Radiotherapy is a key strategy in multidisciplinary cancer care, whose importance in treatments of both radical and palliative intent has been reinforced by the clinical application of innovative technologies such as image-guided and stereotactic intensity-modulated radiotherapy (1). However, these new, more complex techniques, demanding a greater use of time and resources, have led to continuous cost increases (2, 3). In this context, the European Society for Radiotherapy-Health Economics in Radiation Oncology project (ESTRO-HERO) aimed to promote the use of evidence in resource planning in the field of radiation oncology. The costing of healthcare interventions is a necessary first step in the economic evaluations that support reimbursement decisions and policy formulation (4), but there is little evidence describing the costs in radiotherapy in a precise and detailed way (5). Thus, a model that provides evidence on the resource requirements and costs of current practices and technical advances in external beam radiotherapy (EBRT) was developed within the framework of the ESTRO-HERO project.
Using the time-driven activity-based costing (TD-ABC) methodology, a cost accounting model was developed to estimate the cost of EBRT from the perspective of national healthcare providers in Europe. The aim is to enable the assessment of the costs of different EBRT indications, treatments, and techniques as well as of national resource requirements and utilization in order to inform reimbursement decisions and resource planning.
Having completed the model validation stage at the European level, the objective of this study was to estimate the cost of EBRT in public health care centers in Catalonia (Spain), according to the ESTRO-HERO costing model for 2018, and to assess the feasibility of translating the EU model to a national/regional level. To this end, the Working Group on Costs of Radiation Oncology in Catalonia was created, involving professionals from different centers, representative of radiotherapy activity in Catalonia and the Department of Health Cancer Plan.
2 Materials and methods 2.1 ESTRO-HERO costing modelThe cost estimation model developed by the ESTRO-HERO working group uses TD-ABC methodology, in which the cost is allocated according to the activity times related to the radiotherapy indications and techniques (6).
The structure of the model has three main components: (1) activities directly related to the administration of radiotherapy treatment (EBRT-Core), (2) necessary support activities (RO-Support) and (3) activities performed by RO personnel within the multidisciplinary oncology team, not related to EBRT as such (Beyond-EBRT) (Figure 1).
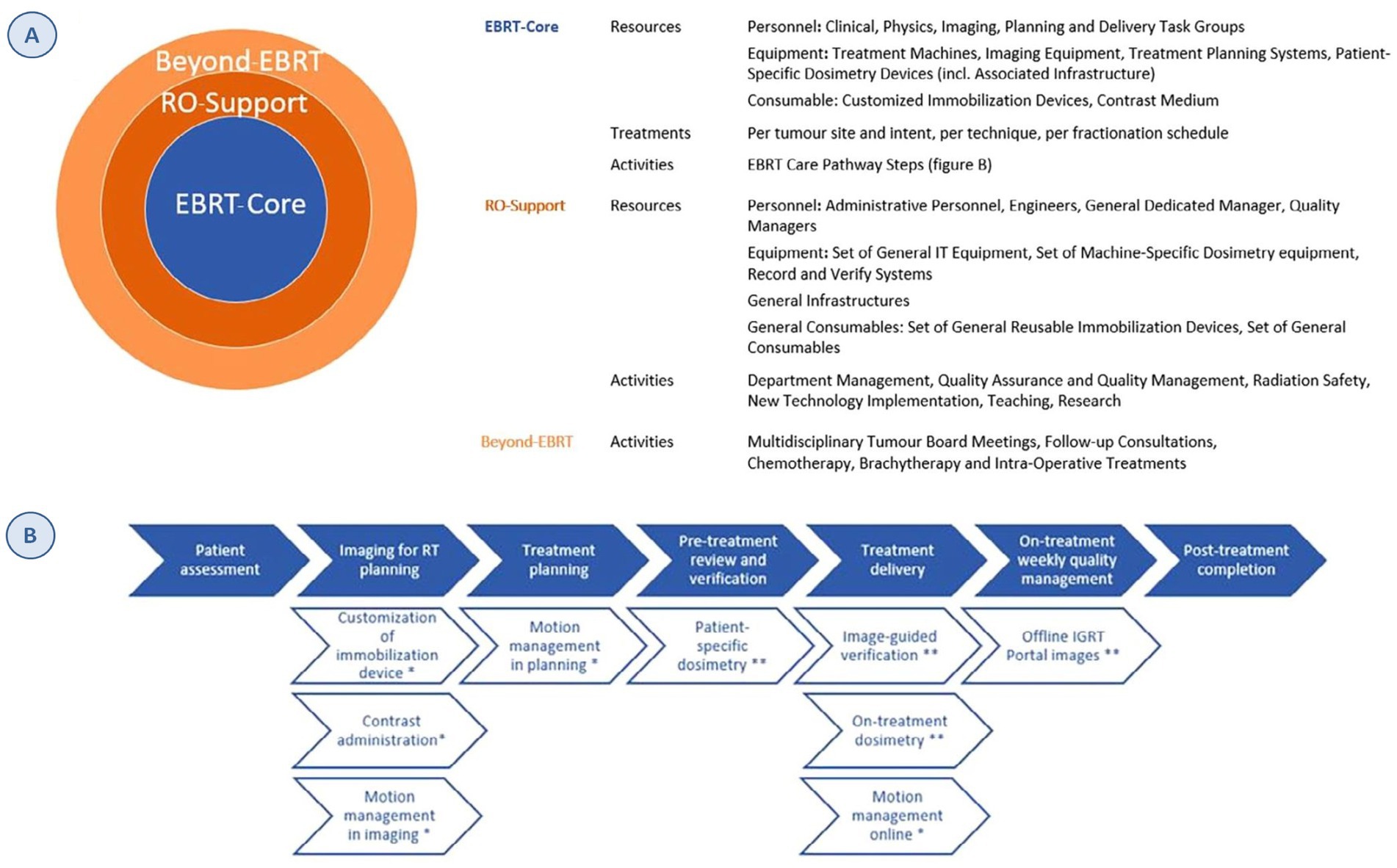
Figure 1. ESTRO-HERO model structure. (A) Inputs for each of the three components of the model. (B) EBRT care-pathway steps. Full arrows: standard activities. Open arrows: optional activities; *OPTIONAL activities defined by tumor site and intent, **optional activities defined based on the technique. Source: Defourny et al. (6). Reprinted with permission from https://www.sciencedirect.com/science/article/abs/pii/S0167814019329512 by Defourny et al. (6), licensed under CC-BY.
