TPS College Patliputra University, Patna India.
Corresponding author email: vinaykumar10121976@gmail.com
Article Publishing HistoryReceived:
Accepted After Revision:
ABSTRACT:The utilization of Cissus quadrangularis and other medicinal plants in traditional and modern medicine holds promise for the prevention and management of oxidative stress-related maladies. Top of FormThe aim of present work was to assess the free radical scavenging potential of the selected bioactive component of stem extract using different solvent (aqueous, methanol, dichloromethane, acetone and chloroform) of the plant Cissus quadrangularis. Antioxidant activity of the selected phytochemical of stem extract of the plant Cissus quadrangularis were done by Ferric reducing antioxidant power assay, DPPH antioxidant power assay and hydroxyl radical scavenging activity. The analysis of ferric reducing antioxidant power assay indicate that all tested solvent extract have good antioxidant potential as compare to ascorbic acid which was taken as reference.
Acetone extract expressed maximum antioxidant potential with EC50 value 0.17 and chloroform extract expressed minimum antioxidant potential with EC50 value 0.35. The order of ferric reducing antioxidant potential among the tested solvent are as s follow: Acetone > Dichloromethane > Methanol > Aqueous > Chloroform. The results of the present study showed that all the extracts exhibited potent antioxidant activity. The analysis of DPPH antioxidant power assay, HRS antioxidant power assay and Ferric reducing antioxidant power assay indicate that all tested solvent extracts have good antioxidant potential and among the five extracts methanolic, dichloromethane, and acetone extract respectively exhibited higher potency of free radical scavenging activity.
KEYWORDS:Antioxidant, Cissus Quadrangularis, DPPH, FRAPA, HRSA, Oxidative Stress. Reactive Oxygen Species.
Download this article as: Copy the following to cite this article:Arvind Kumar A, Kumar V.B. In-vitro assessment of free radical scavenging potential of selected stem extracts of Cissus quadrangularis using different solvents. Biosc.Biotech.Res.Comm. 2024;17(2).
Copy the following to cite this URL:Arvind Kumar A, Kumar V.B. In-vitro assessment of free radical scavenging potential of selected stem extracts of Cissus quadrangularis using different solvents. Biosc.Biotech.Res.Comm. 2023;17(2). Available from: <a href=”https://shorturl.at/MQzVW“>https://shorturl.at/MQzVW</a>
INTRODUCTION
Oxidative stress is one of the major region for the initiation and progression of cancer, mellitus, diabetes, neurodegenerative diseases, cardiovascular diseases and inflammatory diseases among other syndromes (Arika et al., 2019). The condition of oxidative stress arises due to the excessive generation of free oxygen and nitrogen species or their inefficient quenching within the cell (Bhat A.H. et al., 2015). Free radicals, a natural byproduct of cellular metabolism, are continuously generated in the human body as a consequence of oxygen utilization by the cells. This process, known as oxidative metabolism, occurs during various physiological activities, including respiration and energy production. The mitochondria, often referred to as the powerhouse of the cell, are particularly implicated in this process. Free radicals are highly reactive molecules with unpaired electrons, capable of damaging cellular components such as DNA, proteins, and lipids. While the body has defense mechanisms to neutralize these harmful effects, excessive free radical production or inadequate antioxidant defenses can lead to oxidative stress, contributing to various diseases and aging processes (Moriasi et al., 2020).
Free radicals play a fundamental role in any biochemical process and constitute an essential component of aerobic life and metabolism (Tiwari 2001). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) arise from normal cellular metabolism. The prevalent ROS consist of the superoxide anion, hydrogen peroxide (H2O2), peroxyl (ROO) radicals, and reactive hydroxyl (OH) radicals. Nitrogen-derived free radicals include nitric oxide and peroxynitrite anion (ONOO) (Joyce 1987). Reactive oxygen species and reactive nitrogen species are linked to numerous pathological conditions, including atherosclerosis, ischemia, tissue reperfusion injury, central nervous system damage, gastritis, and cancer (Manoharan et al., 2005). Endogenous sources of free radicals comprise electron transfer chain reactions in the mitochondria, the xanthine oxidase pathway, and occurrences during disease states such as inflammation, ischemia, and reperfusion injury (Moriasi et al., 2020).
Many antioxidants are utilized to eliminate these free radicals. They exert a protective effect by neutralizing free radicals, toxic byproducts of natural cell metabolism. The human body employs various mechanisms to counteract oxidative stress by producing antioxidants, which are either naturally produced in situ or externally supplied through foods and/or supplements. These antioxidants function as free radical scavengers, preventing and repairing damages caused by ROS. Consequently, they can enhance immune defense and reduce the risk of cancer and degenerative diseases (Ganapaty et al., 2007). Conventionally, oxidative stress is typically addressed through the utilization of various types of synthetic antioxidant compounds, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and propyl gallate (PG). However, despite their widespread use, these synthetic antioxidant compounds have been linked to undesirable effects (Ndhlala et al 2010). To overcome the harmful effects of synthetic antioxidants, an available alternative is the use of medicinal plants, which offer potent, safer, more affordable, and easily accessible therapies for oxidative stress-related ailments (Goyal et al 2019).Plants are acknowledged as significant sources of novel drugs, offering key molecules of pharmacological interest. Their established applications in traditional medicines have garnered significant attention as a central focus of research (Javaid et al., 2023).
Presently, researchers are directing their focus towards phytochemicals for managing and treating various human diseases. It’s noteworthy that over 50% of all modern clinical drugs originate from natural products, underscoring their pivotal role in the development of pharmaceuticals within the industry (Bardoloi et. al., 2018). Ayurveda, Unani, Siddha, and modern medicinal systems utilize numerous plants in the treatment of various diseases (Jainu et al., 2004). In the Indian subcontinent, there exists a vast array of medicinal plants that are utilized as drugs for treating numerous diseases (Ballabh et al., 2007). Cissus quadrangularis stands out as one of the most beneficial flora among them.
