The transcranial electrical stimulation (tES) is a non-invasive neurostimulation technique, which typically includes transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS) (1–3). It has garnered rapid attention and promotion owing to its ease of use, non-invasiveness, adjustability, and broad range of applications (4–6). Over the past five years, more than 5000 articles have been published on this subject (7). In most clinical scenarios, cortical excitability is modulated by placing one or more pairs of electrodes (8) on the surface of the human scalp, with an applied current intensity of 1-2 mA (9, 10). The tES is generally regarded as non-invasive and well-tolerated by subjects with favorable safety profiles (11–13). However, the weak current produces a low-strength electric field (14), necessitating long-term repetition and accumulation to achieve desired effects. Enhancing the intensity of tES shows the potential to yield improved effectiveness (15–19). Nevertheless, the heightened skin sensation and potential side effects impede further enhancement of the stimulation intensity (15, 16, 20–22).
Numerous types of electrodes exist today that can be utilized for tES, including the commonly used comb dry electrode, sponge saltwater electrode, and gold standard conductive paste electrode. Dry electrodes are easy to use, but have problems with high contact impedance and lack of comfort (23, 24). In contrast, wet electrodes have low contact impedance, but their operation process is time-consuming, and the evaporation of water after long-term use will cause the impedance to increase (25, 26). Moreover, the process of cleaning hair and electrode caps adds to the complexity. Gel electrodes combine the low contact impedance characteristics of wet electrodes with better moisture retention, efficient conductivity and stability (24, 27–29). Unlike wet electrodes, gel electrodes do not leave residues, require minimal preparation time, and do not adhere to the skin (30, 31). Additionally, they maintain relatively stable impedance over extended periods, making them suitable for prolonged brain stimulation experiments. Recently, gel electrodes, as a semi-dry alternative that balances portability and high performance, have seen widespread application in brain stimulation. It achieves a good balance between ease of use and conductivity performance (22, 27, 30). Regardless of the stimulation conditions, ensuring robust safety and tolerability in participants is essential for widespread acceptance (11, 32–34).
The application of tES using gel electrodes, selecting appropriate stimulation parameters is crucial, including stimulation duration, stimulation type, current intensity, and stimulation frequency, among others. Stimulation duration is an important factor that influences tolerability and safety. Especially for potential long-term applications of tES devices, such as chronic therapy or daily interventions. It directly impacts skin responses, cognitive load, and long-term effects on the body (20). Several studies stimulated for more extended periods than 20 minutes without reporting side effects (35). In the tES safety guidelines from Antal et al., tES has been established as safe for up to 60 min duration per day (36). Nevertheless, Alonzo et al. proposed that the repeated tES sessions may result in longer stimulation duration (37), ultimately leading to increased cumulative energy transmission to the brain. This could potentially heighten the risk of heat injury, electrolysis, or fatigue-related discomfort. Conversely, a short duration of stimulation may not be sufficient to induce significant neuromodulation effects (38). Therefore, uncertainty remains regarding the relationship between stimulation duration and both safety and tolerability, especially within the framework of different tES paradigms and individual variances. With the increasing emergence of tES devices, it is essential to study the tolerability and safety of these new devices and treatment modalities before their widespread adoption. However, when designing experiments, multiple variables must often be considered, such as the treatment environment, target population, application methods, and stimulation parameters. The diversity of these conditions, combined with the duration of stimulation, collectively determines the overall duration of the experiment. In this context, stimulation duration becomes a critical variable in research, as it not only dictates the structure and progression of the experiment but also directly impacts the deployment of devices and protocols (39).
Although stimulation duration is a key focus of research, the influence of stimulation type is equally important. In the field of tES, the most commonly used stimulation types are tDCS and tACS (1–3). tDCS can administer a consistent, unidirectional flow of current to influence nerve membrane polarization, regulate the resting membrane potential, and modulate synaptic transmission (40–42), thus altering brain excitability. In contrast, tACS delivers an oscillating current that alternates between positive and negative polarities periodically, potentially synchronizing or interacting with endogenous brain rhythms (43–45). Due to variations in the mechanisms of action between these two types of tES, the physiological responses, side effects, and subjective experiences elicited by each technique may differ, even when employing the same current intensity (46, 47). Previous studies have examined the tolerability and safety of tDCS or tACS using various devices and treatment protocols (11, 16, 20, 47). However, comparative research on the tolerability and safety of these two types of tES using gel-based electrodes remains limited, and no consensus has been reached (48, 49).