The first component has three subcategories: (1) resources: personnel, equipment (including its relevant infrastructure) and consumables; the personnel and equipment are grouped according to the task performed (planning, imaging, etc.); (2) treatments: the number of EBRT treatments by tumor location, treatment intent, technique, and fractionation scheme; and (3) activities: derived from the process map developed by the American Association of Physicists in Medicine (AAPM) (7). The process map structure was adapted to costing purpose of radiotherapy as it was originally developed for quality management. The time estimates also allow the differentiation of six different techniques, capturing the variability and complexity of the treatments (Figure 1).
The second component includes support activities for radiotherapy care, such as quality managers or dosimetry teams, and the third encompasses the activities carried out by RO personnel that are not directly related to the radiotherapy activity. In the cost allocation for these two components, 20% of the costs are assigned using the total number of treatments as the denominator and 80% of the costs using the number of fractions (8, 9).
2.2 Application of the model in Catalonia (Spain)The public health care system in Catalonia has 11 radiotherapy centers covering a population of 7.5 million people (Supplementary Figure 1). For the application of the costing model, the following data from all 11 centers in 2018 were used: N departments, N personnel, equipment, N cases treated according to tumor location, treatment intent (curative/palliative), fractionation scheme, and technique (10). These variables were complemented by a data set for a hypothetical country “Europalia” used in the model validation, incorporating mean values calculated in a European context, which the working group adapted to the Catalan context.
Resources (Table 1). The calculation included full-time equivalent staff and annual salaries, including employer contributions to social security. The cost of the equipment accounted for depreciation and annual maintenance, including costs related to the infrastructure, commissioning personnel, and quality control checks.
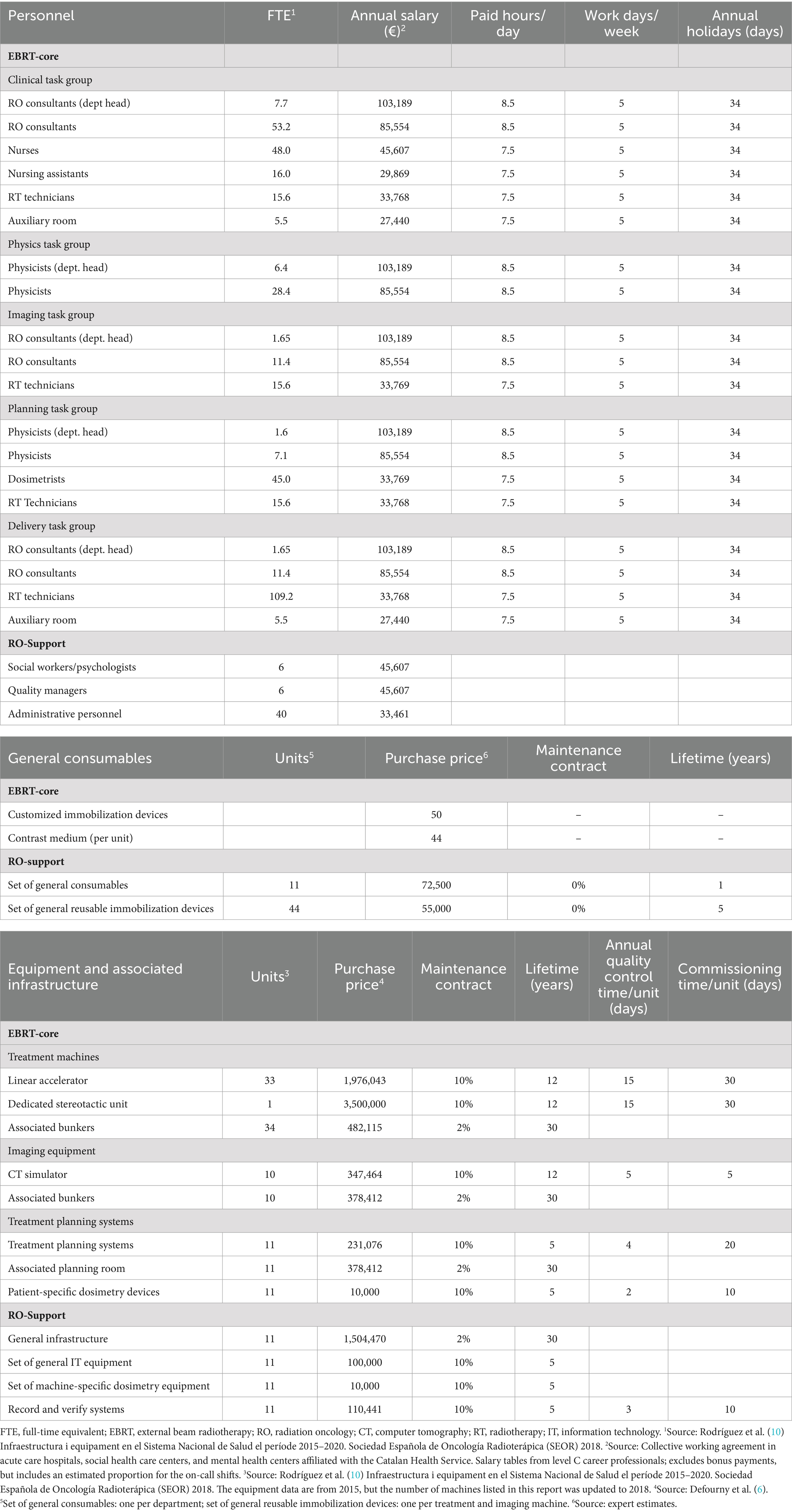
Table 1. Resource input parameters for EBRT-core and RO-support.