Cissus quadrangularis, a perennial herb of the grape family, features a stout, fleshy quadrangular stem with medicinal properties found throughout the tropical regions of the Earth. Also known as Cissus succulent, it is popularly referred to as horjora in Hindi and pirandai in Tamil, and belongs to the family Vitaceae. This plant is extensively utilized in India and is believed to be native to India, Sri Lanka, Malaysia, Java, and West Africa. Widely observed in tropical forest regions of Asia and Africa, Cissus quadrangularis is an evergreen climber that grows at a rapid rate (Ruskin et al., 2014).Cissus quadrangularis has been extensively studied for its phytochemical composition, pharmacological activities, and toxicological evaluation. Numerous phytochemicals, including alkaloids, tannins, lignins, suberins, phenols, flavonoids, resveratrol, piceatannol, pallidol, perthenocissin, phytosterols, and others, have been identified in the plant extract of Cissus quadrangularis (CQ) (Sundaran et al., 2020).
Among these, ascorbic acid, triterpenes, beta-sitosterol, ketosterol, two asymmetrical tetracyclic triterpenoids, and calcium have been recognized as the major phytochemicals of this plant (Jainu et al., 2004). Cissus quadrangularis exhibits a diverse range of beneficial properties, including antimicrobial, antioxidant, anti-inflammatory, anti-cancerous, and cytotoxic effects. Furthermore, it has been observed to promote bone healing, making it particularly valuable in traditional medicinal practices. Its antimicrobial properties make it effective against various pathogens, while its antioxidant and anti-inflammatory actions contribute to overall health and wellness. Additionally, its potential anti-cancer properties offer promise in combating malignancies, and its ability to aid in bone healing underscores its importance in orthopedic medicine. These multifaceted properties highlight the potential of Cissus quadrangularis as a valuable therapeutic agent in the treatment of various ailments., Panche et al 2016, Chinthamani et al 2014, Murthi et al 2003, Kuppuramalingam et al 2018 & Rekha et al 2019, Anwar et al 2021 and Dinesh et al 2021).
MATERIALS AND METHODS
Preparation of stem extract: The fresh stems of Cissus quadrangularis (CQ) were harvested from Supaul district in Bihar, India (Latitude 26.5520640 and Longitude 87.0555330) (Figure 1). Authentication of the plant and stem was conducted by Prof. Rimjhim Sheel, Former Head, University Department of Botany & Dean Faculty of Science and Principal GDM College. Patliputra University, Patna. A voucher specimen has been preserved in the University Department of Botany at Patliputra University, Patna, Bihar, India for future reference. After collection, the stems were cleaned thoroughly by washing them in tap water followed by rinsing with distilled water. Subsequently, the stems were shade dried and ground into a fine powder. The powdered material was then stored in a clean, airtight container for further use. All the chemicals utilized in this study were procured from two suppliers: Bihar Scientific Company and Krishna Scientific, both located in Patna, Bihar, India.
Soxhlet extraction: The dried powder of Cissus quadrangularis stems (50g) was packed into the thimble of a Soxhlet apparatus. Successively, 400 ml of methanol, acetone, chloroform, dichloromethane, hexane, and aqueous solvents (referred to as MACDHA) were employed as solvents one after the other. These solvents were utilized to dissolve the active biomolecules present in the plant material. The stems remained as precipitate while the active biomolecules were extracted into the solvent. The extraction process was continued until the solvent in the thimble appeared clear, typically taking approximately 8 hours on average. Subsequently, the solvent extract was evaporated in a water bath until a dark orange residue was obtained. The percentage yield was 12%, 8%, 7%, 9%, 10% and 12% for methanol, acetone, chloroform, dichloromethane, hexane and aqueous (MACDHA) respectively. The extract were kept at -200 C till further use. All the process of Soxhlet extraction was completed in University Department of botany and Department of Botany TPS College, Patliputra University.
Estimation of free radical scavenging activity: Antioxidant activity of the selected phytochemical of stem extract of the plant Cissus quadrangularis were done by the following methods.
Determination of 1,1, dipheny-2-picrylhydrazyl (DPPH) Radical Scavenging Activities: The DPPH radical scavenging assay was conducted following the protocol outlined by Brand-Williams et al. with certain modifications. In brief, five different concentrations of the plant extracts under investigation (0.0625, 0.125, 0.25, 0.5, and 1 mg/ml) were prepared in methanol (analytical grade). Equivalent concentrations were also prepared for L-ascorbic acid, serving as the standard antioxidant. Subsequently, 1 ml of each plant extract was transferred into a clean test tube, to which 0.5 ml of 0.3 mM DPPH solution in methanol was added. The mixture was then shaken and left to incubate in the dark at room temperature for 15 minutes. Blank solutions, consisting of the plant extract solutions (2.5 ml) and 1 ml of methanol, were utilized as the baseline. The negative control was composed of 2.5 ml of DPPH solution and 1 ml of methanol, while L-ascorbic acid at equivalent concentrations to the plant extracts served as the positive control. Following the incubation period in darkness, the absorbance values were measured at 517 nm using a spectrophotometer. All experiments were conducted in triplicate. The DPPH radical scavenging activity was calculated using the equation as described by Brand-Williams et al.

Where, As is absorbance of the sample and Ac is absorbance of the control. The half maximal inhibitory concentration (IC50) of the extracts was computed from a plot of percentage DPPH free radical inhibition versus the extract concentration.
