The efficacy of immunotherapies that use antibodies to block programmed cell death 1 (PD-1) has been extensively investigated for advanced/metastatic non-small cell lung cancer (NSCLC). Monoclonal antibodies that block PD-1 provide substantial benefit, prolonging both progression-free and overall survival. However, along with reactivation of the patient’s immune response to tumour cells, immune-related adverse effects (iRAEs) with anti-PD1 therapy have been reported.1 Haematological iRAEs have been described occasionally. These include immune thrombocytopenia, autoimmune haemolytic anaemia, agranulocytosis or pure red-cell aplasia.2,3 Severe forms of autoimmune-mediated thrombocytopenia have been reported rarely.
THE CASEAn 80-year-old woman who was diagnosed with primary lung adenocarcinoma (pT1bN2M1a, stage IVA) under treatment with pembrolizumab (Keytruda, on day 11 after initiating pembrolizumab infusion) was admitted to our hospital with severe thrombocytopenia. She had been previously diagnosed with old myocardial infarction and hypertension, without a history of autoimmune or coagulation disorders. Prior to treatment with pembrolizumab, the platelet count was 203×109/L. On admission, the bone marrow aspirate done in another hospital revealed normal plasticity with no obvious morphological abnormalities, phagocytosis or malignant invasion. The number of megakaryocytes was 68 cells/ml. Although megakaryocyte numbers were maintained, the cells were relatively small and immature, and platelets infrequently adhered to them. Laboratory testing showed a platelet count of 13×109/L, white blood cell count of 6.09×109/L, haemoglobin 9.8 g/dl, prothrombin time 14.6 seconds and international normalized ratio of 1.14. On examination, the patient had purpuric rash on the trunk and both upper limbs. She received intravenous immunoglobulin 10 g/day for 3 days, methylprednisolone 80 mg/day for 20 days, thrombopoietin-receptor agonist 15 000 µ/day, for 20 days and 10 units of platelets (Fig. 1). The patient had no improvement in the platelet count. Three days after administration of the immunoglobulin, the patient developed acute left heart failure and progressive decrease of blood pressure and was unable to tolerate rituximab treatment.
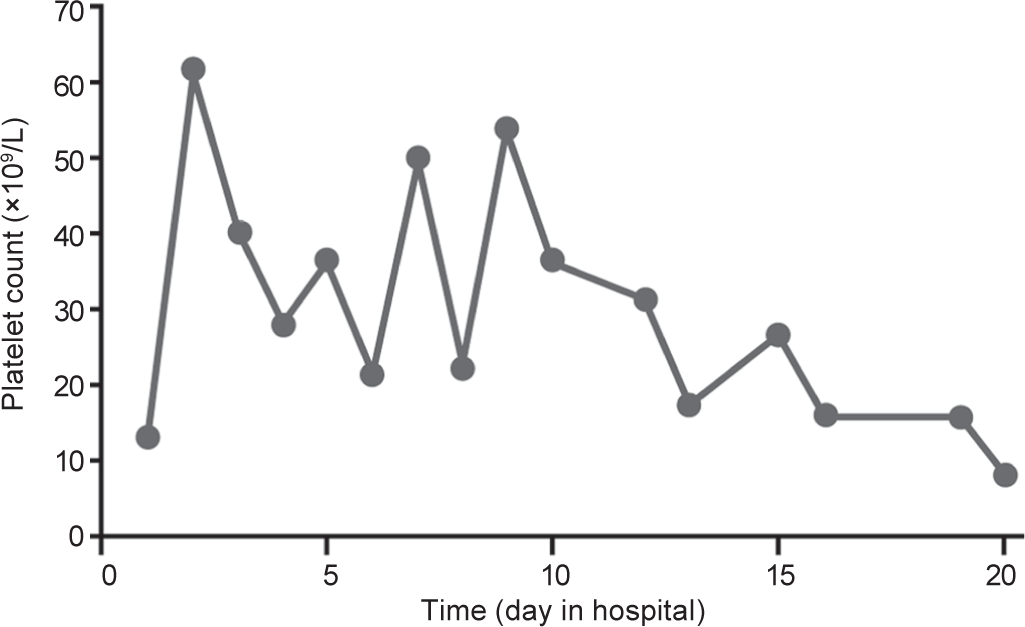
FIG 1.: Changes in platelet count during hospitalization.
Export to PPT
DISCUSSIONProgrammed cell death 1/PD-L1 inhibitors have revolutionized the treatment of malignancies. However, they can trigger various iRAEs, such as interstitial pneumonitis, colitis with gastrointestinal perforation, type 1 diabetes, severe skin reactions, immune thrombocytopenia (ITP), neutropenia and sepsis after corticosteroid therapy, encephalopathy and neurological sequelae, Guillain–Barré syndrome, myelitis, myasthenia gravis, myocarditis and cardiac insufficiency, acute adrenal insufficiency and nephritis.4
Immune thrombocytopenia is acquired thrombocytopenia caused by autoantibody and T cell-mediated platelet destruction and impairment of thrombopoiesis. For patients with NSCLC, PD-1/PD-L1 inhibitors are generally safer and better tolerated than cytotoxic chemotherapy, though a 0.7% incidence of thrombocytopenia has been reported.5 In a retrospective chart review of 2360 patients with melanoma treated with immune checkpoint inhibitors, <1% experienced thrombocytopenia, and of these, most showed spontaneous resolution and did not require treatment.6
In our patient, thrombocytopenia developed shortly after initiating systemic therapy with pembrolizumab, and infusion of platelets, glucocorticoids, gamma globulin and recombinant human thrombopoietin was not effective. In some instances, therapy with pembrolizumab aggravated thrombocytopenia and induced or increased the production of platelet-specific IgG autoantibodies. Due to the clinical course and the laboratory results, it is likely that thrombocytopenia was caused by PD-1 inhibitor-induced platelet autoantibodies through autoimmune activation.
留言 (0)