Treatments (Supplementary Table 1): N annual radiotherapy treatments, according to tumor location and treatment intent (20 indications with curative intent, organized by primary tumor, and 3 palliative indications, organized by metastatic location), technique (single-field radiotherapy, 2D radiotherapy [2D-RT], 3D conformal radiotherapy [3D-CRT], intensity-modulated radiotherapy [IMRT], rotational IMRT and stereotactic techniques), and N fractions administered.
Activities (Supplementary Table 2): Time used (in minutes) by staff and for equipment is defined by activity, differentiating six techniques. For Europalia, the HERO-WP3 expert panel estimated staff discrete time for each core activity (EBRT-Core) based on published evidence (11–13). The equipment costs incurred by the different activities were calculated based on the staff time dedicated to them. For activities involving different task groups working in parallel, the cost estimate is based only on the time used by the reference task group. When task groups work sequentially, time use across all the activities was taken into account. Staff time dedicated to support activities and activities indirectly related to RT (RO-Support and Beyond-EBRT) was defined by the HERO-WP3 panel based on usual practice. These parameters were adapted to the Catalan context by the radiation oncologists and medical physicists in the working group (Supplementary Table 3).
Brain radiosurgery, that is, single-session stereotactic radiosurgery, was not included in this analysis. Respiratory control maneuvers such as deep inspiration or gating were not considered separately, but extra time was added to respiratory control for each disease, especially in breast and lung cancer.
2.3 Incorporation of new techniques and fractionation schemesRecent years have seen an increase in the complexity of the treatments and the use of shorter fractionation schemes, so in a second phase, the costs of the RT treatment were estimated, including the percentages of use of the fractioning techniques and schemes used in 2023 (Supplementary Table 4). The rest of the parameters remain constant; only the percentage of use of the fractionation techniques and schemes were changed.
2.4 Complementary estimates to the ESTRO-HERO modelThe following complementary estimates were included: complementary examinations for planning, stereotactic body radiation therapy (SBRT) fiducial markers, and hospital overhead costs (14). The costs corresponding to the radiation oncology service/department were already included.
3 ResultsThe total cost of EBRT in 2018 was estimated at EUR 42.2 million. Activities directly related to the administration of treatment represented 69.0% of the total cost, while support activities and not directly related EBRT costs represented 20.2 and 10.8% of the total (Figure 2 and Supplementary Table 5).
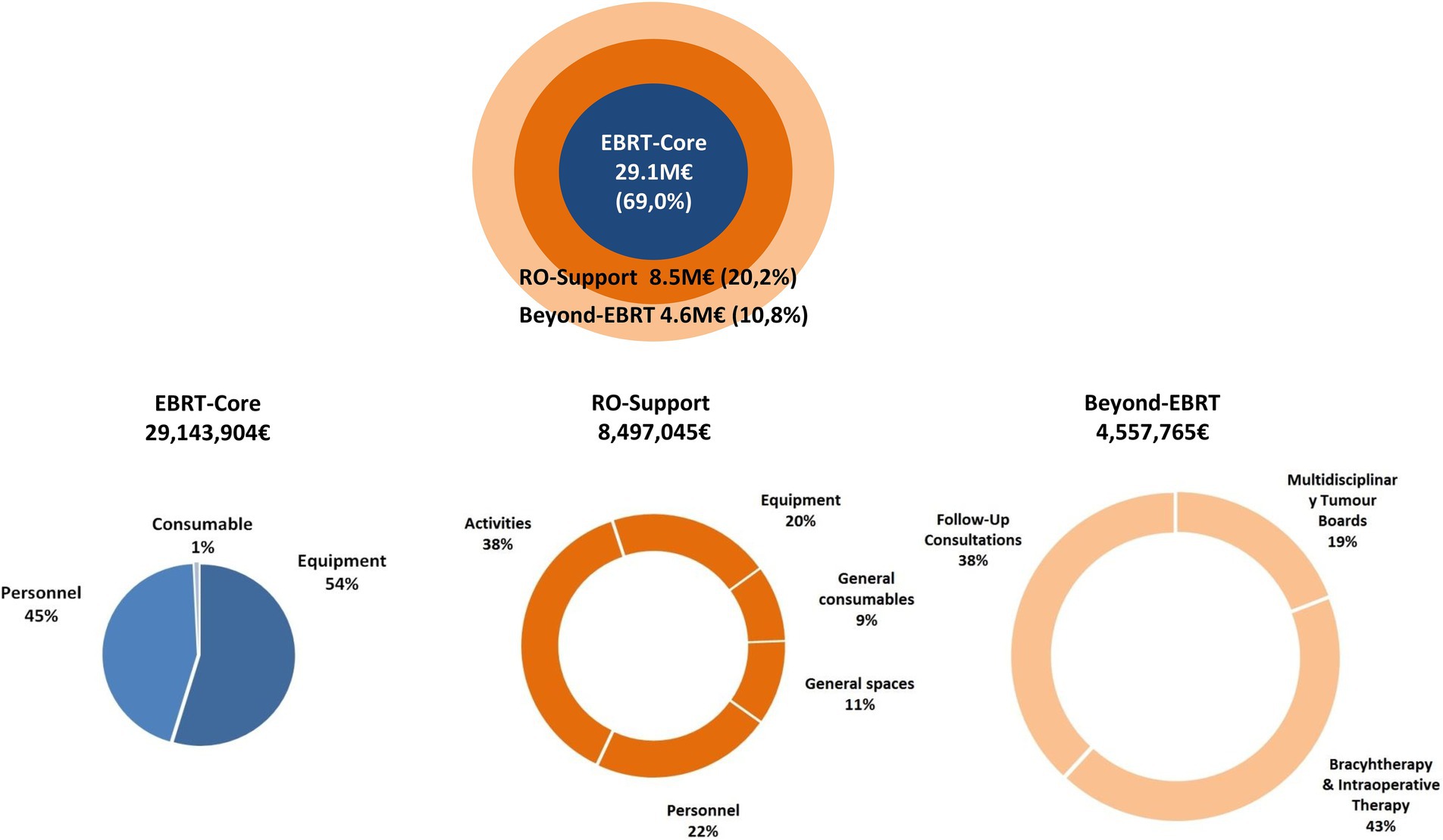
Figure 2. Proportional cost of EBRT-core, RO-support, and beyond-EBRT activities, 2018 (%).