Determination of Hydroxyl Radical Scavenging Activity: The following steps were conducted to determine the Hydroxyl Radical Scavenging Activity of the plant extracts by the method outlined by Klein et al. (1991) with certain modifications: A solution containing 0.13% ferrous ammonium sulfate and 0.26% EDTA was prepared. 1 mL of the prepared Iron-EDTA solution was mixed with various concentrations of plant extracts. To this mixture, 0.5 mL of EDTA solution (0.018%) and 1 mL of DMSO (0.85% v/v in 0.1 M phosphate buffer, pH 7.4) were added. The reaction was initiated by adding 0.5 mL of ascorbic acid (0.22%). The reaction mixture was then incubated at a temperature ranging from 80 to 90°C for 15 minutes in a water bath. After incubation, the reaction was terminated by adding 1 mL of ice-cold trichloroacetic acid (TCA) (17.5% w/v).Following TCA addition, 3 mL of Nash reagent was added to the reaction mixture. The reaction mixture was allowed to stand at room temperature for 15 minutes. The absorbance of the reaction mixture was measured spectrophotometrically at 412 nm against a reagent blank. The percentage of hydroxyl radical scavenging activity was calculated using the following formula:

Where, As is absorbance of the sample and Ac is absorbance of the control. The half maximal inhibitory concentration (IC50) of the extracts was computed from a plot of percentage DPPH free radical inhibition versus the extract concentration.
Ferric Reducing Antioxidant Power Assay: The reducing power of the extracts was assessed following the method outlined by Oyaizu et al. (1986) with slight modifications. Four different concentrations of aqueous extract (1.5 mg, 0.75 mg, 0.38 mg, and 0.19 mg) along with L-ascorbic acid at equivalent concentrations were mixed with 2 ml of phosphate buffer (0.2 M, pH 6.6) and 2 ml of 1% potassium ferricyanide. The mixtures were then incubated at 50°C for 20 minutes. Subsequently, 2 ml of 10% trichloroacetic acid was added, followed by centrifugation at 1000 rpm for 10 minutes. The supernatant (2 ml) was aspirated and mixed with 2 ml of distilled water and 1 ml of 0.1% ferric chloride. The absorbances were measured at 700 nm using a UV-Vis spectrophotometer and recorded. The concentration of each extract capable of producing an absorbance value of 0.5 was determined from the graph of absorbance at 700 nm against extract concentration. This concentration was considered as the median effective concentration (EC50).
RESULT AND DISCUSSION
The stems of CQ were freshly collected from Supaul district of Bihar, India (Latitude 26.5520640 and Longitude 87.0555330). Authentication of the plant and stem was performed by Prof. Rimjhim Sheel, Former Head, University Department of Botany & Dean Faculty of Science and Principal GDM College, Patliputra University, Patna.
Reactive oxygen species (ROS) or free radicals are natural byproducts of cellular metabolic reactions. However, when they accumulate in cells, they can transform into highly toxic substances that damage essential cellular components such as DNA, RNA, proteins, and lipids (Ali S. S. et al., 2020). These accumulated damages can contribute to the development of chronic diseases including cancer, diabetes, and heart diseases (Hajam Y. A. et al., 2022). Balancing the effects of ROS is crucial, and external antioxidants play a significant role in this process. Plants, being a major part of our diet, serve as the primary source of these antioxidants (Nwozo O. S. et al., 2023).
The processing and extraction of the leaves and stems of CQ were conducted accordingly, and the extracts were obtained using various solvents (Acetone, Chloroform, Methanol, Dichloromethane, and Aqueous). These extracts were then subjected to further analysis for radical scavenging activity. The results of the scavenging activity are tabulated below.
The stems of CQ were freshly collected from Supaul district of Bihar, India (Latitude 26.5520640 and Longitude 87.0555330). Authentication of the plant and stem was performed by Prof. Rimjhim Sheel, Former Head, University Department of Botany & Dean Faculty of Science and Principal GDM College, Patliputra University, Patna.
Reactive oxygen species (ROS) or free radicals are natural byproducts of cellular metabolic reactions. However, when they accumulate in cells, they can transform into highly toxic substances that damage essential cellular components such as DNA, RNA, proteins, and lipids (Ali S. S. et al., 2020). These accumulated damages can contribute to the development of chronic diseases including cancer, diabetes, and heart diseases (Hajam Y. A. et al., 2022). Balancing the effects of ROS is crucial, and external antioxidants play a significant role in this process. Plants, being a major part of our diet, serve as the primary source of these antioxidants (Nwozo O. S. et al., 2023).
The processing and extraction of the leaves and stems of CQ were conducted accordingly, and the extracts were obtained using various solvents (Acetone, Chloroform, Methanol, Dichloromethane, and Aqueous). These extracts were then subjected to further analysis for radical scavenging activity. The results of the scavenging activity are tabulated below.
DPPH antioxidant power assay
Comparative analysis of % inhibition by plant extract of different solvents. (Table 1)
The stems of CQ were freshly collected from Supaul district of Bihar, India (Latitude 26.5520640 and Longitude 87.0555330). Authentication of the plant and stem was performed by Prof. Rimjhim Sheel, Former Head, University Department of Botany & Dean Faculty of Science and Principal GDM College, Patliputra University, Patna.
Reactive oxygen species (ROS) or free radicals are natural byproducts of cellular metabolic reactions. However, when they accumulate in cells, they can transform into highly toxic substances that damage essential cellular components such as DNA, RNA, proteins, and lipids (Ali S. S. et al., 2020). These accumulated damages can contribute to the development of chronic diseases including cancer, diabetes, and heart diseases (Hajam Y. A. et al., 2022). Balancing the effects of ROS is crucial, and external antioxidants play a significant role in this process. Plants, being a major part of our diet, serve as the primary source of these antioxidants (Nwozo O. S. et al., 2023).