In this context, the present study aims to investigate the effects of stimulation duration and type on the tolerability and safety of tES using gel-based electrodes. This research will provide valuable reference points for selecting appropriate stimulation parameters in the design and clinical application of tES devices, laying the foundation for tailoring stimulation protocols to different clinical indications.
2 Materials and methodsIn this study, tES was performed using preconfigured gel particle electrodes to gather experimental measurements, including self-report questionnaire data and in-experiment behavioral keystroke data from participants. These measurements included adverse events (AEs) and the relationship of adverse events with tES (AEs-rela) in terms of tolerability, the Visual Analog Scale (VAS) and the Skin Sensation Rating (SSR) ratings in terms of safety, mental health evaluation and behavioral experimental outcomes from the Montreal Cognitive Assessment (MoCA), the Self-Rating Anxiety Scale (SAS), the Visual Analog Mood Scale-Revised (VAMS-R), and Stroop task performance data.
2.1 ParticipantsIn the study, 17 healthy subjects were recruited, comprising 6 females and 11 males. One female was excluded due to a history of head nerve pain, and one male was excluded due to data collection errors related to experimental parameters. Ultimately, the remaining 15 healthy subjects (5 females and 10 males, mean age ± SD: 24.00 ± 1.36 years, average education duration: 17.53 ± 1.19 years.) successfully completed the entire experiment.
All participants with normal or corrected-to-normal vision provided their informed consent forms and basic information forms, and specified the scales and behavioral tasks to be completed during the experiment. Prior to the experiment, no participants reported a history of craniotomy or head injury, personal or family history of neurological or psychiatric disorders, metal implants or implanted electronic devices, skin sensitivities, or drug use. For safety reasons, individuals who are pregnant or may become pregnant were excluded from participation. Informed consent was obtained before engaging in the research. This study was approved by the Human Ethics Committee of the University of Science and Technology of China (IRB No. 2022KY275).
2.2 Experimental procedureParticipants voluntarily participated in a total of 10 stimulation sessions, comprising five sessions of tDCS and five sessions of tACS, each stimulus type with varying durations (2 minutes, 5 minutes, 7 minutes, 10 minutes, and 20 minutes). In our study, the interval between each stimulation session is 5 minutes. No washout period was included. Therefore, we implemented a Latin square design to balance the sequence of experimental conditions. This design ensures that the effects of different conditions are evenly distributed across participants, effectively minimizing potential carryover or order effects on the results. The experiment adhered to a single-blind multi-condition experimental approach.
Participants were instructed to complete neuropsychological scales such as MoCA and SAS prior to the formal stimulation. During the stimulation process, participants engaged in a behavioral keystroke Stroop task and stopped the Stroop task at the end of the stimulation. The VAS, SSR, AEs, AEs-rela, and VAMS-R scales were completed immediately after each stimulation session. After the completion of all the stimulations, each subject underwent neuropsychological scale assessments such as MoCA and SAS, as illustrated in Figure 1.
