of the okra varieties, two significant parameters, namely chlorophyll content and relative water content (RWC), were evaluated (Table 4).
Chlorophyll A, chlorophyll B, and total chlorophyll (chlorophyll ab) were determined and recorded. Among the treatments, the highest chlorophyll content was observed in the highest treatment of EMS + Sodium azide (T4), with a value of 1.90, surpassing other treatments and the control (Table 4).
Table 4. Synergetic impact of EMS and Sodium Azide on Physiological Parameters.
Treatments (T) Chlorophyll Content Relative Water content (%) Chl. A Chl. B Chl. AB T0 0.96 0.98 1.95 63.36 T1 0.53 0.54 1.18 43.30 T2 0.49 0.48 1.06 35.76 T3 0.62 0.69 1.25 41.09 T4 0.90 0.97 1.90 51.81 Mean 0.70 0.73 1.47 47.06 SE 1.93 2.34 3.73 7.393 CD (0.05) 8.45 8.04 9.69 11.046The relative water content (RWC) was calculated using a specific formula, and observations were recorded. The highest RWC was observed in the EMS + Sodium azide T4 treatment, with a value of 51.81%, surpassing other treatments and the control. This indicates that the RWC was significantly influenced by the mutagenic treatment. The complete data and calculations for RWC should be provided in your research, as it’s crucial for understanding the physiological response of the okra seedlings to the treatments. The formula used to calculate RWC and the specific values for each treatment should be included (Table 4).
The Relative Water Content (RWC) was calculated for different treatments of okra seedlings. The results show variations in RWC among the treatments. The highest RWC was observed in the EMS + Sodium azide T4 treatment, with a value of 51.81%. In contrast, the control (T0) had an RWC of 63.36%. The significant differences in RWC among the treatments suggest that the mutagenic treatment had an impact on the water content of the seedlings. This physiological parameter provides insights into the response of okra seedlings to the mutagenic agents. The complete data and the statistical calculations are essential for a comprehensive understanding of the physiological changes induced by these treatments.
The formula used for calculating RWC and the specific values for each treatment should be presented in your research for clarity and reference (Table 4). Significant variations were observed in almost all morphological traits, including seed germination, shoot length, and the number of leaves, as well as physiological traits such as chlorophyll content and relative water content in wheat plants exposed to salt and drought stress. These traits exhibited substantial changes, particularly in the varieties NIAW-917, NIAW-295, and MACS-6222, when compared to NIAW-301 and NIAW-3170. Notably, the relative water content was highest in NIAW-3170, measuring at 78.03%, but it decreased to 71.25% under the influence of water and salt stress and chlorophyll content experienced a significant reduction under stress conditions (More et al., 2023).
This DNA isolation process is essential for subsequent molecular analyses and genetic studies, ensuring that high-quality DNA is obtained for accurate and reliable results. This DNA isolation process is essential for subsequent molecular analyses and genetic studies, ensuring that high-quality DNA is obtained for accurate and reliable results. The genomic DNA extracted from the okra seedlings was quantified using a spectrophotometer. The quantification was performed to ensure the quality and quantity of the DNA samples shown in (figure 5). The data are presented in the following (Table 5).
Table 5. Synergistic Influence of EMS and Sodium Azide on DNA Quantification
Treatments (T) Concentration (ng/μl) T0 94 T1 85.5 T2 74 T3 72.5 T4 86.5 Mean 82.5 C.D(0.5) 1.52 S.E 12.74The values of the DNA concentration for all treatments (T0, T1, T2, T3, and T4), ranging from 72.5 ng/μl to 94.0 ng/μl. The highest DNA concentration is found in T0 (94), while the lowest concentration is observed in T3 (72.5). This quantification data is vital for subsequent research and ensures the availability of suitable DNA samples for further study (Table 5), (figure5).
Figure 5 Agorse gel electrophoresis.
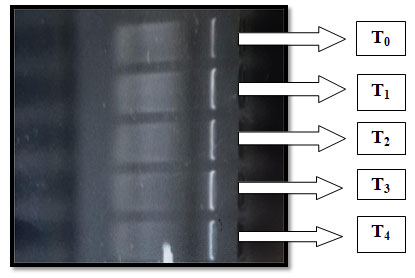
PCR Analysis: The assessment of genetic purity is vital in maintaining seed quality during okra hybrid seed production and ensured the genetic purity of the seeds; SSR (Simple Sequence Repeat) markers are used to differentiate the allelic size of 260 and 300 base pairs. These markers help confirm the specificity of allelic size in the seeds and their association with both the seed and pollen parents of the hybrid. Following chemical mutagen treatment, the DNA sequence may be altered, leading to changes in the SSR marker patterns. The results indicate that the first marker is linked only to the control (T0) and not to the DNA bands of other treatments. In certain conditions, the marker may be linked to other markers, resulting in the appearance of double bands on the agarose gel.