The processing and extraction of the leaves and stems of CQ were conducted accordingly, and the extracts were obtained using various solvents (Acetone, Chloroform, Methanol, Dichloromethane, and Aqueous). These extracts were then subjected to further analysis for radical scavenging activity. The results of the scavenging activity are tabulated below.
DPPH antioxidant power assay
Comparative analysis of % inhibition by plant extract of different solvents. (Table 1)
Table 1. DPPH radical scavenging activity showing % inhibition of different plant extracts of the plant Cissus quadrangularis.
Data were expressed as Mean ± Standard Deviations (SD). Values differ significantly at p<0.05
(in mgs)
% inhibition (By plant extract of different solvents) Aqueous Methanol Dichlorome-thane Acetone Chloroform Ascorbic acid 01 1.50 74.43±0.1691.88±0.08
78.42±0.22
89.64±0.14
68.72±0.22
96.72±0.06
02 0.75 63.82±0.20 82.59±0.08
58.67±0.16
81.32±0.12
53.90±0.12
82.33±0.04
03 0.38 50.46±0.19
70.87±0.06
43.37±0.17
70.42±0.06
35.30±0.23
72.88±0.07
04 0.19 43.98±0.11
60.77±0.11
30.14±0.19
62.39±0.22
21.45±0.11
66.06±0.09
IC50
(in mgs)
00.28 00.09 00.51 00.08 00.63 00.07Graphical presentation of % inhibition by plant extract of different solvents.
Figure 1: DPPH radical scavenging activity of different stem extracts of the plant Cissus quadrangularis.
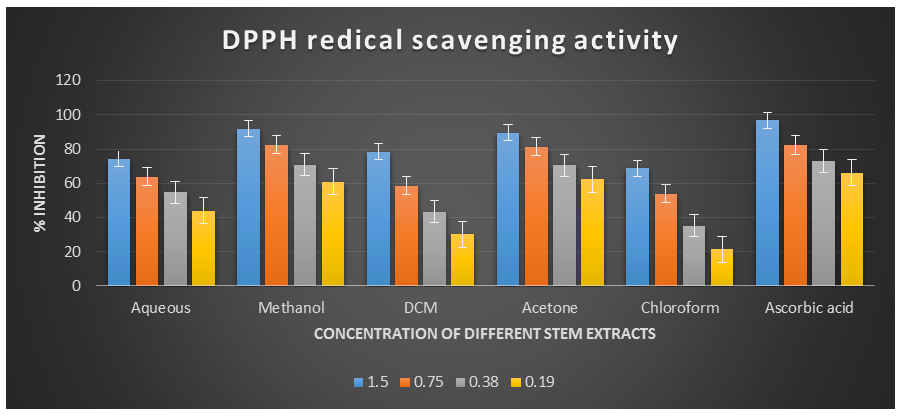
Figure 2: DPPH radical scavenging activity of different plant extracts of the plant Cissus quadrangularis.
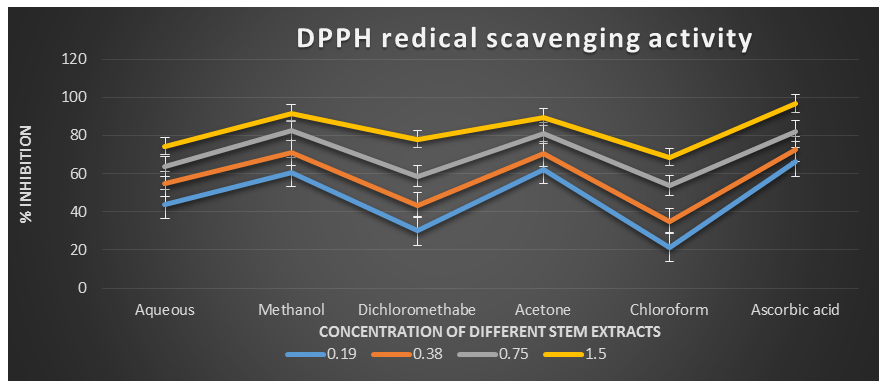
DPPH˙ (1,1-Diphenyl-2-picrylhydrazyl) is a stable nitrogen-centered free radical with an unpaired electron at one atom of the nitrogen bridge. The scavenging of DPPH free radicals is a widely used method for assessing antioxidant activity. This assay measures the ability of antioxidants to directly scavenge DPPH˙ radicals by monitoring changes in absorbance using a spectrophotometer at a wavelength of 517 nm (Kedare S. B. et al., 2011). The DPPH radical scavenging assay offers a quick and simple way to evaluate the antioxidant activity of various plant extracts. In this study, the Acetone, Aqueous, Chloroform, Dichloromethane, and Methanol extracts of Cissus quadrangularis stems were assessed for their ability to scavenge free radicals using DPPH˙ as the substrate. This assay measures the hydrogen or electron donating ability of the stem extracts. The extracts of Cissus quadrangularis stems were found to reduce the stable purple color of DPPH (1,1-diphenyl-2-picrylhydrazyl) free radicals to yellow-colored 1,1-diphenyl-2-picrylhydrazine. The reduction capacity increased with increasing concentration of the extract. Analysis of the DPPH antioxidant power assay indicated that all tested solvent extracts exhibited good antioxidant potential compared to ascorbic acid, which was used as a reference antioxidant.
