Oral lichen planus (OLP) is a common, chronic inflammatory dermatologic condition that often involves the skin, oral mucosa and/or genital mucosa. It is considered to be a dysregulated T-cell-mediated disorder that occurs in response to exogenous triggers.1 Worldwide, the prevalence of OLP is estimated to be 0.22% to 5%, and the incidence is up to 2.2%.2 Of those who develop OLP, a subset of patients (0–37%, mean 4.59%) may develop oral squamous cell carcinoma.3-11 However, there is still controversy regarding the rate of malignant transformation of OLP and the need for and frequency of monitoring. This highlights the need for better understanding of the factors related to the development of this condition and its malignant transformation.8,12-14
The etiology of OLP is poorly understood. It is thought that genetic factors, environmental triggers and the immune system play an important role.15-18 Several factors are considered to be associated with the development of OLP including psychological stress, drug intake and anxiety, as well as comorbidities such as hepatitis C (HCV) infection, hypertension, diabetes and thyroid disease.15,19,20 Geographic differences exist in the prevalence, presentation and risk factors associated with development of OLP.4,15 Currently, there is a lack of evidence on the risk factors that may be associated with the development of OLP in Canada. The main objective of this is study was to characterize such factors and the malignant transformation of OLP in 2 Canadian provinces.
Materials and MethodsStudy Design and Study Sample
To answer the clinical question, we conducted a retrospective, observational study. We reviewed the dental charts of patients who presented to oral medicine and oral pathology at the Montreal General Hospital (MGH) in Montréal, Quebec, and to a private oral and maxillofacial pathology clinic, the Atlantic Oral Surgery & Dental Implant Centre in St. John’s, Newfoundland. All adult patients (≥18 years of age) who presented to the MGH clinic or the Atlantic Oral Surgery & Dental Implant Centre between 1 Sept. 2016 and 31 Jan. 2020 for an oral pathology appointment were screened for eligibility.
The patients were seen by a specialist in oral medicine and oral pathology for diagnosis and management. The visit was documented in their dental charts, and patients with a clinical diagnosis of OLP were included in the study. OLP diagnosis was based on the American Academy of Oral and Maxillofacial Pathology position paper.2 Lesions were considered to be OLP if the presentation was symmetric, with well-defined white reticular lines or striae on an erythematous base with any 1 of the recognized patterns (reticular, atrophic, erosive, papular, plaque or bullous).2 Two oral pathology specialists were involved in the diagnosis of patients. When a diagnosis was difficult to ascertain clinically at the initial visit, a biopsy was performed and histology conducted to confirm the diagnosis. Patients were excluded if they did not have a diagnosis of OLP, if a drug- or dental-restoration related lichenoid reaction diagnosis was made and/or they had unilateral lesions.
The study protocol was reviewed and approved by the McGill University Health Centre Research Ethics Board and the Newfoundland and Labrador Health Research Ethics Board. We followed the Helsinki Declaration guidelines in this investigation.
Study Variables
From patient charts, we recorded age, sex, medical history including comorbid conditions and previous surgeries, medications, allergies, tobacco and alcohol consumption, site of OLP lesion(s), disease duration, OLP-associated symptoms, treatments prescribed and investigations performed. Where follow up was available, the response to the treatment was recorded.
Data Analysis
Data were entered into an electronic data collection document and descriptive statistical data (mean, frequency, range) were computed, using Excel, v. 16.38 (Microsoft Corp., Redmond, Wash., USA), for each study variable when appropriate.
SubtResultsitleDemographic Characteristics
Among those who presented to the clinics in Montréal and St. John’s during the study period, 94 OLP patients were identified. Their mean age was 62.2 years (range 35–92 years); 73% of the patients with OLP were female.
Medical History
The most frequent comorbidities were hypertension (32%); thyroid disorders (28%), most frequently hypothyroidism; and diabetes (17%), with 6 patients with type II diabetes mellitus and 1 with a history of gestational diabetes. A significant proportion of patients (47%) noted that they had had a major surgical procedure, such as a mastectomy, hysterectomy, oophorectomy with or without salpingectomy, cholecystectomy, thyroidectomy, cardiac bypass surgery, hip and/or knee replacement or other types of musculoskeletal surgeries including knee replacement and back surgeries. Most of these surgeries were among women.