To assess the purity of Parbhani Kranti seeds, bulked samples from both rows and columns were analyzed on the agarose gel. The amplification of both allelic sizes (260 and 300 bp) was expected. The presence of double bands in specific coordinates indicated potential contamination. To confirm the identity of off-type individuals, leaf samples from seedlings at these coordinates were analyzed. The results showed that the allele size of seedlings at these coordinates was similar to the off-type coordinate. Therefore, based on the presence of double bands, it was possible to confirm them as off-type seedlings (Table 6).
In the agarose gel, when five contaminated samples were added, they also exhibited double bands, indicating that they were off-type seedlings. Based on the presence of double bands in three coordinates, it was inferred that these samples were also contaminated and classified as off-type seedlings. To test the genetic purity of Parbhani Kranti subjected to chemical mutagenic treatments, they were analyzed for single or double bands. The presence of double bands, as seen in T1-R4 and T3-R1, R4, R5 coordinates, indicated the presence of off-type seedlings. Based on these results, the percentage of genetic purity was calculated and is presented in the following (Table 6) shown in (figure 6).
Table 6. Assessment of Genetic Purity and Total Off-Type
Percentage in Cultivated Plant Varieties
The table provides the percentage of off-type plants and the corresponding genetic purity for each treatment. These results are crucial for evaluating the genetic purity of Parbhani Kranti and identifying off-type seedlings.
Figure 6 Combine effect of sodium azide and EMS on genetic purity of Okra.

(L – Ladder, Hightlight part – Off type, R – Replication, T – Treatment)
The study investigated the synergetic impact of Sodium azide and EMS mutagen treatment on the Parbhani Kranti okra variety. Several key findings emerged: Germination rates decreased as mutagen concentration increased. Control (T0) had the highest germination rate at 97%, while T4 (highest mutagen concentration) had the lowest at 70%.Shoot and root length decreased in mutagen-treated plants. The lowest values were observed in T4 table 6.
Higher mutagen concentrations led to increased flower and fruit numbers. However, these fruits were smaller and lighter in weight. Chlorophyll content increased with higher mutagen concentrations. RWC also increased with mutagen concentration.
Genomic DNA was successfully isolated, and DNA concentrations varied among treatments.SSR markers revealed contamination in some treatments, impacting genetic purity. T3 showed the highest off-type percentage. These findings highlight the complex Synergetic impact of mutagen treatment on okra, with implications for breeding and genomic diversity preservation in this crop. Assessment of genetic purity of seed is crucial for maintaining the seed quality in okra hybrid seed production. Among the two SSR marker differentiated with allelic size of 260 and 300 base pairs respectively.
Thus ensuring that allelic size are specific to the seed and Pollen parent of the hybrid. The increase in chlorophyll content and relative water content with an increase in mutagenic elements concentrations suggests a complex response in plants. The exposure seed of the plant species one hour, the interpretation is as – Plants may be responding to the presence of mutagenic elements by activating adaptive or tolerance mechanisms. This could involve an increased production of chlorophyll, which is crucial for photosynthesis, and an enhancement of water retention to cope with potential stress. The increase in chlorophyll and water content could be a stress response.
In some cases, exposure to mutagenic elements might trigger stress responses that include physiological changes in an attempt to mitigate the negative Synergetic impact s of the stressor. Plants strive to maintain internal balance or homeostasis. The increase in chlorophyll content and water retention may be a part of the plant’s efforts to regulate internal conditions and sustain essential processes. Mutagenic elements have the potential to induce genetic changes. The observed increase in chlorophyll and water content may be a result of genetic alterations that influence the plant’s physiological characteristics. Different plant species may respond differently to mutagenic elements, and even within a species, individual plants may exhibit varying responses.
Therefore, the interpretation of these changes would be more accurate if specific details about the plant species and the mutagenic elements involved are known. It’s important to consider that an increase in chlorophyll and water content may not necessarily indicate a positive or beneficial outcome for the plant. It could be an adaptive response to stress, and prolonged exposure to mutagenic elements might have negative consequences on overall plant health and productivity. Further research and analysis are needed to understand the specific mechanisms at play and the long-term Synergetic impact on the plants in question.
CONCLUSION
In summary, exploration into the Synergetic impact of sodium azide and EMS mutagens on Parbhani Kranti okra illuminates the intricate dynamics between genetic alterations and plant response. Higher concentrations of mutagens emerged as catalysts for reduced germination rates and distinct shifts in plant morphology, exposing the vulnerability of okra to mutagenic stress.
The chlorophyll and water content hints at a genetic recalibration, suggesting an adaptive mechanism triggered by environmental stress. This dual emphasis on mutagenic impacts and genetic purity enhances our understanding of the intricate dance between genetic modification and sustainable crop breeding, echoing a resounding imperative for precision and foresight in the pursuit of resilient and high-yielding okra
Conflict of Interest:Author declare no conflicts of interests to disclose.
