Acute myocardial infarction (AMI) is a prevalent acute and severe cardiovascular diseases, characterized by its sudden onset, high mortality rate, and unfavorable prognosis (1–3). With the rapid advancement of medical technology, the level of early diagnosis and effective treatment of AMI has been improved (4, 5). However, Xining is situated in a region of middle to high altitude and due to its unique geographical environment, coupled with the population's limited health awareness, unequal medical standards and other factors, there remain some AMI patients who are difficult to diagnose early on. This results in delayed or suboptimal treatment as well as inadequate postoperative patient management which ultimately leads to heart failure, sudden cardiac death or other serious complications. The presence of type 2 diabetes not only signifies a chronic metabolic disorder, but also serves as a catalyst for various cardiovascular and cerebrovascular ailments such as coronary heart disease and stroke (6, 7).
The literature (8–10) has consistently demonstrated that diabetes is an independent risk factor for poor prognosis in cardiovascular disease. Moreover, a significant majority of acute myocardial infarction (AMI) patients often present with comorbid type 2 diabetes, which not only poses challenges in clinical management but also significantly escalates cardiovascular mortality among such individuals. Then, the efficacy in the prevention and treatment of acute myocardial infarction (AMI) combined with type 2 diabetes is suboptimal, failing to effectively reduce the incidence of cardiovascular events and improve patient prognosis (11). However, the introduction of a novel sodium-glucose co-transporter 2 (SGLT-2) inhibitor called dapagliflozin has sparked high expectations among clinicians regarding its potential for treating patients with type 2 diabetes who have experienced acute myocardial infarction (AMI) (12).
Currently, there is a scarcity of domestic research on the significant enhancement and improvement in quality of life as well as long-term prognosis for AMI patients with type 2 diabetes mellitus through dapagliflozin treatment, and there exists an insufficiency of evidence-based medical foundation (13). The objective of this study is to investigate the impact of dapagliflozin on the prognosis of patients with acute myocardial infarction (AMI) complicated by type 2 diabetes, aiming to provide robust evidence supporting dapagliflozin as the preferred choice for clinical management in such patients.
Methods Research objectA total of 415 patients with AMI combined with type 2 diabetes who were hospitalized in the Department of Cardiovascular Disease of Qinghai Provincial People's Hospital from January 2018 to January 2020 were analyzed, and 245 patients qualified for the study were incorporated into the group after screening by inclusion and exclusion criteria. Inclusion criteria: (1) Patients who fulfill the diagnostic criteria for acute myocardial infarction (AMI) as outlined in the fourth edition of the Global Definition of Myocardial Infarction (14), including typical symptoms and signs of myocardial ischemia, dynamic changes in electrocardiogram, elevated markers of myocardial injury, etc.; (2) Patients diagnosed with type 2 diabetes according to the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2020 Edition) (15) and currently receiving hypoglycemic medication for glycemic control. Exclusion criteria: (1) Patients with lost follow-up and poor compliance will be excluded from the study. (2) Patients with severe liver and renal insufficiency will not be included in the study population. (3) Patients who exhibit intolerance to the drugs used in this research will be excluded. (4) Patients presenting severe respiratory tract infection, urinary tract infection, fever, or other systemic infections combined will not be considered for participation. (5) Individuals diagnosed with other types of diabetes will be excluded from the study group. (6) Tumor patients are not eligible for inclusion in this research project. (7) Patients lacking complete clinical data will also be excluded. The present study adhered to the principles of medical ethics, and the acquisition of medical records was authorized by our hospital's Ethics Committee, thereby waiving the need for informed consent.
The index of observation and its categorizationThe observation measures included patients’ demographic information, admission blood pressure, comorbidities (such as hypertension, atrial fibrillation, stroke), blood biochemical markers (fasting blood glucose, glycated hemoglobin, liver and kidney function indicators), echocardiographic parameters (left ventricular ejection fraction, left ventricular end-diastolic volume), cardiac functional grade (Killip grade), treatment plan, and medication regimen. Patients were categorized into two groups: the dapagliflozin group (patients who had taken dapagliflozin either in the past or during hospitalization) and the control group (patients taking other hypoglycemic agents excluding dapagliflozin).