The mean cost of radical treatment ranged from €1714 (leukemia) to €4,645 (pancreas), and with a palliative intent, from €938 (bone metastases) to €1753 (brain metastases) (Table 2). According to the technique used, costs ranged from €1,475 (3D conformal) to €3,608 (rotational IMRT), and by fractionation scheme, from €1,308 (extreme hypofractionation) to €4,094 (standard fractionation).
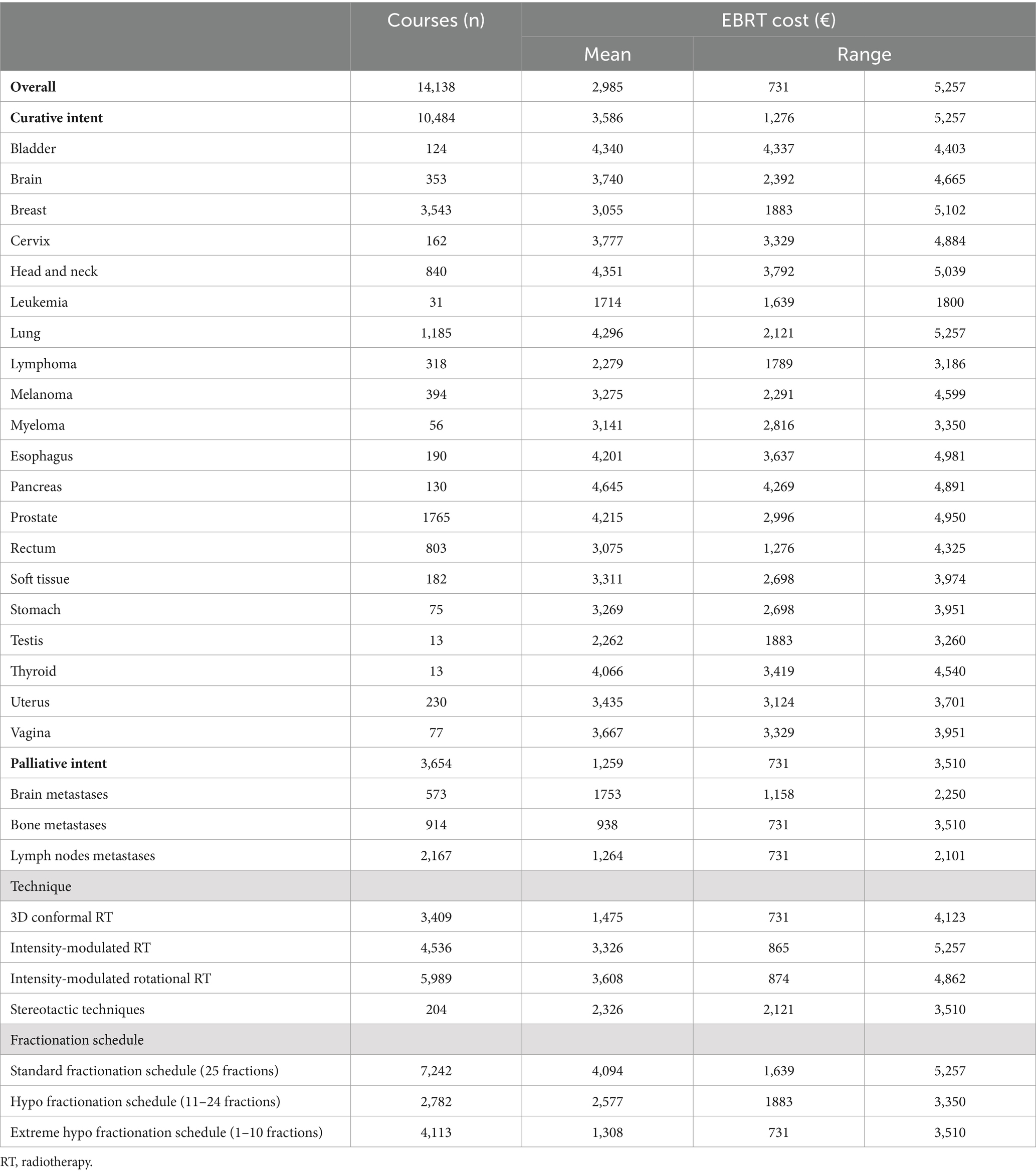
Table 2. Mean cost per external beam radiotherapy treatment, according to intent, technique, and fractionation schedule, 2018 (€).
Figure 3 shows the mean cost of RT treatment according to tumor location and type of activity for the year 2018. The mean cost of RT treatment was also estimated according to tumor location, technique, fractionation scheme, and type of activity for all indications (Supplementary Table 6).