Authors contribution: Formulation of the research concept, experimental design, provision of experimental materials, execution of field and laboratory experiments, data collection, analysis, interpretation of data, and manuscript preparation.
ACKNOWLEDGEMENT: The support and facilities provided by IBT, MGM University, are greatly appreciated.
REFERENCES
Abdel Baki, A. A., and Anderson, J. D. (1973). Quantitative trait loci pyramiding for fruit quality traits in okra. Molecular Breeding, 31, 217-222.
Adiger, S. S. (2011). Association studies in okra (Abelmoschus esculentus (L.) Moench). Electronic Journal of Plant Breeding, 2(4), 568-573.
Akinyele, B. O., & Osekita, O. S. (2006). Studies on the Performance and Morphological Characterization of Okra (Abelmoschus esculentus L. Moench) Genotypes for Yield and Yellow Vein Mosaic Viruses. International Journal of Current Microbiology and Applied Sciences, 6(7), 1102-1106.
Dahot, M., Umar, M., Rafiq, A., Arif, A. M., and Naqvi, S. H. A. (2012). Effect of sodium azide on the growth of Capsicum annuum (chilli). Pak. J. Biotechnol., 9 (1), 13-20.
Ellis, T. H. N., and Roberts, J. A., et al. (1981). Induction of mutation in tomato (Solanum lycopersicum L.) by gamma irradiation and EMS. Indian J. Genet., 4, 392-399.
Gandhi, E. S., Devi, A. S., and Mullainathan, L. (2014). The effect of ethyl methane sulphonates and diethyl sulphate on chilli (Capsicum annuum L.) in M1 generation. International Letters of Natural Sciences, 10, 18-23.
Gupta, N., et al. (2019). Mutation breeding in vegetable crops: A review. International Journal of Chemical Studies, 3(6), 3516-3519.
Khan, M. K., Butt, S. J., et al. (2017). Morphological and physico-biochemical characterization of various tomato cultivars in a simplified soilless media. Annals of Agriculture Sciences, 62, 139-143.
Kumar, G., Verma, S., et al. (2011). Comparative effect of individual and sequential treatment of gamma rays and sodium azide in Vigna unguiculata. Chromosome Botany International Society of Chromosome Botany, 6, 33-36.
Kundan N. More, Saurabh M. Patil, and Ashwinikumar B. Kshirsagar. (2023). Evaluation of Wheat under Salt and Drought Stress. Bioinfolet, 20(3 A), 419-422, 2023.
Matthew, C. O., Onsigbere, V., and Osawar, M. (2018). Okra germplasm collection from Southern Nigeria and their morphological characterization. Makara Journal of Science, 22(2).
Minoia, S., Petrozza, A., et al. (2010). A new mutant genetic resource for tomato crop improvement by TILLING technology. BMC Research Note, 3, 69.
Mishra, S., et al. (2015). Characterization of three Okra [Abelmoschus (L.)] accessions using morphology and SDS-PAGE for the basis of conservation. Egyptian Academic Journal of Biological Sciences, 5 (1), 55-65.
Oladosu, Y., et al. (2016). Principles and application of plant mutagenesis in crop improvement: A review. Biotechnology and Biotechnological Equipment, 30(1), 1-16.
Panse, V. G., and Sukhmate, N. K., et al. (1968). Studies on biochemical composition of various tomato genotypes. International Journal of Current Microbiology and Applied Sciences, 12, 977-987.
Pharmawati. (2018). Morphological changes of Capsicum annuum L. induced by Ethyl Methane Sulfonate (EMS). Current Agriculture Research Journal, 6, 1-7.
Rohlf, F. J. (1998). NTSYS-PC. Numerical taxonomy and multivariate analysis system, version 2.02. Exeter Software.
Saifullah, R., and Rabbani, M. (2009). Inheritance studies of morphological characteristics in okra (Abelmoschus esculentus L. Moench) under drought conditions. Pakistan Journal of Scientific and Industrial Research, 50 (2), 138-142.
Sun, Z., Wang, X., Liu, Z., Gu, Q., Zhang, Y., Li, Z., Ke, H., Yang, J., Wu, J., Wu, L., Zhang, G., Zhang, C., and Ma, Z. (2017). Genome-wide association study discovered genetic variation and candidate genes of fiber quality traits in Gossypium hirsutum L. Plant Biotechnology Journal, 15, 982-996.
Tiwari, K. N., and Chattopadhyay, A. (1998). Response of okra (Abelmoschus esculentus (L.) Moench) to drip irrigation under mulch and non-mulch conditions. Agricultural Water Management, 38(2), 91-102.
Yafizham, and Herwibawa, B. (2018). Effects of sodium azide on seed germination and seedling growth of chili pepper (Capsicum annuum L. Cv. Landung). IOP Publishing, 10, 450-455.
留言 (0)