Follow-up and study endpointThe enrolled patients were monitored through telephone follow-up, outpatient visits or inpatient records. The primary endpoint was defined as the composite outcome of cardiac mortality, heart failure events, and cerebrovascular insult stroke. The secondary endpoint was defined as death resulting from cardiac and cerebrovascular causes, excluding other factors. Adverse events associated with daglipzin included hypoglycemia, hypovolemia, diabetic ketoacidosis, acute kidney injury, and genitourinary infection. The follow-up period concluded on December 31, 2023, with a median duration of 42 months.
Statistical approachThe statistical software SPSS 25.0 was utilized for conducting data analysis. T-test was employed to compare groups when dealing with measurement data following a normal distribution. For measurement data that did not follow a normal distribution, they were represented as M(Q25,Q75), and the Mann-Whitney test was used for group comparisons. Count data were expressed as rates or component ratios, and group comparisons were conducted using either χ2 test or Fisher exact probability method. Survival analysis was performed using the Kaplan-Meier method, and the Log-rank test was employed to compare survival rates between groups. A significance level of P < 0.05 indicated statistical difference.
Results The clinical data of dapagliflozin group and control group were comparedA total of 245 patients, ranging in age from 34 to 94 years with an average age of (61 ± 11) years, were enrolled in this study. Among them, there were 200 males (81.63%) and 45 females (18.37%). In the daglipzin group, there were 92 males (77.97%), while the control group consisted of 102 males (85.04%). No statistically significant differences were observed between the two groups in terms of clinical data including age, gender, diabetes duration, comorbidities, blood biochemical parameters and echocardiographic indices (P > 0.05), as presented in Table 1.
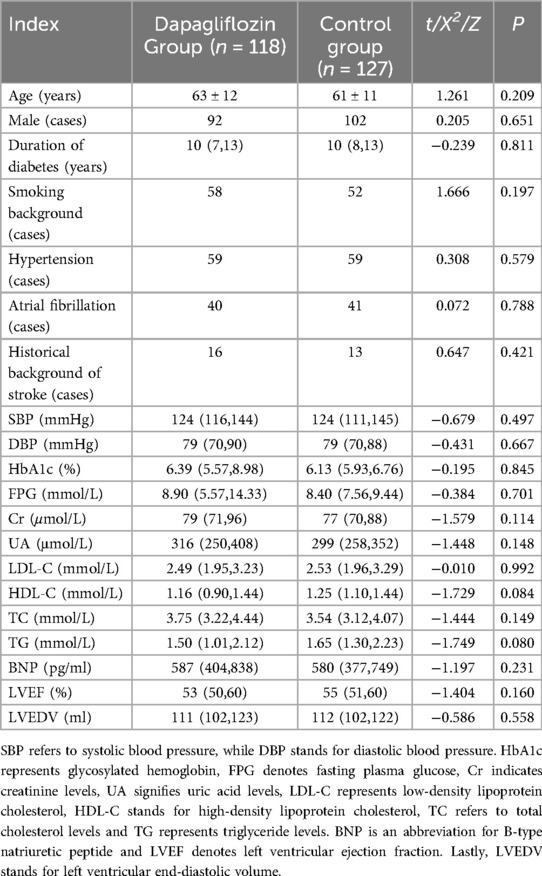
Table 1. Comparison of clinical data between dapagliflozin group and control group.
The cardiac function classification, treatment plan and incidence of endpoint events were compared between dapagliflozin group and control groupThe number of coronary artery lesions, treatment regimen, cardiovascular and other hypoglycemic drugs did not differ significantly between the dapagliflozin group and the control group (P > 0.05). However, the incidence rates for both primary endpoint and secondary endpoint were lower in the dapagliflozin group compared to the control group with statistically significant differences (P < 0.05). Please refer to Table 2.