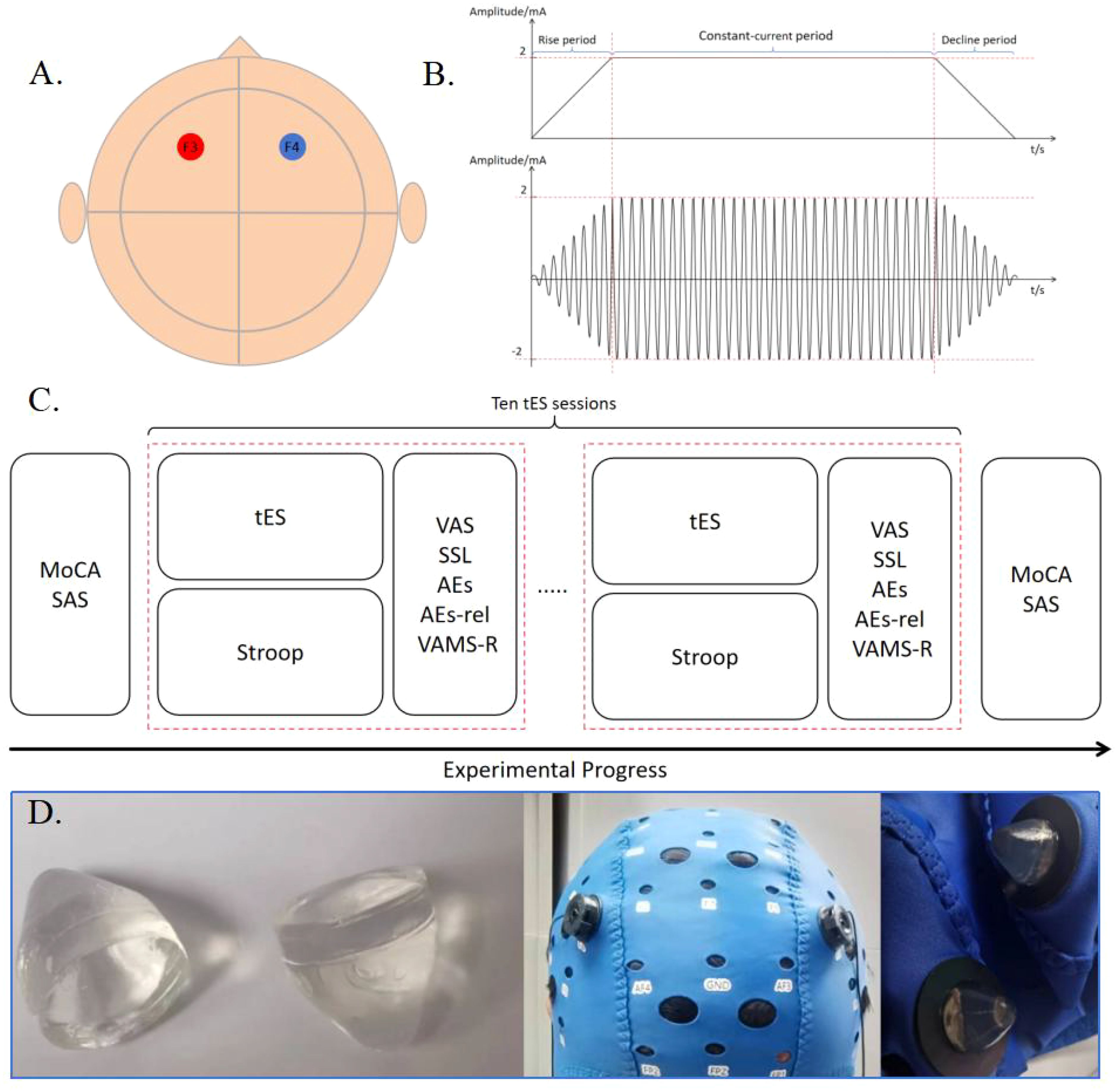
Figure 1. (A) Locations of the stimulation electrodes. The positive and negative electrodes of a pair are placed in F3 and F4, respectively. (B) Schematic diagram of tDCS and tACS stimulation with a current intensity of 2mA. (C) Experimental Procedure. (D) Preset gel particle electrode used in this experiment.
2.3 Transcranial electrical stimulationThe selected electrode is a preconfigured gel particle electrode, which is connected to the output port of the stimulator using a silver/silver chloride powder sintered electrode and a gel-fixed shell, with a diameter of 10 mm. It is secured on the scalp using a professional EEG electrode cap, ensuring that the impedance value during electrical stimulation remains below 15 kΩ. tDCS was set to 2 mA, and tACS was set to 2 mA at 10 Hz. The current linearly increased to 2 mA for 30 seconds at the beginning of the stimulation and then decreased to 0 mA for 30 seconds at the end of the stimulation. The intensity of stimulation at 2 mA was maintained throughout the duration of this study. Real-time monitoring of impedance changes during the stimulation process is conducted by analyzing impedance return values from software on the tablet. If the impedance levels become too high, the relay will be automatically disconnected to protect the subject. At the end of each stimulation session, participants were required to complete a self-reported preparation form before commencing the next stimulation program. Participants were seated in a comfortable chair, which they adjusted according to their preference, facing a screen displaying the Stroop task.