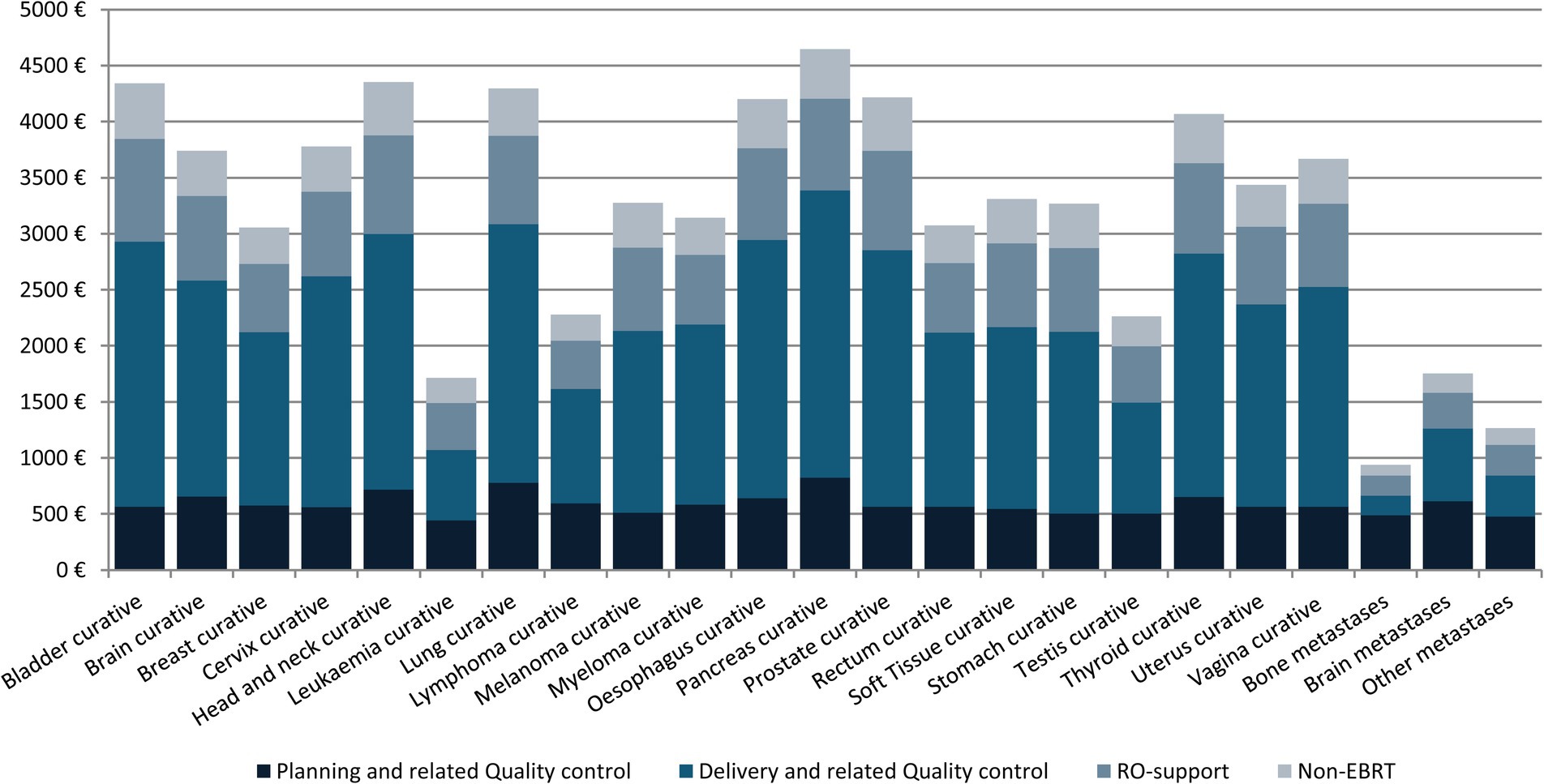
Figure 3. Average EBRT cost per treatment and activity, 2018 (€).
Taking into account the level of complexity in 2023, the mean cost of treatment rose by 0.9% overall, but it ranged widely according to tumor site, from a 13% rise in the case of stomach cancer, to a fall of −15.0, −24.4, and −17.2% in myeloma, pancreas, and lung cancer, respectively (Table 3; Supplementary Tables 7, 8). The mean cost of the treatment increased by 15.6% once complementary examinations and hospital overhead costs were included (Supplementary Table 9).
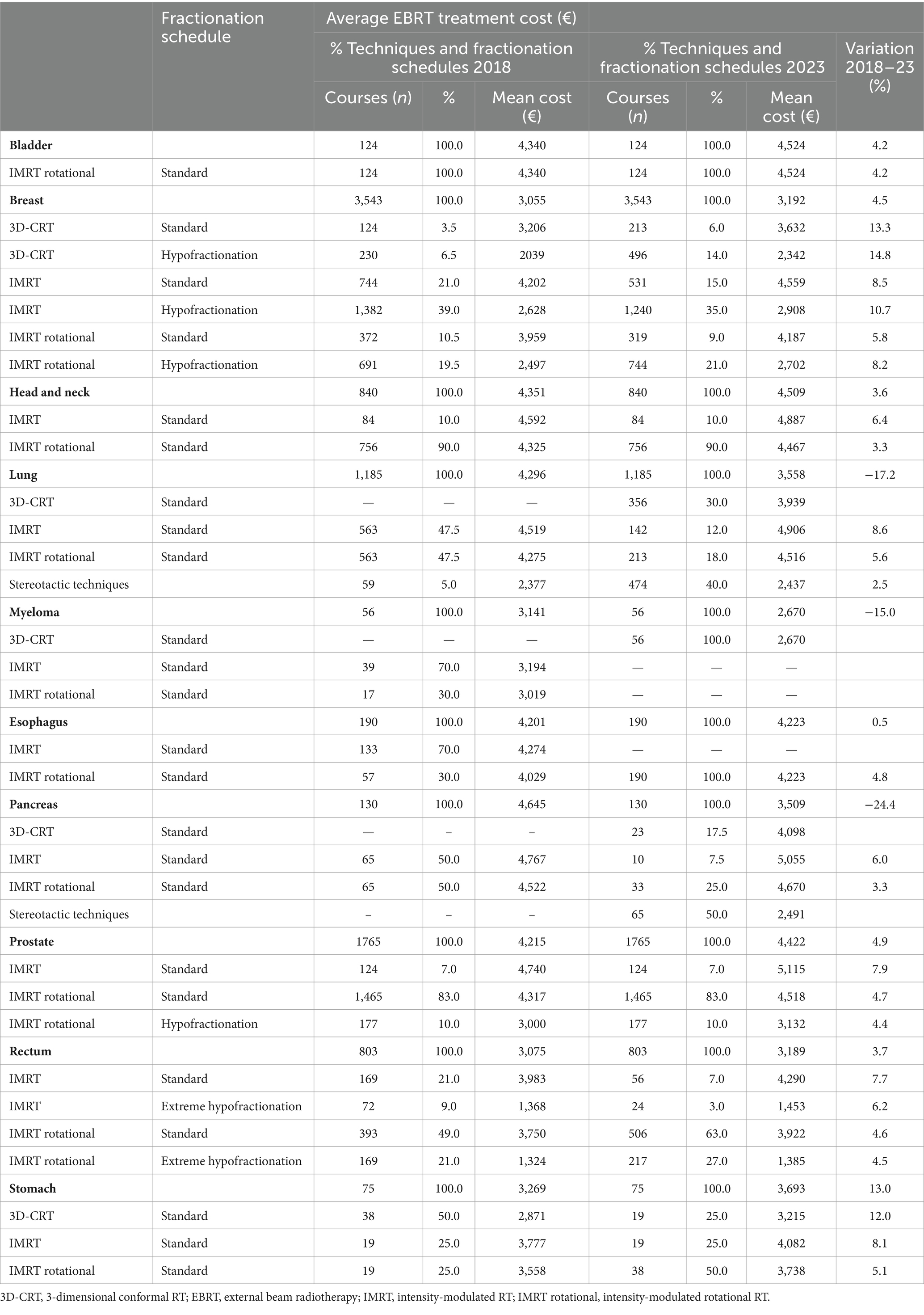
Table 3. Comparison of average EBRT cost per treatment, technique, and fractionation schedule in selected tumors by techniques and fractionation schedules 2018 and 2023.