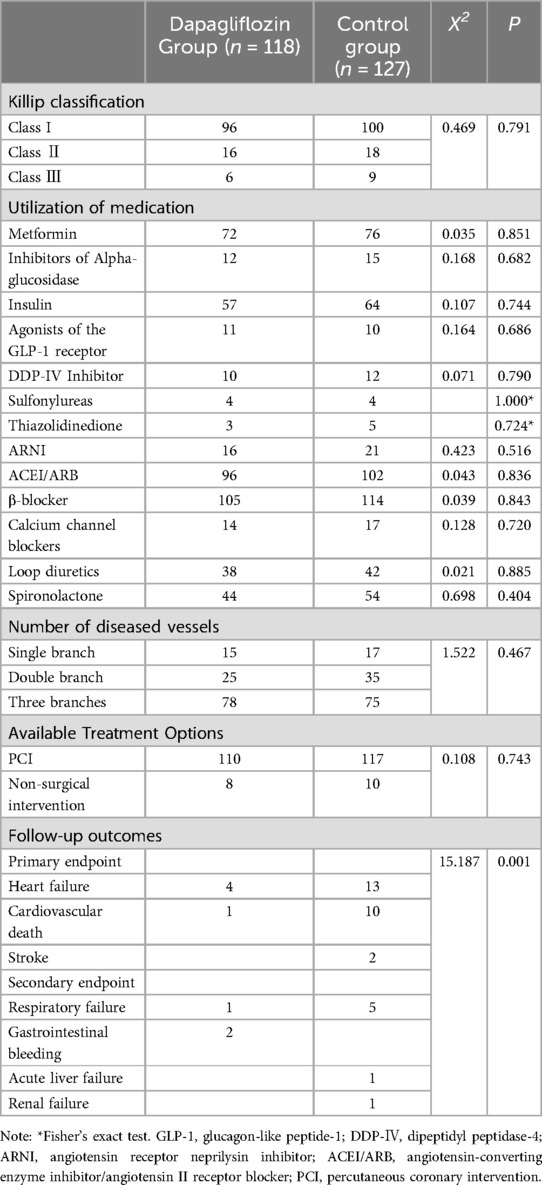
Table 2. Comparison of cardiac function, treatment plan and drug use between dapagliflozin group and control group.
Comparison of follow-up status and overall survival analysis between dagliferen group and control group of patientsAll enrolled patients completed follow-up, ranging from 12 to 71 months, with a mean of 42 ± 11 months. The Kaplan-Meier survival analysis showed no statistically significant difference in the incidence rates of secondary endpoints between the Dapagliflozin group and the control group (P > 0.05). However, the incidence rate of the primary endpoint was significantly lower in the Dapagliflozin group than in the control group, with a statistically significant difference (P < 0.05). Furthermore, the overall survival rate was significantly higher in the Dapagliflozin group than in the control group, with a statistically significant difference (P < 0.05), as shown in Figures 1–3.
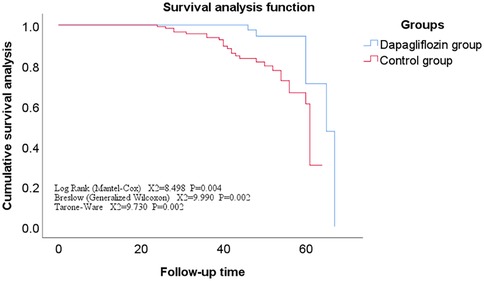
Figure 1. Survival curve of the main endpoints events in the dapagliflozin group versus the control group.
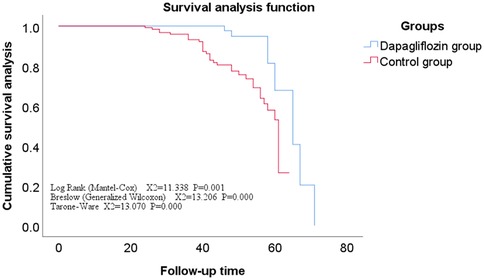
Figure 2. Survival curves for secondary endpoints in the dapagliflozin group versus the control group.
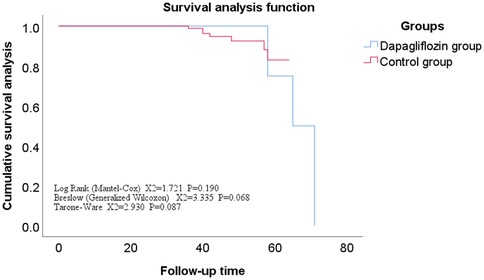
Figure 3. Overall survival curve for dapagliflozin group vs. control group.