The transcranial electrical stimulation device utilized is a lithium battery-powered stimulator. The stimulator communicates with the software on the tablet computer via Bluetooth, and the software establishes specific parameters, which are then transmitted to the stimulator for execution. The stimulator has been isolated from the main power supply, and batteries are utilized to ensure the safety of the subjects. The performance of the stimulator has been previously verified in the literature (50). The F3 and F4 electrodes in the 10-20 EEG system were selected as stimulation sites in this experiment, which are located in the left and right prefrontal lobes, respectively. The reason of the selection of the stimulation site was that the function of the brain region underlying the F3 and F4 electrodes aligns with the cognitive abilities verified by the Stroop task (51, 52).
The participant is blinded to the stimulus conditions, and the control software for the stimulus parameters is installed on a tablet computer, ensuring that the participant does not have access to information regarding the stimulus parameters.
2.4 Neuropsychology testsThe MoCA Scale is a convenient and efficient cognitive screening tool that encompasses various cognitive domains, including attention and concentration, executive function, memory, language, visual-spatial skills, abstract thinking, calculation, and orientation. To minimize the impact of pre-test memory on subjects’ performance, an alternate version of the test was administered both before and after the experiment.
The SAS scale is a psychometric tool utilized for assessing anxiety state and severity. Participants respond to a series of self-reported questions regarding feelings of anxiety, encompassing sensations such as nervousness, worry, fear, and physical discomfort. Each item is rated on a Likert scale from 1 to 4 (1 = absent or rarely present, 2 = sometimes present, 3 = mostly present, 4 = almost always or always present). They then select a rating that best corresponds to their current situation. Subsequently, the scores for each item are totaled and calculated to derive an overall score. This score is used to determine the presence of anxiety disorders and evaluate the level of anxiety symptoms in individuals.
The VAMS-R scale were utilized to assess the emotional states of the participants. Emotional states, including changes in visual perception of sorrow, bewilderment, fear, happiness, fatigue, anger, strain, and full of energy, were gauged by indicating the intensity of feelings on a specified emotional continuum line. After measurement and quantification, each item was scored on a scale ranging from 0 to 100, with a high score indicating that emotion was strong and a low score indicating the opposite.
2.5 The Stroop taskThe Stroop task, also known as the Stroop Effect experiment, is a classic psychological study initially proposed by John Ridley Stroop in 1935 (53). This experiment is primarily utilized to investigate human cognitive processes, particularly in the areas of attention, perception, reaction time, and executive function. In this study, the performance in the Stroop task was used to indicate whether subjects concentrate enough during the experiment (51, 54).
The Stroop task used in this study was compiled by PsychoPy 3.8.10. Instructions will be displayed when it was initiated. Please wait until the participant is ready and press the ‘space’ key to begin. A series of color words will appear on the screen. However, it is important to note that the task is not to read the meaning of the word, but rather to quickly and accurately report the color of the word by pressing the corresponding button. Specifically, ‘red’ corresponds to the key ‘R’, ‘green’ corresponds to the key ‘G’, ‘blue’ corresponds to the key ‘B’, and ‘yellow’ corresponds to the key ‘Y’.
The experimental procedure involved consistent and inconsistent colors. Participants were instructed to focus on the colors and ignore the words. Clear prompts were provided between the beginning of the experiment and each stage. If participants had any questions, they were encouraged to ask the interviewer. After becoming familiar with the task, participants were instructed to promptly and accurately identify and provide feedback on the word font color in the formal stimulus experiment. Following the conclusion of the experiment, data on both accuracy rate and reaction time were collected by the Stroop task stop program from participant responses. This study focuses specifically on two key results: percentage of correct responses and reaction time.