The IC50 values for aqueous, methanol, dichloromethane, acetone and chloroform extracts are 0.28, 0.09, 0.51, 0.08, and 0.63 mg/ml respectively was compared with standard ascorbic acid (IC50 = 0.07 mg/ml) (p < 0.05; Table 1). Furthermore, it was demonstrated that the IC50 value for L-ascorbic acid was lower than the IC50 values of all the studied plant extracts. At all the tested concentration the maximum DPPH˙ radical scavenging activity was 91.88±0.08 % for methanol extract and minimum was 21.45±0.11 for chloroform extract among the plant extracts (p < 0.05; Table 1 and Fig 1). Furthermore, it was demonstrated that the DPPH˙ radical scavenging activity of ascorbic acid was maximum at all the concentration against plant extracts. The decreasing order of 1,1-diphenyl-2-picrylhydrazyle radical scavenging activity of the different extract was found to be Methanol > Acetone > Dichloromethane > Aqueous > Chloroform (p < 0.05; Table 1 and Fig 1) with correlation coefficient 0.95, 0.96, 0.98, 0.96 and 0.96 respectively.
Hydroxyl radical scavenging activity.
Hydroxyl radical scavenging activity (Table 2).
Table 2. Hydroxyl radical scavenging activity showing % inhibition of different plant extracts of the plant Cissus quadrangularis.
Data were expressed as Mean ± Standard Deviations (SD). Values differ significantly at p<0.05.
(in mgs)
% inhibition (By plant extract of different solvents) Aqueous Methanol Dichlorome-thane Acetone Chloroform Ascorbic acid 01 1.50 73.03±0.0768.38±0.21
78.90±0.26
54.82±0.10
68.09±0.29
81.45±0.20
02 0.75 65.35±0.03
62.61±0.17
65.97±0.10
41.69±0.40
58.46±0.21
73.00±0.21
03 0.38 59.34±0.07
55.61±0.20
50.31±0.20
31.49±0.32
49.32±0.16
65.00±0.20
04 0.19 52.65±0.17
49.69±0.17
37.19±0.69
25.43±0.08
46.93±0.23
57.26±0.20
IC50
(in mgs)
00.16 00.19 00.37 01.23 00.36 00.11Graphical presentation of % inhibition by plant extract of different solvents.
Figure 3: Hydroxyl radical scavenging activity of different plant extracts of the plant Cissus quadrangularis.
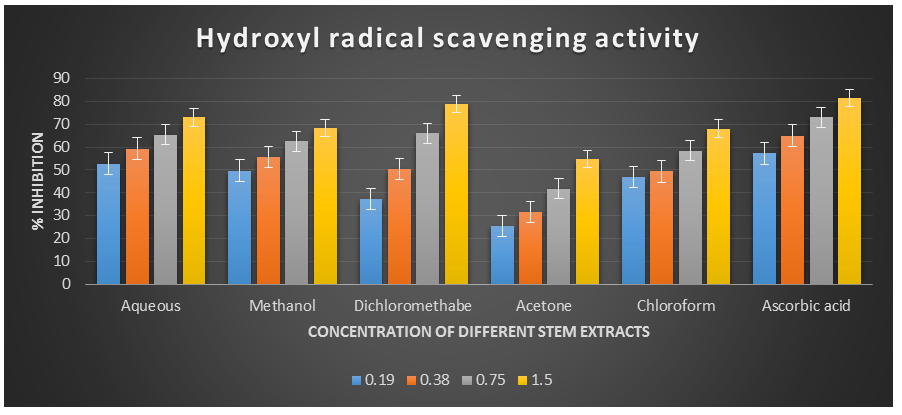
Figure 4: Hydroxyl radical scavenging activity of different plant extracts of the plant Cissus quadrangularis.
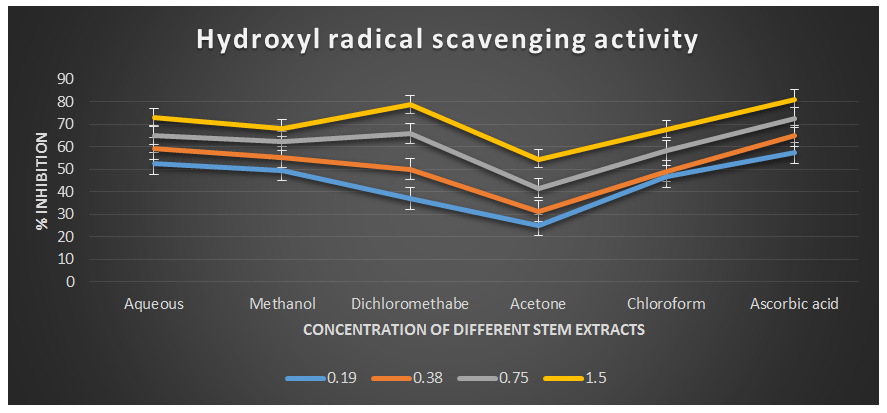
The hydroxyl radical, being the most reactive oxygen-centered species, can cause significant damage to adjacent biomolecules. In this study, the hydroxyl radical scavenging assay was conducted by generating hydroxyl radicals using ascorbic acid and EDTA. The hydroxyl radicals were formed through an oxidation reaction with dimethyl sulfoxide (DMSO), resulting in the production of formaldehyde, which provided a convenient method for detecting hydroxyl radicals by treatment with Nash reagent. All the extracts of Cissus quadrangularis, when added to the reaction mixture, exhibited the ability to scavenge hydroxyl radicals in a concentration-dependent manner. This scavenging activity could be attributed to the presence of phenolic compounds in the extracts, which possess hydrogen-donating abilities. Analysis of the hydroxyl radical antioxidant power assay revealed that all tested solvent extracts (aqueous, methanol, dichloromethane, acetone, and chloroform) displayed significant antioxidant potential compared to ascorbic acid, which served as the reference antioxidant in this study.