Comparison of adverse events between dagliferen group and control group patientsDuring the hospitalization period, a total of 5 drug-related adverse reactions occurred in both groups. Among them, 1 case of hypoglycemia and 1 case of urinary tract infection occurred in the group treated with dapagliflozin; 2 cases of hypoglycemia and 1 case of urinary tract infection occurred in the control group. No other serious drug-related adverse reactions occurred in either group. There was no statistically significant difference in the incidence of drug-related adverse reactions between the two groups (P > 0.05).
DiscussionXining, located at an average altitude of 2,300 meters, is known as the eastern gateway to the Tibetan Plateau and is one of the high-altitude cities in the world. Xining has a continental plateau semi-arid climate or a high-altitude cold and temperate climate, characterized by low pressure, low oxygen, low temperature, and low humidity. Several studies have shown (16, 17), due to its unique climate environment, the incidence and mortality rate of cardiovascular diseases in Xining are lower than those in the plains, especially the incidence and mortality rate of AMI are lower than the national level. However, some studies have shown (18), the high-altitude environment can produce transient or long-term effects on blood pressure, heart rate, cardiac structure and function, and the long-term residence altitude is positively correlated with blood pressure level. However, some scholars conducted a population-based epidemiological investigation on the incidence of AMI in Xining area over the past 10 years and pointed out that due to the gradual improvement of people's living standards in the area, the incidence of AMI and diabetes has shown an upward trend (19–21). Type 2 diabetes is a metabolic disease characterized by chronic hyperglycemia, and AMI is a common complication of type 2 diabetes. Moreover, when type 2 diabetes patients suffer from AMI, they usually have multiple vessel lesions, which makes them more prone to heart failure, ultimately leading to poor treatment outcomes, high mortality rate, and poor prognosis. Studies at home and abroad have shown that (22–25) dapagliflozin, a novel anti-diabetic drug, not only has the function of lowering blood sugar but also improves left ventricular remodeling. Its safety and reliability for AMI patients with type 2 diabetes are better than those of other similar anti-diabetic drugs, and it can effectively improve and enhance the cardiac function and quality of life of these patients.
The results of this study indicate that men account for a larger proportion of patients with AMI and type 2 diabetes, and that high-risk factors such as smoking, hypertension, and atrial fibrillation account for about 50% of the total. Secondly, the study included patients with Killip functional classification of II or III, which accounted for about 18.6%–21.3% of the total. These patients had varying degrees of congestive heart failure during their hospital stay. However, after being treated with dapagliflozin and other cardiovascular drugs, AMI patients with type 2 diabetes showed a significant improvement in their heart failure symptoms compared to those treated with other therapies. This is consistent with the findings of many domestic scholars, who have found that dapagliflozin can reduce the volume of interstitial fluid without affecting blood volume, thereby significantly reducing the degree of pulmonary edema in patients (26). Moreover, the findings of this study indicate that instances of heart failure occurred in both the Dapagliflozin group and the control group following discharge. Nevertheless, the incidence rates of heart failure, cardiovascular mortality, and other related cardiovascular events were significantly lower in the Dapagliflozin cohort compared to the control group. This is corroborated by international research (9, 27), which demonstrates that Dapagliflozin can substantially diminish the risk of cardiovascular death or hospitalization due to heart failure in patients