2.6 Sensation and adverse eventsThe pain score is measured by the VAS, which ranges from 0 to 10 points from left to right. A higher score indicates a greater level of pain. The relation between the score and pain intensity is as follows: 1-3 = mild pain; minimal impact on activities of daily living; 4-6 = moderate pain; moderate impact on the ability to live daily; 7-10 = severe pain; significant impact on the ability to live daily.
The SSR scale was used to indicate the tolerance effect of tES on the subject by providing evaluation options of ‘no sensation,’ ‘slight,’ ‘moderate,’ ‘severe,’ and ‘extreme,’ corresponding to discrete values of 1, 2, 3, 4, and 5, respectively. Subjects are allowed to stop the stimulation process at any time if they are unable to tolerate it.
AEs were evaluated for symptoms such as headache, neck pain, scalp pain, scalp pressure, tingling, burning sensation, itching sensation, sleeping issues, trouble concentrating, dizziness, nausea, and vibration intensity on a scale of 1 to 4 (1 = absent, 2 = mild, 3 = moderate, 4 = severe). Simultaneously, participants rated the relationship between adverse events and tES (AEs-relation) on a scale of 1 to 5 (1 = none, 2 = remote, 3 = possible, 4 = probable, 5 = definite).
2.7 Statistical analysisAll analyses were conducted using OriginPro 2021 software (OriginLab Corp, Northampton, MA, USA) and IBM SPSS Statistics 26.0 (IBM Corp, Armonk, NY, USA). Based on the normality and homogeneity of variance of the data in each condition, the paired-sample Wilcoxon signed-rank test with non-parametric analysis was utilized for the comparison between tDCS and tACS. Friedman analysis of variance (ANOVA) was performed for the comparison between five stimulation durations.
3 ResultsBy applying tES to F3 and F4 on the scalp surface of healthy subjects using preset gel particle electrodes, we investigated the safety and tolerability of stimulation types (tDCS and tACS), as well as stimulation durations (2 minutes, 5 minutes, 7 minutes, 10 minutes, and 20 minutes). In the study, MoCA, SSA, VAS, and SSR, AEs, AEs-rela, VAMS-R and Stroop analysis based on participants’ rating and response were analyzed.