In the study group, 69 patients (73%) had had at least 1 surgery. In the general Canadian population, approximately, 1.8% of adults over the age of 18 years have had at least 1 surgery.21,22 Among the patients in this study who had a surgical procedure, the mean number of surgeries per person was 1.57. An additional 7% of patients self-reported that they had undergone a major stressful life event before development and diagnosis of OLP. Only 4 patients in our study reported a history of hepatitis, whether treated or active, and only 1 of these had HCV; 2 had autoimmune hepatitis and 1 had a history of hepatitis A.
The mean number of medications per patient was 3.62 (assessed in 93 of 94 patients as 1 patient’s file did not include specific medications). The most frequent types of medications were antihypertensives (32%), statins (30%), proton-pump inhibitors (29%), levothyroxine (22%) and antihyperglycemic agents (14%). The antihypertensive medications, taken by 32% of patients, consisted of angiotensin II receptor blockers (24%), beta-blockers (22%), calcium-channel blockers (22%), angiotensin-converting enzyme inhibitors (18%), diuretics (14%); 2% could not remember the specific medication names. Of the 14% of patients on antidiabetic medications, most were taking metformin (32%), dipeptidyl peptidase 4 inhibitors (27%), sulfonylurea (18%), sodium-glucose cotransporter-2 inhibitors (18%) and insulin (5%). Most patients (63%) indicated that they did not have a history of allergies to medication.
Social History
Smoking and alcohol use data were available for 64 patients. Approximately 25% of these patients (16) had a positive smoking history as current or previous smokers. Pack history was available for 10 of these 16 patients, and the mean was 14.6 pack years. Details on the relation between smoking history and OLP onset was not available. One patient reported marijuana use; however, the frequency of use was not specified.
Approximately 53% (34 of 64 patients) reported that they did not consume alcohol, while 27% (17) reported social alcohol use. For patients who reported regular alcohol consumption, 19% (12) indicated moderate alcohol use, defined as up to 1 drink/day for women or 2 drinks/day for men. Only 1 patient had a history of high alcohol use, defined as alcohol consumption greater than the threshold defining moderate use. Table 1 summarizes medical and social history.
Table 1: Demographic data for study population.**Note: mean age, years: 62.2 (range 35–92).
† Moderate alcohol use is defined as up to 1 drink/day for women and 2 drinks/day for men. High use is defined as >1 drink/day for women and >2 drinks/day for men.
OLP Lesions and Symptoms
A significant proportion of patients (78%) was affected by OLP at ≥2 sites, with gingivae and buccal mucosa being the most common sites (46% of patients each; Figure 1). The lesions were bilateral and symmetric in distribution. Other areas that were involved included the dorsum of the tongue (21%), lateral tongue (12%), ventral tongue (11%), lips (6%), hard palate (4%) and soft palate (1%). No differences in lesion location were based on sex or disease duration. A small proportion of patients also had skin (5%) and genital (4%) involvement. Approximately 6% of patients had erosive OLP, and most patients (94%) had lesions that were described as white reticular lines on an erythematous base.
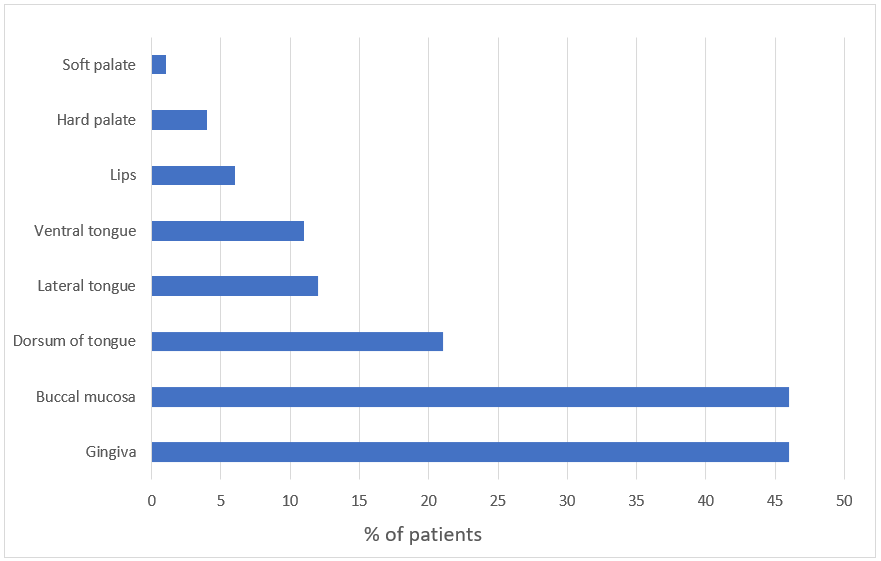
Figure 1: Site of oral lichen planus lesions in our study population.