with type 2 diabetes by 17%. Furthermore, among patients with acute myocardial infarction (AMI), Dapagliflozin has been shown to reduce the likelihood of major adverse cardiovascular events by approximately 16%, thereby markedly enhancing cardiovascular outcomes for individuals with type 2 diabetes and AMI. There is also evidence from several domestic studies (28) that dapagliflozin has similar findings in Chinese patients with AMI and type 2 diabetes as in foreign studies. Therefore, early use of dapagliflozin can not only better control blood sugar and blood pressure in patients with AMI and type 2 diabetes, but also improve their cardiac function, reverse left ventricular remodeling, and reduce the incidence of cardiovascular adverse events (29, 30). Additionally, other studies have shown (31) that regardless of whether they have type 2 diabetes, dapagliflozin used in combination with conventional anti-heart failure drugs can significantly improve the cardiac function of AMI patients, reduce the incidence of cardiovascular adverse events, and improve the long-term prognosis of patients. At the same time, the results of this study show that there were a total of 5 drug-related adverse reactions in the dapagliflozin group and the control group during hospitalization, but there was no significant difference in the incidence of adverse reactions between the two groups, and no other serious drug-related adverse reactions occurred.The findings of numerous domestic and international studies (32–34) have also corroborated the aforementioned theory derived from this study, namely that dapagliflozin exhibits adverse reactions similar to other hypoglycemic drugs, including urinary tract infection, hypoglycemia, ketoacidosis, and hypotension. However, no study has demonstrated a significantly higher incidence of adverse reactions with dapagliflozin compared to other antidiabetic medications. Therefore, in terms of drug safety, dapagliflozin is deemed as safe and reliable as other hypoglycemic drugs (35). Nevertheless, in order to mitigate the occurrence of adverse reactions associated with dapagliflozin usage, the dosage of dapagliflozin should be strictly controlled according to the specific conditions of the patients, and patients are advised to increase their water intake, maintain perineal hygiene practices diligently and engage in regular physical activity (36).
In conclusion, the combination of dapagliflozin with conventional anti-heart failure drugs is not only safe but also effective in reducing cardiovascular adverse events and improving the long-term prognosis of patients with AMI and type 2 diabetes. However, this study has several limitations, such as being a single-center retrospective study with a limited sample size, incomplete clinical observation indicators, and failure to conduct a multivariate analysis of confounding factors. Therefore, in order to obtain more reliable research results, we plan to conduct a large-sample, multicenter prospective study in the future.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statementThe studies involving humans were approved by Ethics Committee of the Qinghai Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsJC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DL: Software, Writing – review & editing. YL: Funding acquisition, Project administration, Supervision, Writing – review & editing. XS: Funding acquisition, Resources, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Special Program for Innovation Platform Construction and Cultivation in Qinghai Province; Principal Research Areas in the Health and Healthcare System of Qinghai Province (No: 2023-wjzd-01).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Wang L, Ma YL, Wang WM, Zhu TG, Jin WY, Zhao H, et al. Study on the clinical features and long-term prognosis of patients with coronary microcirculation dysfunction after acute myocardial infarction in the elderly. Chin J Geriatr Cardiovasc Cerebrovasc Dis. (2022) 24:603–6. doi: 10.3969/j.issn.1009-0126.2022.06.012
Crossref Full Text | Google Scholar