The results of MoCA and SAS scales showed no significant changes in cognitive function and anxiety levels before and after the stimulation sessions (see Figure 2A), suggesting that the subjects remained in a cognitively normal and non-anxious state throughout the study. The accuracy in the Stroop task under different conditions was larger than 85%, with an averaged accuracy was larger than 97%, indicating that subjects were quite engaged in the experiment, minimizing potential discrepancies in self-reported outcomes due to distractions (15).
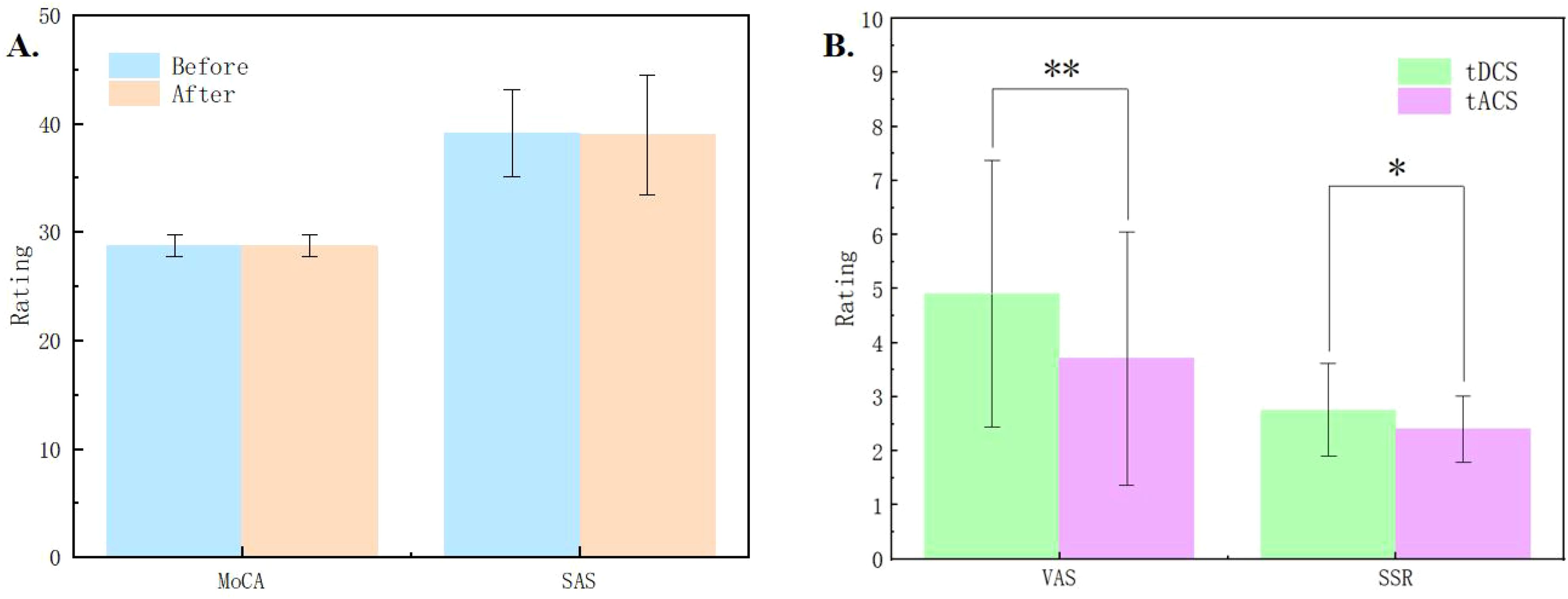
Figure 2. (A) Results of the Montreal Cognitive Assessment (MoCA) and Self-Rating Anxiety Scale (SAS) before and after the experiment. (B) Comparisons of the pain measured by Visual Analog Scale (VAS) and skin sensation rating (SSR) between tDCS and tACS. * indicates p < 0.05, ** indicates p < 0.01.
3.1 Stimulation durationFor the VAS, and SSR, AEs, AEs-rela, VAMS-R and Stroop, no significant differences were found between stimulation durations (p > 0.05), except for the fatigue item and the full of energy item of the VAMS-R (see Supplementary Table S1). Specifically, with the increase in the duration of stimulation, fatigue of subjects significantly increased (χ2 = 13.667, p = 0.008), while energy significantly decreased (χ2 = 9.906, p = 0.042).
3.2 Stimulation typeIn the experiment, we collected VAS pain ratings and skin sensation ratings of the subjects in each session. The average VAS rating was below 5 points, and the skin sensation rating was also below 3 points. The results indicated that tDCS was significantly higher than tACS in terms of both VAS (Z = 2.867, p = 0.001) and SSR (Z = 2.048, p = 0.033) ratings, and subjects felt more painful and less tolerable under the stimulation of tDCS (see Figure 2B).
For the AEs, significant differences were found between tDCS and tACS for headache, scalp pain, scalp pressure, tingling, burning sensation, trouble concentrating, dizziness, and vibration (Z = -5.976 to 5.526, p = <0.001 to 0.029). Specifically, headache, scalp pain, tingling, and burning sensation were more intense under tDCS than those under tACS. On the other hand, scalp pressure, trouble concentrating, dizziness, and vibration were more intense under tACS than under tDCS (see Supplementary Table S2; Figure 3A). Detailed data revealed that adverse events with a rating higher than half of the total value included scalp pain, tingling, and burning sensation under tDCS, as well as scalp pain, scalp pressure, tingling, and vibration under tACS.