The IC50 values for aqueous, methanol, dichloromethane, acetone and chloroform extracts are 0.16, 0.19, 0.37, 1.23, and 0.36 mg/ml respectively was compared with standard ascorbic acid (IC50 = 0.11 mg/ml) (p < 0.05; Table 3) Furthermore, it was demonstrated that the IC50 value for L-ascorbic acid was lower than the IC50 values of all the studied plant extracts. At all the tested concentration the maximum hydroxyl radical scavenging activity was 78.90±0.26 % for dichloromethane extract and minimum was 25.43±0.08 for acetone extract among the plant extracts (p < 0.05; Table 3 and Fig 3). The decreasing order of hydroxyl radical scavenging activity of the different extract was found to be Dichloromethane > Aqueous > Methanol > Chloroform > Acetone (p < 0.05; Table 3 and Fig 3) with correlation coefficient 0.96, 0.97, 0.96, 0.99, and 0.99 respectively.
Ferric reducing antioxidant power assay.
Comparative analysis of absorption of different solvents (Table 3).
Table 3. Ferric reducing antioxidant power assay showing absorbance of different plant extracts of the plant Cissus quadrangularis.
Data were expressed as Mean ± Standard Deviations (SD). Values differ significantly at p<0.05.
methane
Acetone Chloroform Ascorbic acid 01 1.50 1.638±0.0011.880±0.001
1.901±0.001
2.089±0.002
1.003±0.003
3.192±0.002
02 0.75 1.112±0.001
1.137±0.001
1.306±0.004
1.332±0.002
0.716±0.005
2.455±0.002
03 0.39 0.693±0.001
0.712±0.001
0.9560.002
0.884±0.003
0.540±0.001
1.885±0.001
04 0.19 0.342±0.001
0.394±0.001
0.62±0.001
0.692±0±0 0.383±0.003
1.664±0.004
EC50
(in mgs)
00.23 00.23 00.18 00.17 00.35 00.05Graphical presentation of absorbance value of Ferric reducing antioxidant power assay by plant extract of different solvents.
Figure 5: Ferric reducing antioxidant power assay of different plant extracts of the plant Cissus quadrangularis.
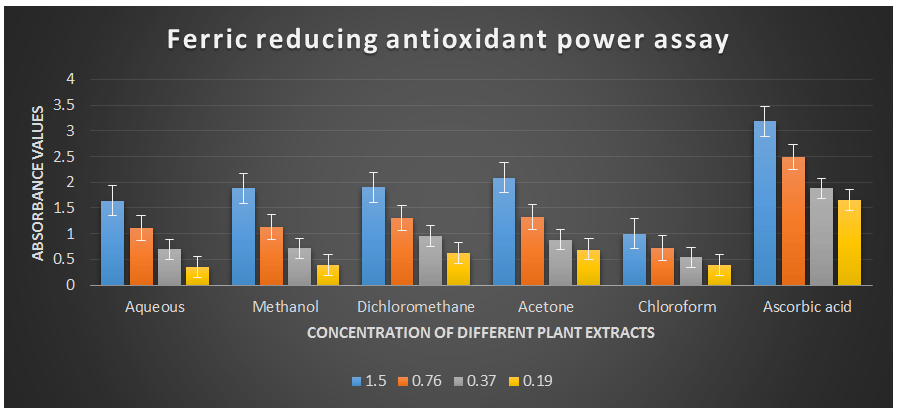
Figure 6: Ferric reducing antioxidant power assay of different plant extracts of the plant Cissus quadrangularis.
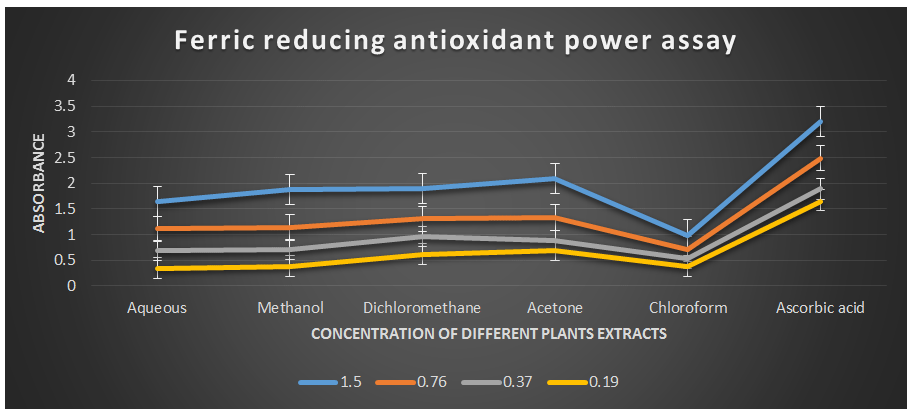
This method is based on the ability of the analyte to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) (Gulcin I., 2010 & MacDonald-Wicks L. K. et al., 2006). Therefore, the formation of Fe2+ can be assessed by measuring the absorbance capacity at 700 nm. Increases in absorbance at this wavelength indicate an increase in reducing power. The analysis of the ferric reducing antioxidant power assay revealed remarkable concentration-dependent increases in absorbance values at a wavelength of 700 nm (Table 2) compared to ascorbic acid, which was used as the reference antioxidant in this study. The half-effective concentrations (EC50) of the studied plant extracts required to produce an absorbance value of 0.5 were determined in this study.