2. Zhou P, Zhao YT, Gong SY, Liu ZY, Chen X, Wan D, et al. Establishment of risk factors and prediction model for perioperative myocardial infarction in percutaneous coronary intervention. Chin Circ J. (2020) 35:450–4. doi: 10.3969/j.issn.1000-3614.2020.05.006
Crossref Full Text | Google Scholar
3. Cui QX, Chen H. Advances in genetic research on early onset myocardial infarction. Chin J Geriatr Cardiovasc Dis. (2017) 19:541–4. doi: 10.3969/j.issn.1009-0126.2017.05.023
Crossref Full Text | Google Scholar
5. Zeymer U. Hat der patient einen herzinfarkt? [Diagnosis and initial management of acute myocardial infarction]. MMW Fortschr Med. (2019) 161:34–6. (In German). doi: 10.1007/s15006-019-0223-3
PubMed Abstract | Crossref Full Text | Google Scholar
8. James S, Erlinge D, Storey RF, McGuire DK, de Belder M, Björkgren I, et al. Rationale and design of the DAPA-MI trial: dapagliflozin in patients without diabetes mellitus with acute myocardial infarction. Am Heart J. (2023) 266:188–97. doi: 10.1016/j.ahj.2023.08.008
PubMed Abstract | Crossref Full Text | Google Scholar
9. Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes Mellitus and previous myocardial infarction. Circulation. (2019) 139:2516–27. doi: 10.1161/CIRCULATIONAHA.119.039996
PubMed Abstract | Crossref Full Text | Google Scholar
10. Han E. The effect of dapagliflozin on insulin resistance index in patients with type 2 diabetes receiving short-term intensive insulin pump therapy. J Clin Mil Med. (2020) 48:437–8. doi: 10.16680/j.1671-3826.2020.04.29
Crossref Full Text | Google Scholar
11. Nauck MA, Wefers J, Meier JJ. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. (2021) 9:525–44. doi: 10.1016/S2213-8587(21)00113-3
PubMed Abstract | Crossref Full Text | Google Scholar
12. Marfella R, Sardu C, D'Onofrio N, Fumagalli C, Scisciola L, Sasso FC, et al. SGLT-2 inhibitors and in-stent restenosis-related events after acute myocardial infarction: an observational study in patients with type 2 diabetes. BMC Med. (2023) 21:71. doi: 10.1186/s12916-023-02781-2
PubMed Abstract | Crossref Full Text | Google Scholar
13. Huo ZC, Zhang J, Liu FC. Effect of daglizin on prognosis of patients with acute myocardial infarction complicated with type 2 diabetes. Chin J Cardiol. (2022) 27:107–11. (In Chinese). doi: 10.3969/j.issn.1007-5410.2022.02.002
Crossref Full Text | Google Scholar
14. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction(2018). Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617 Erratum in: Circulation. (2018);138(20):e652.30571511
PubMed Abstract | Crossref Full Text | Google Scholar
15. Chinese Medical Association Diabetes Division. Chinese guidelines for the prevention and treatment of type 2 diabetes (2020 edition). Chin J Endocrinol Metab. (2021) 37:311–98. doi: 10.3760/cma.j.cn115791-20210221-00095
Crossref Full Text | Google Scholar
16. Liu GY, Su XL, Li W, Zhang YP, Zhou BL, Luo R. Preliminary analysis of the composition ratio of acute myocardial infarction in Xining area in the past 10 years (2002–2012). J Plateau Med. (2012) 22:31–3. doi: 10.3969/j.issn.1007-3809.2012.03.009
Crossref Full Text | Google Scholar
17. Li L, Liu Y, Fan SM, Liu LJ, Liu WJ. Analysis of the application of selective coronary angiography and interventional treatment in high altitude conditions. Chin J Pract Int Med. (2003) 23:609–11. doi: 10.3969/j.issn.1005-2194.2003.10.016
Crossref Full Text | Google Scholar
18. Fan YY, Wu CQ, Zu LY. The effect of high altitude environment on cardiovascular system physiological indicators and diseases. Chin J Evid Based Cardiovasc Med. (2021) 13:1267–9. doi: 10.3969/j.issn.1674-4055.2021.10.33
Crossref Full Text | Google Scholar
19. Li WL, She HL, Tong YF. Effect of reperfusion therapy strategy on prognosis of acute myocardial infarction with ST segment elevation in Xining area. J High Altitude Med. (2016) 26:39–41. CNKI:SUN:GYYZ.0.2016-04-013
20. Shang ZL. Study on common risk factors and coronary artery lesion severity in Tibetan and Han patients with coronary heart disease in Xining area. J Plateau Med. (2013) 23:19–21. doi: 10.3969/j.issn.1007-3809.2013.04.006