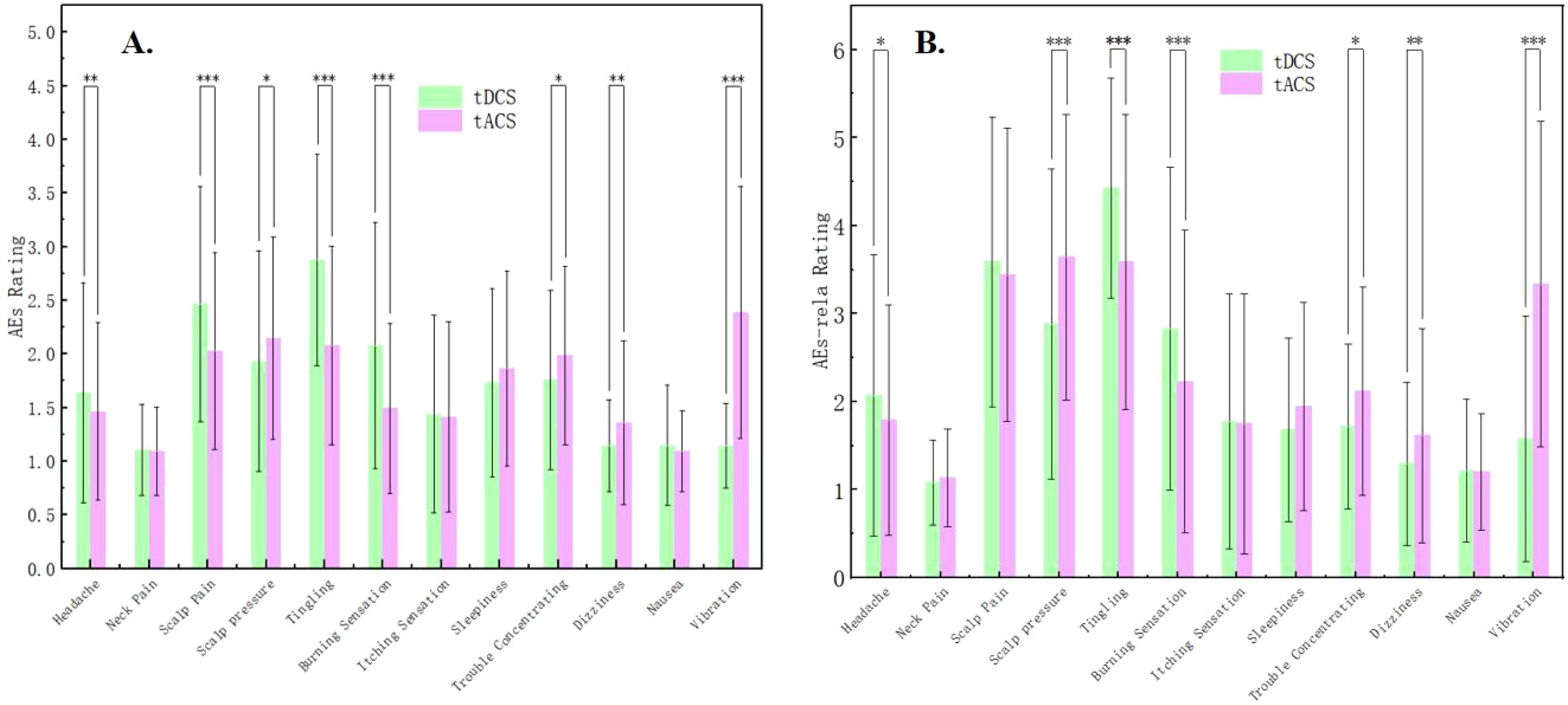
Figure 3. (A) Comparisons of Adverse events (AEs) between tDCS and tACS. (B) Comparisons of Adverse events relation (AEs-rela) of tES between tDCS and tACS. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
For the AEs-relation, consistent with those for AEs, significant differences between tDCS and tACS were found regarding headache, scalp pressure, tingling, burning sensation, trouble concentrating, dizziness, and vibration (Z = -5.569 to 4.495, p = <0.001 to 0.013), indicating that subjects believed that these adverse effects were more likely to be related to the stimulation of tDCS or tACS (Supplementary Table S2; Figure 3B). Specifically, the belief of the relation between AEs and tES was stronger for tDCS than tACS regarding headache, tingling, and burning sensation while weaker regarding scalp pressure, trouble concentrating, dizziness, and vibration.