4 DiscussionThe European Society for Radiotherapy and Oncology - Health Economics in Radiation Oncology project (ESTRO-HERO) developed a cost accounting model to estimate the cost of external beam radiotherapy (EBRT) treatment from the perspective of healthcare providers at a national level in Europe, using the time-driven activity-based costing methodology. Subsequently, a working group on costs of radiation oncology, created under the auspices of the Department of Health Cancer Plan, applied the ESTRO-HERO model to estimate the cost of EBRT in Catalonia. The working group was made up of expert radiation oncologists and medical physicists from different centers, representative of radiotherapy activity in Catalonia and under the Cancer Plan.
The ESTRO-HERO model comprises three components: the first is directly related to the administration of external radiotherapy, the second encompasses support activities, and the third accounts for the activities carried out by RO personnel that are not directly related to the radiotherapy activity in the context of the multidisciplinary oncology teams. As seen in Figure 2, the costs derived from the first component—related to administering EBRT—have the greatest weight in the total cost. The overall mean cost of treatment with a curative intent was €3,586, ranging from €1714 to €4,645 depending on the tumor site (Table 2). These figures are lower than for other cancer treatments, demonstrating once again that despite the upfront investment required, radiotherapy continues to be very cost-effective.
Regarding the mean cost of treatment by primary tumor site, results show that for the five tumors with the highest incidence (breast, prostate, lung, head and neck, and rectum), the average cost ranges from €3,055 for breast cancer to €4,351 for tumors of the head and neck. These differences are a direct result of the widespread use of hypofractionated schemes for breast cancer, and the negligible use of these techniques for head and neck cancer. As for the rest of the tumors, the highest treatment costs were for pancreatic cancers (€4,645), which is consistent with the use of classic fractionation schemes and the great technical complexity.
The technique-specific results are unsurprising (Table 2). The 3D techniques have a mean cost of €1,475, and this increases with complexity, reaching €3,326 for IMRT and €3,608 for rotational IMRT. The apparently low cost of stereotactic techniques is somewhat unexpected (€2,326 euros), but as the table shows, the cost of treatment with classic fractionation is €4,094; with hypofractionation, €2,577; and with extreme hypofractionation, €1,308, and stereotactic techniques are always used with extreme hypofractionation schemes. Likewise, most of the costs, across all diseases, are associated with the administration of the treatment (Figure 3), which explains why administering fewer fractions has such an important impact on the overall cost. This result is also found in a multicenter time-driven activity-based costing study in Belgium conducted by the Belgian Health Care Knowledge Centre, as well as in another study analyzing the financial impact of SBRT for oligometastatic disease in Belgium (8, 15). The weak relationship between treatment complexity and cost is also partly due to the widespread use of simulation units, treatment planning systems, and in general, technology that allows performing techniques of any complexity. Extreme hypofractionation requires a high-performance linear accelerator that incorporates an excellent image-guided RT system, rotational IMRT, a 6-degree-of-freedom table, etc. While such a complete accelerator is not necessary for a classically fractionated treatment, the upgrading of equipment carried out in recent years in Catalonia has afforded the region a park of technologically advanced accelerators that can be used for any treatment, regardless of complexity.
The average costs of treatments with a palliative intent are lower than for radical ones. However, some treatments that are considered palliative can be relatively expensive, as evidenced from the higher end of the ranges seen in Table 2. This elevated cost is due to the use of complex techniques such as hippocampal preservation in palliative cerebral irradiation, which has shown a positive impact on cognition, or the use of SBRT techniques in some bone metastases. Thus, it is important to base any increase in complexity on evidence; otherwise it can increase costs without benefitting patients.
Taking into account the change complexity of treatments delivered in 2023, there was a notable decrease in costs for pancreatic and lung cancer. This decrease can be attributed to the adoption of hypofractionated techniques, which generally reduce the cost despite increasing the complexity, for example from using respiratory control systems (Table 3). When comparing the results of 2018 and 2023 in relation to the indication and the technique used, the greater weight of fractionation compared to complexity in the cost of treatment is clear.
Various studies have estimated the costs of external radiotherapy in different contexts and with different methodologies (2, 5, 8, 14–20). Our results show significantly lower costs than those calculated in studies using activity-based costing. This is mainly due to the difference in salaries of health professionals between European countries (21). Compared to the studies by Defourny et al. (6, 18) conducted in Europalia (a hypothetical country utilized in the validation of the HERO cost model, incorporating mean values calculated within a European context) and Belgium, the salaries of Catalonia’s clinical task force are 49 and 66% lower, respectively. Similarly, the salaries of the physics task force in Catalonia are between 4 and 17% lower, and the salaries of the imaging task group are between 31 and 54% lower. On the other hand, the estimates of equipment costs are similar. Alternatively, it is important to highlight that, despite the fact that the costs are lower, the proportional cost of SBRT compared to longer schedules in Catalonia is similar to that found in the study of Nevens et al. (15). Using a microcosting methodology, the estimated cost of RT in rectal cancer in the study by Hanly et al. (19) was similar to ours; however, this was not the case in the study by Perrier et al. (20) for head and neck cancers, where the estimated cost was much lower.