The EC50 values for aqueous, methanol, dichloromethane, acetone and chloroform extracts are 0.23, 0.23, 0.18, 0.17, and 0.35 mg/ml respectively was compared with standard ascorbic acid (IC50 = 0.07 mg/ml) (P < 0.05; Table 2). Furthermore, it was demonstrated that the EC50 value for L-ascorbic acid was lower than the EC50 values of all the studied plant extracts. At all the tested concentration the maximum absorbance value for ferric reducing antioxidant activity was 2.089±0.002 for acetone extract and minimum was 0.342±0.001 for aqueous extract (p < 0.05; Table 2 and Fig 2). Furthermore, it was demonstrated that the absorbance value for ferric reducing antioxidant activity of ascorbic acid was significantly maximum at all the concentration against plant extracts (table 2). The decreasing order of ferric reducing antioxidant activity of the different extract was found to be Acetone > Dichloromethane > Methanol > Aqueous > Chloroform (p < 0.05; Table 2 and Fig 2) with correlation coefficient 0.99, 0.99, 0.99, 0.98 and 0.99 respectively.
CONCLUSION
Today, there is a growing interest in the antioxidative properties of plants due to their potential use as natural additives to replace synthetic ones. The results of the present study demonstrate that all the extracts exhibited potent antioxidant activity. Analysis of the DPPH antioxidant power assay, HRS antioxidant power assay, and Ferric reducing antioxidant power assay indicated that all tested solvent extracts possessed good antioxidant potential. Among the five extracts, methanolic, dichloromethane, and acetone extracts respectively exhibited higher potency of free radical scavenging activity. These findings suggest that the stem extract of the plant Cissus quadrangularis could serve as a valuable source of natural antioxidants for promoting health benefits. Further isolation of bioactive compounds is recommended to identify the unknown compounds and establish their pharmacological properties.
ACKNOWLEDGEMENT
Authors thank the Head Department of Botany and Principal T.P.S. College, PPU and HOD, University Department of Botany, PPU for their support and encouragement.
Conflict of interest: We declare that there is no conflict of interest.
REFERENCES
Ali, S.S, Ahsan, H, Zia, M.K, Siddiqui, T, Khan, F.H. 2020. Understanding oxidants and antioxidants: Classical team with new players. J Food Biochem. 44(3):e13145. DOI: https://doi.org/10.1111/ jfbc.13145
Anwar, P., Sezhian, U., & Narasingam, A. 2021. Molecular docking of components from the extracts of endophytic bacteria of Cissus quadrangularis against aurora b kinase. Journal of Advanced Scientific Research, 12(02), 40-48. DOI: https://doi.org/10.55218/JASR.202112207.
Arika, W., Kibiti, C. M., Njagi, J. M., and Ngugi, M. P. 2019. In vitroantioxidant proper- ties of dichloromethanolic leaf extract of Gnidia glauca (Fresen) as a promising antiobesity drug, Journal of Evidence-Based Integrative Medicine, vol. 24. DOI: http://doi.org/10.1177/2515690X19883258.
Ballabh, S. Chaurasia, O.P. 2007. Traditional medicinal plant of cold desert Ladakh-used in treatment of cold, cough and fever. Journal of Ethenopharmacology, 112: 341- 349. . DOI: http://doi.org/10.1016/j.jep.2007.03.020.
Bhat, A.H., Dar, K.B., Anees, S., Zargar, M.A., Masood, A., Sofi, M.A. and Ganie, S.A., 2015. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomedicine & Pharmacotherapy, 74, pp.101-110.DOI: http://doi.org/10.1016/j.biopha.2015.07.025.
Bordoloi, P., Devi, T. and Lahkar, M. 2018. Evaluation of anti-inflammatory and anti-arthritic activity o ethanolic extract of leaves of Nyctanthes arbor-tristis an experimental animal mode”. Journal of Evolution Medicinal and Dental Science, 7(10):1247-1251. DOI: https://doi.org/10.14260/jemds/2018/284.
Chinthamani, J. and Srinath, N., 2014. Antioxidant potential determination of Pipper betle and Cissus quadrangularis Kong. Res J. 1(1): 95-99. DOI: http://doi.org/10.26524/krj21.
Dinesh, Y., Abilasha, R., Ramani, P. and Rajeshkumar, S., 2021. Assessment of cytotoxic, antioxidant, thrombolytic, anti-Inflammatory and antimicrobial activity of curcuma longa linn, Cissus quadrangularis and Boerhaavia diffusa herbal mixture-an In vitro Study. J Pharm Res Int, 33, pp.1766-77.DOI: http://doi.org/10.9734/JPRI/2021/v33i60B34805
Goyal, M. R. and Suleria, H. A. R. 2019. Eds. Human Health Benefits of Plant Bioactive Compounds: Potentials and Prospects”, CRC Press, Boca Raton, FL, USA. Books Google.com.
Gulçin, I.(2010. “Antioxidant properties of resveratrol: a structure-activity insight,” Innovative Food Science & Emerging Technologies, vol. 11, no. 1, pp. 210–218. DOI: http://doi.org/10.1016/j.ifset.2009.07.002.
Hajam, Y.A, Rani, R, Ganie, S.Y, Sheikh, T.A, Javaid, D, Qadri, S.S, Pramodh, S, Alsulimani, A, Alkhanani, M.F, Harakeh, S, Hussain, A. 2022. “Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives”. Cells. 11(3):552. DOI: https:// doi.org/10.3390/cells11030552
Jainu, M. and Devi, C.S.S., 2004. “Effect of Cissus quadrangularis on gastric mucosal defensive factors in experimentally induced gastric ulcer comparative study with Su- cralfate”.Journal of medicinal food, 7(3), 372- 376. DOI: http://doi.org/10.1089/jmf.2004.7.372.