Mean disease duration, as assessed in the 85 (of 94) patients for whom this was recorded in their dental chart, was 3.06 years at the time the patient presented for an oral pathology consultation. The other 9 patients had indicated that they had OLP for “many years.”
Of the patients, 73% presented with symptoms, such as sensitivity to hot and spicy foods, and an additional 7% indicated that they had sensitivity to toothpaste, particularly Sensodyne (GlaxoSmithKline Inc., Mississauga, Ont.), mouthwash or fluoride rinse. A fifth (20%) of patients indicated that they were asymptomatic and that their OLP lesions had been noticed by their dental professional during a routine check-up.
OLP Management
All symptomatic patients were treated with topical corticosteroids, and all patients, regardless of symptoms, were provided with information on optimal mouth care for OLP lesions. In addition, 1 patient (1%) was given a corticosteroid injection for severe presentation of erosive OLP at the initial appointment, and 10 patients (11%) had incisional punch biopsies taken of their lesions. These lesions were bilateral and symmetric, and solitary lesions were excluded to reduce the risk of inadvertent inclusion of lichenoid lesions. The patients who had incisional biopsies to rule out dysplasia tended to present with suspicious lesions and/or had a history of smoking and/or ethanol use and/or long-standing OLP. Finally, 4 patients (4%) had lesions that were excised at the initial presentation and sent for pathology. As these lesions were OLP, the entire lesion was not excised, only the suspicious erythematous portions that were isolated and <1 mm in size.
Follow Up
For 65 patients (69%), follow-up information was available over an average duration of 5.10 months. For 8 patients (9%) follow up was still pending at the time of the data analysis, and 2 patients (2%) refused follow up. The remaining 19 patients (20%) were asymptomatic, with non-suspicious lesions at the initial presentation, and short-term follow up was deemed not required.
For patients with follow-up data available, 68% (44 of 65) had no symptoms, were stable or experienced improvement in their symptoms after topical corticosteroid use. Only 5 (8%) still had active OLP requiring corticosteroid injection or systemic treatment. Finally, 2 patients (3%) had suspicious lesions requiring incisional biopsy.
Squamous Cell Carcinoma and Dysplasia
In our cohort of 94 OLP patients, 13 had either incisional or excisional biopsies taken during the course of their treatment. Of these, 3 resulted in a diagnosis of squamous cell carcinoma or dysplasia; 2 of these patients were female and 1 was male and their average age was 63.7 years. These 3 patients had a prior history of OLP with a mean disease duration of 4.06 years (range 2 months to 10 years). Two were previous smokers, although they had quit at the time of the biopsies. Two patients stated that they consumed alcohol socially, and the third reported zero alcohol consumption. The lesions were located on the lateral tongue in 2 patients and the third patient’s lesion was on the hard palate. One patient did not have any concomitant medications or comorbidities, and the other 2 had multiple medical conditions, including diabetes, hypertension and a history of myocardial infarction, and were taking antihypertensives, antihyperglycemics, a statin and aspirin to treat these conditions.
DiscussionIn this study, we aimed to describe the characteristics of patients with OLP in 2 provinces, as there is a lack of Canadian-specific data. Most of the OLP patients in our study were female (73%), which is consistent with the proportion observed in other geographic areas.15,23 In our cohort, the mean age was 62 years, representing a slightly older patient population than previously described, which typically ranged from 30 to 60 years.23,24 Race and ethnicity were not documented in the charts.