One limitation of the study is that the cost model used may lead to an underestimation of the resources and costs of EBRT at the regional or national level, since the hospital overhead costs are not included, only those corresponding to the departments (6). Although there is some variability in relation to the weight of this type of cost over the total, in this study we made a complementary estimate of the overhead costs based on a recent study carried out by Spencer et al. (14) in the UK.
In the context of personalized radiotherapy in the era of precision medicine, one of the most imminent developments is the systemic deployment of adaptive techniques during the course of treatment (22), which will substantially increase the cost of activities directly related to treatment administration. Process automation tools, including those based on artificial intelligence, can mitigate this extra cost by reducing the time required of staff and for the treatment itself, but their adoption will in turn mean a new expense to be taken into account (23).
On the other hand, this costing study shows that, in our setting, the most important contributor to the cost of EBRT is the occupation of the treatment unit, that is, the number of sessions, hence the tendency to try to hypofractionate as much as possible. Moreover, this modality is also more comfortable for patients, and it also helps reduce waiting lists. However, hypofractionation schemes should mainly be applied with an eye toward minimizing toxicity in healthy tissue, ensuring that the patient’s overall quality of life is improved.
Both adaptive radiotherapy (and especially the set of tools that it involves) and the increasing use of hypofractionation in most tumor sites will entail changes in the roles of radiation oncologists, radiotherapy technicians and medical physicists. Professionals will have to adapt to a changing environment, without forgetting to center the patient and their needs, adopting appropriate measures to ultimately enhance their quality of life. Additional studies will be required to assess the costs in this new environment.
Finally, the application of the HERO costing model can support health care cancer policy and decision-making in Catalonia. Firstly, it promotes the utilization of evidence in resource planning in the field of radiation oncology. Secondly, the TD-ABC methodology contributes significantly by streamlining and optimizing the entire care cycle in radiotherapy. By accurately mapping and analyzing each step of the process, TD-ABC helps identify inefficiencies, reduce costs, and improve resource allocation, ultimately enhancing patient outcomes and care quality throughout the radiotherapy treatment pathway (24). And, finally, by enabling the costing of radiotherapy treatments at a regional level, it provides support for reimbursement decisions and policy formulation (25). This approach allows policymakers to better understand the financial implications of radiotherapy services, promoting a sustainable allocation of healthcare funds. Additionally, it aids in developing standardized reimbursement rates that reflect the actual costs of providing high-quality care, ensuring that financial resources are allocated in a way that supports both efficiency and accessibility. This data-driven approach not only guides reimbursement strategies but also facilitates long-term planning for radiotherapy infrastructure and workforce development, ultimately improving access to cancer care across the region.
In conclusion, this study is a first approximation to the cost of external beam radiotherapy using time-driven activity-based costing (TD-ABC) in Catalonia. For each indication, the average cost per treatment increases with the associated complexity. Moreover, costs decrease when hypofractionation schemes are used, mainly due to the lower weight of the equipment in the treatment cost. Consequently, the adoption of stereotactic techniques is driving cost decreases. All in all, this model represents a powerful tool to analyze different possible scenarios, such as changes in fractionation and complexity.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributionsJC: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MA: Methodology, Writing – review & editing. CM-M: Methodology, Writing – review & editing. AE: Methodology, Writing – review & editing. JG: Methodology, Writing – review & editing. ND: Conceptualization, Methodology, Writing – review & editing. YL: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JB: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funded by the Departament de Recerca i Universitats de la Generalitat de Catalunya i l’AGAUR” (grant number 2021 SGR 00808). We thank CERCA Programme/Generalitat de Catalunya for institutional support.
AcknowledgmentsThe administrative support made by Meritxell Nomen is gratefully acknowledged, as well as Megan Harris for her English editorial support.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1474376/full#supplementary-material
References2. van de Werf, E, Verstraete, J, and Lievens, Y. The cost of radiotherapy in a decade of technology evolution. Radiother Oncol. (2012) 102:148–53. doi: 10.1016/j.radonc.2011.07.033
Crossref Full Text | Google Scholar
3. Lievens, Y, Dunscombe, P, Defourny, N, Gasparotto, C, Borras, J, and Grau, C. HERO (health economics in radiation oncology): a Pan-European project on radiotherapy resources and needs. Clin Oncol. (2015) 27:115–24. doi: 10.1016/j.clon.2014.10.010
PubMed Abstract | Crossref Full Text | Google Scholar
4. Lievens, Y, Borras, J, and Grau, C. Cost calculation, a necessary step towards widespread adoption of advanced radiotherapy technology. Acta Oncol. (2015) 54:1275–81. doi: 10.3109/0284186X.2015.1066932
PubMed Abstract | Crossref Full Text | Google Scholar
5. Defourny, N, Dunscombe, P, Perrier, L, Grau, C, and Lievens, Y. Cost evaluations of radiotherapy: what do we know? An ESTRO-HERO analysis. Clin Transl Rad Oncol. (2016) 121:468–74. doi: 10.1016/j.radonc.2016.12.002
PubMed Abstract | Crossref Full Text | Google Scholar
6. Defourny, N, Perrier, L, Borras, JM, Coffey, M, Corral, J, Hoozée, S, et al. National costs and resource requirements of external beam radiotherapy: a time-driven activity-based costing model from the ESTRO-HERO project. Radiother Oncol. (2019) 138:187–94. doi: 10.1016/j.radonc.2019.06.015
PubMed Abstract | Crossref Full Text | Google Scholar
7. Ford, EC, Fong de Los Santos, L, Pawlicki, T, Sutlief, S, and Dunscombe, P. Consensus recommendations for incident learning database structures in radiation oncology. Med Phys. (2012) 39:7272–90. doi: 10.1118/1.4764914
PubMed Abstract | Crossref Full Text | Google Scholar
8. Hulstaert, F, Mertens, AS, Obyn, C, et al. Innovative radiotherapy techniques: a multicentre time-driven activity-based costing study. Brussels: Health technology assessment (HTA) Belgian health care knowledge Centre (KCE) (2013).