Crossref Full Text | Google Scholar
21. Jiang YP, Luo W, Yao YL, Fan PY, Wang SQ, Fan XX, et al. Analysis of type 2 diabetes incidence among different types of obese patients in Xining area. Chin Med. (2022) 17:535–8. doi: 10.3760/j.issn.1673-4777.2022.04.014
Crossref Full Text | Google Scholar
22. Dawwas GK, Smith SM, Park H. Cardiovascular outcomes of sodium glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Diabetes Obes Metab. (2019) 21:28–36. doi: 10.1111/dom.13477
PubMed Abstract | Crossref Full Text | Google Scholar
23. Paolisso P, Bergamaschi L, Gragnano F, Gallinoro E, Cesaro A, Sardu C, et al. Outcomes in diabetic patients treated with SGLT2-inhibitors with acute myocardial infarction undergoing PCI: the SGLT2-I AMI PROTECT registry. Pharmacol Res. (2023) 187:106597. doi: 10.1016/j.phrs.2022.106597
PubMed Abstract | Crossref Full Text | Google Scholar
24. Yang Z, Dong J, Zhang JT, Shan QM. Clinical evaluation of dapagliflozin in patients with type 2 diabetes and heart failure in China: a meta-analysis. Chongqing Med J. (2019) 48:2861–3. doi: 10.3969/j.issn.1671-8348.2019.16.041
Crossref Full Text | Google Scholar
25. Chen XJ, Zheng RL, Yang ZX, Xu ZW, Li WZ. The effect of dapagliflozin on cardiac function in patients with acute myocardial infarction and diabetes: a study based on the data from the DAPA-CKD trial. J Integr Cardiovasc Cerebrovasc Dis. (2022) 20:493–1496. doi: 10.12102/j.issn.1672-1349.2022.08.034
Crossref Full Text | Google Scholar
27. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
PubMed Abstract | Crossref Full Text | Google Scholar
28. Lin JJ, Wang LL, Wang D, Fan XD, Dai YN, Tang N, et al. The effect of dapagliflozin on cardiac function and left ventricular remodeling in patients with acute myocardial infarction and type 2 diabetes. Chin Evid Based Cardiovasc Med J. (2023) 15:1076–80. doi: 10.3969/j.issn.1674-4055.2023.09.12
Crossref Full Text | Google Scholar
29. Huo ZC, Zhang J, Liu FC. Effect of dapagliflozin on prognosis of patients with acute myocardial infarction and type 2 diabetes mellitus. Chin J Cardiovasc Dis. (2022) 27:107–11. doi: 10.3969/j.issn.1007-5410.2022.02.002
Crossref Full Text | Google Scholar
30. Li YF, Si JN, Liu YY, Jiao H, Guo WM, Liang JH. The effect of dapagliflozin on the incidence of major cardiovascular adverse events in patients with acute myocardial infarction and type 2 diabetes. Chin J Modern Pharmacy. (2023) 17:6–9. doi: 10.14164/j.cnki.cn11-5581/r.2023.06.002
Crossref Full Text | Google Scholar
31. Liu KL, Zhang Y. The effect of dapagliflozin on cardiac function and MACE in older patients with acute myocardial infarction and heart failure. J PLA Med J. (2023) 48:1427–32. doi: 10.11855/j.issn.0577-7402.2162.2023.0523
Crossref Full Text | Google Scholar
32. Zheng QM, Zhou CF. Glycemic indicators and adverse reactions of dapagliflozin and saxagliptin in the treatment of type 2 diabetes. Jilin Med J. (2023) 44:3120–2. doi: 10.3969/j.issn.1004-0412.2023.11.036
Crossref Full Text | Google Scholar
33. Zhou L, Geng X, Hu Q, Gu J, Chen Y. Effects of dapagliflozin on cardiac function and short-term prognosis of patients with both acute myocardial infarction and type 2 diabetes mellitus. Minerva Cardiol Angiol. (2024) 72:292–8. doi: 10.23736/S2724-5683.23.06478-5
PubMed Abstract | Crossref Full Text | Google Scholar
34. Lim J, Choi YJ, Kim BS, Rhee TM, Lee HJ, Han KD, et al. Comparative cardiovascular outcomes in type 2 diabetes patients taking dapagliflozin versus empagliflozin: a nationwide population-based cohort study. Cardiovasc Diabetol. (2023) 22:188. doi: 10.1186/s12933-023-01911-7
PubMed Abstract | Crossref Full Text | Google Scholar
35. Ge T, Yang Y, Zhao Y. A study of the efficacy of sacubitril/valsartan plus dapagliflozin combination treatment in pulmonary arterial hypertension due to left heart disease. Perfusion. (2023) 38:1697–704. doi: 10.1177/02676591221127924
PubMed Abstract | Crossref Full Text | Google Scholar
36. Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. (2018) 55:503–14. doi: 10.1007/s00592-018-1116-0
留言 (0)