In terms of comorbid medical conditions, most patients in our cohort had hypertension, thyroid disease, specifically hypothyroidism, and diabetes. This is consistent with what has been observed in other populations.15,19,25 Some antihypertensive medications, such as methyldopa; beta-blockers, such as propranolol; amlodipine; and antihyperglycemics, such as metformin and sulfonylureas, have been associated with lichenoid drug reactions.26,27 Given that such reactions may be difficult to differentiate clinically and histologically from OLP, they may have been inadvertently included in our study, although every attempt was made to identify them. Because of the retrospective nature of our study, a temporal relationship is also difficult to establish. However, an association for patients with a history of HCV and OLP, as seen in some Japanese and Italian studies,28,29 was not observed in our Canadian cohort. This may be a result of the treatment options for HCV that have been adopted in the last 5–10 years, thus reducing the number of active HCV infections in our cohort. Another possible explanation is an epidemiological difference between HCV and OLP in the Canadian population, as only 4 patients in our study reported a history of hepatitis whether treated or active, and only 1 of these was HCV.
A significant proportion of patients (47%) noted that they had had a major surgical procedure, such as a mastectomy, hysterectomy, oophorectomy with or without salpingectomy, cholecystectomy, thyroidectomy, cardiac bypass surgery, hip and/or knee replacement and other types of musculoskeletal surgeries. A possible explanation is that medical conditions requiring these surgeries may be correlated with the characteristics associated with development of OLP, such as female sex and older age. Among the patients who had had a surgical procedure, the mean number of surgeries per person was 1.57. However, a temporal association between the surgeries and the onset of OLP is difficult to establish in this retrospective study and may be an area of future investigation.
An additional 7% of patients noted that they had undergone a major stressful life event before their development of OLP. Stress may be a triggering factor for OLP flares.15,30 In fact, in one study, 77% of patients reported that emotional stress was a trigger for OLP.30 Furthermore, a recent systematic review found evidence that psychological disorders, specifically stress, anxiety and depression, were associated with the development of OLP.31
It would be of interest to investigate whether the physical, mental and emotional stress of serious medical conditions and their associated major surgical procedures have an impact on the development of OLP in susceptible patients. Because of the retrospective nature of this study, it was not feasible to evaluate whether surgical procedures and their associated stress also preceded the development of OLP. Also, although OLP patients were typically asked about their surgical history and stressful life events at the initial visit in both study sites, this information was not always documented in the charts, making it difficult to ascertain whether such lack of information indicated a negative response from the patient or a failure of the clinician to record it. Overall, the charts of about 65% of patients from the St. John’s study site and 54% of the MGH patients documented a response to the question relating to stressful life events.
In terms of the association of previous or current smoking history with OLP, 75% of the patients (48 of 64 patients) indicated that they did not currently or previously smoke. This result was limited by the fact that 30 patients did not have smoking data available. To account for this, as a sensitivity analysis, we assumed that the patients with data missing were either all non-smokers or all smokers. Thus, the range of patients in our cohort with a current or previous smoking history would be 17% (16 smokers out of 94 patients) to 49% (46 smokers out of 94 patients). Therefore, as a conservative measurement, we can assume that at least 51% of our patients with OLP are non-smokers, and this value can go up to a maximum of 83% of the study population. Based on these calculations, it appears that most of the patients who developed OLP are non-smokers. Another study conducted in patients in Israel reported similar results, suggesting that OLP development was independent of smoking history.32
In contrast, there may be a correlation with malignant transformation of OLP lesions in smokers, as 2 of 3 of our patients who had positive biopsies for squamous cell carcinoma and/or dysplasia had previous smoking history. Of interest, the 2 patients had both quit smoking several years before their development of the dysplasia and/or carcinoma. However, these numbers are too small to conclude that smoking history is associated with malignant transformation, and additional studies are required to evaluate the role of smoking in such transformation in Canadian patients.