Javaid, A, Khan, I.H, Ferdosi, M.F.H, Manzoor, M, Anwar, A. 2023. “Medically important compounds in Ipomoea carnea flowers”. Pak J Weed Sci Res. 29(2):115-21. DOI: https://doi.org/10.17582/journal.PJWsr/2023/29.2.115.121.
Joyce, D. A.1987. “Oxygen radicals in disease”. Adv. Drug React. Bull. 127 476–479. DOI: http://doi.org/10.1097/00012995-198712000-00001.
Kedare, S. B. & Singh, R.P. 2011. “Genesis and development of DPPH method of antioxidant assay”. J Food Sci Technol. 48:412-22. DOI: http://doi.org/10.1007/s13197-011-0251-1.
Klein, S. M., G.and Cohen, A. I. 1991. “Cederbaum. Production of formaldehyde during metabolism of dimethyl sulphoxide by hydroxyl radical radical generating system”. Biochemistry. 20: 6006-6012. DOI: http://doi.org/10.1021/bi00524a013.
Kuppuramalingam, A. P., & Ramesh, B. 2018. “Antioxidant activity of cissus quadrangularis l. Stem in-vitro”. World Journal of Pharmaceutical Research, 7(11), 759-765. DOI: http://doi.org/10.20959/wjpr201811-12381.
MacDonald-Wicks, L. K., Wood, L. G. and Garg, M. L. 2006. “Methodology for the determination of biological antioxidant capacityin vitro: a review,” Journal of the Science of Food and Agriculture, vol. 86, no. 13, pp. 2046–2056. DOI: http://doi.org/10.1002/jsfa.2603
Jainu, M. and Devi, C.S.S. 2004. “Effect of Cissus quadrangularis on Gastric Mucosal Defensive Factors in Experimentally Induced Gastric Ulcer—A Comparative Study with Sucralfate”. Journal of Medicinal Food. Sep 372-376. DOI: http://doi.org/10.1089/jmf.2004.7.372.
Manoharan, S., Kolanjiappan, K., Suresh, K., and Panjamurthy, K. 2005. “Lipid peroxidation & antioxidants status in patients with oral squamous cell carcinoma,” Indian Journal of Medical Research, vol. 122, no. 6, pp. 529–534,. Google scholar
Mathew, B. B., Jatawa, S. K., & Tiwari, A. 2012. “Phytochemical analysis of Citrus limonum pulp and peel”. Int J Pharm Pharm Sci, 4(2), 369-71.Google scholar.
Moriasi, G. A, Ireri, A. M., and Ngugi, M. P., 2020. “In vivo cognitive- enhancing, ex vivo malondialdehyde-lowering activities and phytochemical profiles of aqueous and methanolic stem bark extracts of Piliostigma thonningii (schum.),” International Journal of Alzheimer’s Disease, vol. Article ID 1367075, 15 pages. DOI: http://doi.org/10.1155/2020/1367075.
Murthy, K.N.C, Vanitha, A, Swamy, M.M, Ravishanka,r G.A., 2003. “Antioxidant and anti- microbial activity of Cissus quadrangularis L”. Journal of Medicinal Food: 6(2):99- 105. DOI: http://doi.org/10.1089/109662003322233495.
Ndhlala, A., Moyo, M., and Van Staden, J., 2010. “Natural antioxi- dants: fascinating or mythical biomolecules?” Molecules, vol. 15, no. 10, pp. 6905–6930. DOI: http://doi.org/10.3390/molecules15106905.
Nwozo, O.S, Effiong, E.M, Aja, P.M, Awuchi, C.G. 2023. “Antioxidant, phytochemical and therapeutic properties of medicinal plants: A review”. Int J Food Prop.; 26(1):359-88. DOI: https:// doi.org/10.1080/10942912.2022.2157425
Oyaizu, M., 1986. “Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine,”The Japanese Journal of Nutrition and Dietetics, vol. 44, no. 6, pp. 307–315. DOI: http://doi.org/10.5264/eiyogakuzashi.44.307.
Panche, A. N., Diwan, A. D., and Chandra, S. R., 2016. “Flavonoids: an overview,” Jour- nal of Nutritional Science, vol. 5. DOI: http://doi.org/10.1017/jns.2016.41.
Rekha, G.V.S. and Devika, P.T. 2019. “Antioxidant activity and GC-MS analysis of ethanol extract of creeper stem of Cissus quadrangularis”. Journal of Pharmacog- nocy and Phytochemistry, 8(4): 760-765. Google scholar.
Ganapaty, S., Chandrashekhar, V. M., Chitme, H. R. and Lakashmi Narsu, M. 2007. “Free radical scavenging activity of gossypin and nevadensin: an in-vitro evaluation,” Indian Journal of Pharmacology, vol. 39, no. 6, pp. 281–283. DOI: http://doi.org/10.4103/0253-7613.39147.
Ruskin, R.S., Priya Kumari, V.M., Gopukumar, S.T. and Praseetha, P.K., 2014. ”Evaluation of phytochemical, antibacterial and anti-cancerous activity of Cissus quadrangularis from South Western Ghats regions of India”. Int. J. Pharm. Sci. Rev. Res, 28(1), pp.12-15.Google scholar.
Sundaran, J, Begum, R, Vasanthi, M, Kamalapathy, M, Bupesh, G, Sahoo, U. 2020. “A short review on pharmacological activity of Cissus quadrangularis”. Bioinformation. Aug 31;16(8):579-585. DOI: https://doi.org/10.6026/97320630016579.
Tiwari, I.2001. “Imbalance in antioxidant defense and human diseases: multiple approach of natural antioxidants therapy”. Curr. Sci. 81 1179–1187. http://www.jstor.org/stable/24106434.
留言 (0)