9. Lievens, Y, Obyn, C, Mertens, AS, Van Halewyck, D, and Hulstaert, F. Stereotactic body radiotherapy for lung cancer: how much does it really cost? JTO. (2015) 10:454–61. doi: 10.1097/JTO.0000000000000421
PubMed Abstract | Crossref Full Text | Google Scholar
10. Rodríguez, A, Algara, M, Monge, D, López-Torrecilla, J, Caballero, F, Morera, R, et al. Infrastructure and equipment for radiation oncology in the Spanish National Health System: analysis of external beam radiotherapy 2015-2020. Clin Transl Oncol. (2018) 20:402–10. doi: 10.1007/s12094-017-1727-x
Crossref Full Text | Google Scholar
11. Remonnay, R, Morelle, M, Giraud, P, and Carrere, MO. The cost of respiration-gated radiotherapy in the framework of a clinical research programme “STIC”. Cancer Radiother. (2009) 13:281–90. doi: 10.1016/j.canrad.2009.03.001
PubMed Abstract | Crossref Full Text | Google Scholar
12. Fietkau, R, Budach, W, Zamboglou, N, Thiel, HJ, Sack, H, and Popp, W. Time management in radiation oncology: development and evaluation of a modular system based on the example of rectal cancer treatment. Strahlenther Onkol. (2012) 188:5–11. doi: 10.1007/s00066-011-0003-1
PubMed Abstract | Crossref Full Text | Google Scholar
13. Vorwerk, H, Zink, K, Schiller, R, Budach, V, Böhmer, D, Kampfer, S, et al. Protection of quality and innovation in radiation oncology: the prospective multicenter trial the German Society of Radiation Oncology (DEGRO-QUIRO study). Strahlenther Onkol. (2014) 190:433–43. doi: 10.1007/s00066-014-0634-0
PubMed Abstract | Crossref Full Text | Google Scholar
14. Spencer, K, Defourny, N, Tunstall, D, Cosgrove, V, Kirkby, K, Henry, A, et al. Variable and fixed costs in NHS radiotherapy; consequences for increasing hypo fractionation. Radiother Oncol. (2022) 166:180–8. doi: 10.1016/j.radonc.2021.11.035
PubMed Abstract | Crossref Full Text | Google Scholar
15. Nevens, D, Kindts, I, Defourny, N, Boesmans, L, Van Damme, N, Engels, H, et al. The financial impact of SBRT for oligometastatic disease: a population-level analysis in Belgium. Radiother Oncol. (2020) 145:215–22. doi: 10.1016/j.radonc.2020.01.024
PubMed Abstract | Crossref Full Text | Google Scholar
16. Van Dyk, J, Zubizarreta, E, and Lievens, Y. Cost evaluation to optimise radiation therapy implementation in different income settings: A time-driven activity-based analysis. Radiother Oncol. (2017) 125:178–85. doi: 10.1016/j.radonc.2017.08.021
PubMed Abstract | Crossref Full Text | Google Scholar
18. Defourny, N, Hoozée, S, Daisne, JF, and Lievens, Y. Developing time-driven activity-based costing at the national level to support policy recommendations for radiation oncology in Belgium. J Account Public Policy. (2023) 42:107013. doi: 10.1016/j.jaccpubpol.2022.107013
Crossref Full Text | Google Scholar
19. Hanly, P, Céilleachair, AÓ, Skally, M, O’Neill, C, and Sharp, L. Direct costs of radiotherapy for rectal cancer: a microcosting study. BMC Health Serv Res. (2015) 15:184. doi: 10.1186/s12913-015-0845-9
PubMed Abstract | Crossref Full Text | Google Scholar
20. Perrier, L, Morelle, M, Pommier, P, Boisselier, P, Coche-Dequeant, B, Gallocher, O, et al. Cost analysis of complex radiation therapy for patients with head and neck Cancer. Int J Radiat Oncol Biol Phys. (2016) 95:654–62. doi: 10.1016/j.ijrobp.2016.02.013
PubMed Abstract | Crossref Full Text | Google Scholar
21. Atun, R, Jaffray, DA, Barton, MB, Bray, F, Baumann, M, Vikram, B, et al. Expanding global access to radiotherapy. Lancet Oncol. (2015) 16:1153–86. doi: 10.1016/S1470-2045(15)00222-3
Crossref Full Text | Google Scholar
22. Baumann, M, Krause, M, Overgaard, J, Debus, J, Bentzen, SM, Daartz, J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. (2016) 16:234–49. doi: 10.1038/nrc.2016.18
Crossref Full Text | Google Scholar
23. de Dios N, R, Moñino A, M, Liu, C, Jiménez, R, Antón, N, Prieto, M, et al. Machine learning-based automated planning for hippocampal avoidance prophylactic cranial irradiation. Clin Transl Oncol. (2023) 25:503–9. doi: 10.1007/s12094-022-02963-z
PubMed Abstract | Crossref Full Text | Google Scholar
24. da Silva, B, Etges, AP, de Lara, LR, Sapper, SL, Frankenberg Berger, AV, Streck, M, et al. Redesign of radiotherapy for prostate cancer: a proposal for universal healthcare systems. J Comp Effect Res. (2023) 12:e230023. doi: 10.57264/cer-2023-0023
PubMed Abstract | Crossref Full Text | Google Scholar
25. Borras, JM, Corral, J, Aggarwal, A, Audisio, R, Espinas, JA, Figueras, J, et al. Innovation, value and reimbursement in radiation and complex surgical oncology: time to rethink. Radiother Oncol. (2022) 169:114–23. doi: 10.1016/j.radonc.2021.08.002
留言 (0)