The duration of disease in patients who had malignant transformation ranged from 2 months to 10 years from the time of the diagnosis of OLP to the time of positive biopsy results, suggesting that malignant transformation can occur rapidly. This could be because many patients are asymptomatic; so, the lesions may be present for some time before they are diagnosed by a health care professional. At this time, in the Canadian setting, it may be prudent to evaluate all patients, smokers and non-smokers alike, for the development of OLP and to screen patients, including non-smokers, current smokers and those who have previously quit smoking, for the risk of malignant transformation of OLP regardless of disease duration.
Alcohol consumption did not appear to be correlated with OLP in our study as most patients (80%) indicated no or infrequent alcohol consumption. However, these findings are limited by the fact that the social history in the dental charts was collected from patient self-reports and, thus, may be prone to an under-reporting bias. Other studies conducted in the elderly and/or in women have noted that some patients may under-report their drinking habits to be perceived as socially acceptable.33,34 Thus, additional studies are required to investigate the association of alcohol and OLP in Canada.
In our study, 3 patients (3.2%) developed malignant transformation of OLP lesions. This rate is consistent with the range of values quoted in other studies.8 In contrast, in a recent publication,9 the rate of malignant transformation of OLP was reduced from 1.2% to 0.44% when stricter criteria were applied to the definition of lesion that could lead to malignant transformation. This could also be the case in our study and, thus, a limitation: if stricter criteria were applied, the rate of malignant transformation observed may be reduced.
On follow up, most patients in our study demonstrated significant improvement in their symptoms after use of topical corticosteroids. A small percentage of patients required corticosteroid injections. Follow up was also important to assess suspicious lesions and conduct incisional biopsies to assess malignant transformation of these lesions in some patients.
ConclusionsOur study is 1 of the first assessments of the characteristics of patients with OLP in Canada. It suggests that Canadian patients with OLP tend to be older females, and hypertension, thyroid disorders and diabetes were common in these patients. Malignant transformation was observed in 3% of patients and, thus, these lesions should be followed for changes. This study serves as an important preliminary analysis and suggests areas for future investigation.
THE AUTHORS

Dr. Iskander is a DMD graduate, faculty of dentistry, McGill University, Montreal, Quebec.

Dr. Samim is a certified specialist in oral medicine/oral and maxillofacial pathologist, clinical assistant professor, faculty of dentistry, McGill University, Montreal, Quebec.
Corresponding author: Dr. Firoozeh Samim, Faculty of Dentistry, McGill University, 2001 McGill College, Room 502, Montreal QC H3A 1G1. Email: firoozeh.samim@mcgill.ca
Funding for this project was provided by McGill University, Faculty of Dentistry.
This article has been peer reviewed.
References Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(1):72-80. Cheng YSL, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(3):332-54. Aghbari SMH, Abushouk AI, Attia A, Elmaraezy A, Menshawy A, Ahmed MS, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19(5):1161-7. Olson MA, Rogers 3rd RS, Bruce AJ. Oral lichen planus. Clin Dermatol. 2016;34(4):495-504. Bermejo-Fenoll A, Sanchez-Siles M, López-Jornet P, Camacho-Alonso F, Salazar-Sanchez N. Premalignant nature of oral lichen planus. A retrospective study of 550 oral lichen planus patients from south-eastern Spain. Oral Oncol. 2009;45(8):e54-6. Agha-Hosseini F, Sheykhbahaei N, SadrZadeh-Afshar MS. Evaluation of potential risk factors that contribute to malignant transformation of oral lichen planus: a literature review. J Contemp Dent Pract. 2016;17(8):692-701. Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K. Current controversies in oral lichen planus: report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(2):164-78. Idrees M, Kujan O, Shearston K, Farah CS. Oral lichen planus has a very low malignant transformation rate: a systematic review and meta-analysis using strict diagnostic and inclusion criteria. J Oral Pathol Med. 2021;50(3):287-98. Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145(1):45-56. van der Meij EH, Schepman KP, Smeele LE, van der Wal JE, Bezemer PD, van der Waal I. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(3):307-10. Mattsson U, Jontell M, Holmstrup P. Oral lichen planus and malignant transformation: is a recall of patients justified? Crit Rev Oral Biol Med. 2002;13(5):390-6. Larsson Å, Warfvinge G. Malignant transformation of oral lichen planus. Oral Oncol. 2003;39(6):630-1. Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis. 2008;14(3):229-43. Li C, Tang X, Zheng X, Ge S, Wen H, Lin X, et al. Global prevalence and incidence estimates of oral lichen planus: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(2):172-81. Al-Mohaya MA, Al-Harthi F, Arfin M, Al-Asmari A. TNF-α, TNF-β and IL-10 gene polymorphism and association with oral lichen planus risk in Saudi patients. J Appl Oral Sci. 2015;23(3):295-301. Carrozzo M, Uboldi de Capei M, Dametto E, Fasano ME, Arduino P, Broccoletti R, et al. Tumor necrosis factor-alpha and interferon-gamma polymorphisms contribute to susceptibility to oral lichen planus. J Invest Dermatol. 2004;122(1):87-94. Fujita H, Kobayashi T, Tai H, Nagata M, Hoshina H, Nishizawa R, et al. Assessment of 14 functional gene polymorphisms in Japanese patients with oral lichen planus: a pilot case–control study. Int J Oral Maxillofac Surg. 2009;38(9):978-83. Alqahtani M, Woods TR, Smith MH, Bhattacharyya I, Cohen DM, Islam MN, et al. Medication use and medical history of 155 patients with oral lichenoid lesions: a retrospective study. Gen Dent. 2018;66(2):40-5. De Carli JP, Linden MSS, da Silva SO, Trentin MS, Matos F de S, Paranhos LR. Hepatitis C and oral lichen planus: evaluation of their correlation and risk factors in a longitudinal clinical study. J Contemp Dent Pract. 2016;17(1):27-31. Inpatient hospitalizations, surgeries and newborn indicators, 2016-2017. Ottawa: Canadian Institute for Health Information; 2017. Population estimates on July 1st, by age and sex: Table 17-10-00501. Ottawa: Statistics Canada; 2021. Available: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501 Lozada-Nur F, Miranda C. Oral lichen planus: epidemiology, clinical characteristics, and associated diseases. Semin Cutan Med Surg. 1997;16(4):273-7. Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60(3):222-9. Garcia-Pola MJ, Llorente-Pendás S, Seoane-Romero JM, Berasaluce MJ, García-Martín JM. Thyroid disease and oral lichen planus as comorbidity: a prospective case–control study. Dermatology. 2016;232(2):214-9. Do Prado RF, Marocchio LS, Felipini RC. Oral lichen planus versus oral lichenoid reaction: difficulties in the diagnosis. Indian J Dent Res. 2009;20(3):361-4. Fortuna G, Aria M, Schiavo JH. Drug-induced oral lichenoid reactions: a real clinical entity? A systematic review. Eur J Clin Pharmacol. 2017;73(12):1523-37. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46(2):207-14. Nagao Y, Sata M. A retrospective case–control study of hepatitis C virus infection and oral lichen planus in Japan: association study with mutations in the core and NS5A region of hepatitis C virus. BMC Gastroenterol. 2012;12:31. Chen HX, Blasiak R, Kim E, Padilla R, Culton DA. Triggers of oral lichen planus flares and the potential role of trigger avoidance in disease management. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(3):248-52. Cerqueira JDM, Moura JR, Arsati F, Lima-Arsati YBO, Bittencourt RA, Freitas VS. Psychological disorders and oral lichen planus: a systematic review. J Investig Clin Dent. 2018;9(4):e12363. Gorsky M, Epstein JB, Hasson-Kanfi H, Kaufman E. Smoking habits among patients diagnosed with oral lichen planus. Tob Induc Dis. 2004;2(2):103-8. Hunsberger M, Mehlig K, Björkelund C, Lissner L. Regular versus episodic drinking in Swedish women: reporting of regular drinking may be less biased by social desirability. Alcohol. 2020;86:57-63. Rigler SK. Alcoholism in the elderly. Am Fam Physician. 2000;61(6):1710-6, 883-4, 887-8 passim.
留言 (0)