In this experiment, the subjects were evaluated for their emotional responses after each stimulation session. The result shown in Figure 4 indicated that there was no significant difference in emotional response between tDCS and tACS. As illustrated in Figure 5, the Stroop behavioral keystroke experiment showed no significant variance across different stimulus types.

Figure 4. Comparisons of items in Visual Analogue Mood Scales-Revised (VAMS-R) between tDCS and tACS.
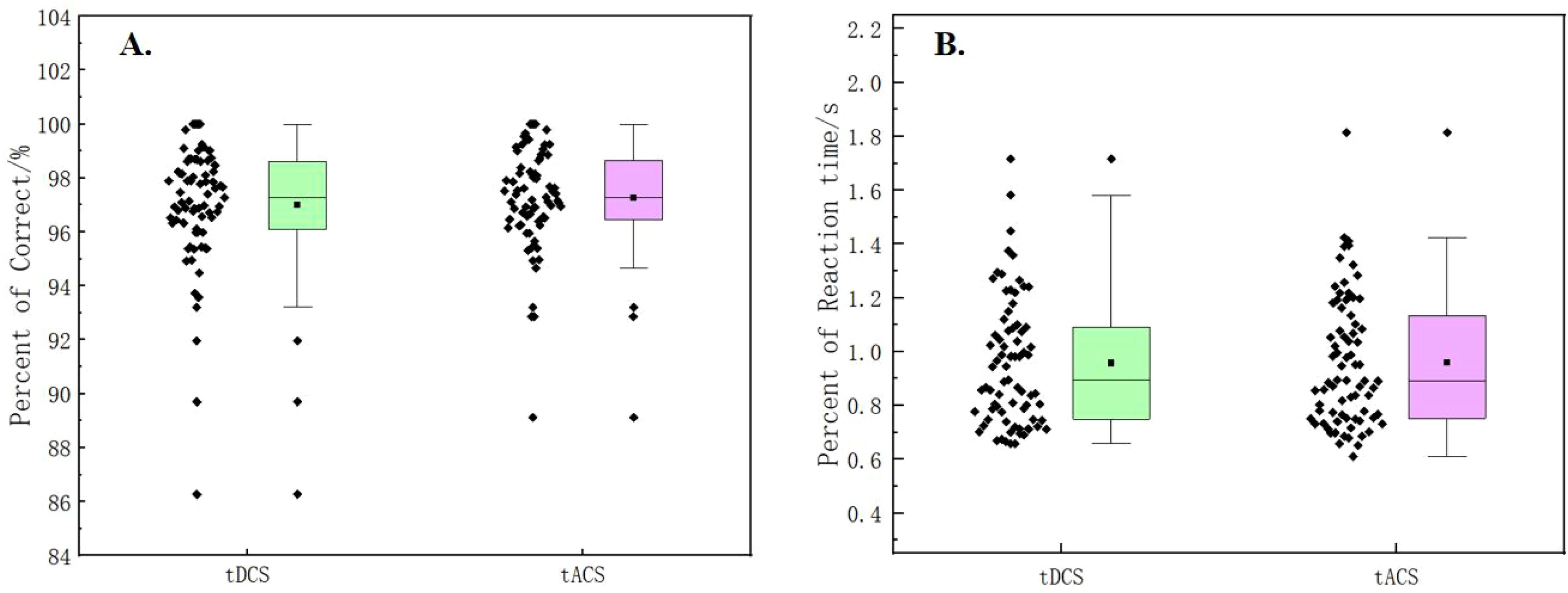
Figure 5. (A) Comparisosn of percent of correct in the Stroop task between tDCS and tACS. (B) Comparisons of reaction time in the Stroop task between tDCS and tACS.
4 DiscussionWith the advancement of technology, gel particle electrodes have gradually emerged in our field of vision. Due to their portability, comfort, hygiene, and ease of operation, they have become an important option for brain-computer interface electrodes. In this study, we examine the tolerability and safety with 2mA current of different stimulation durations and types using preset gel particle electrodes. There were no significant differences between different durations but significant differences between stimulation types, stressing on the attention to the stimulation type rather than stimulation durations when accessing the tolerability and safety.
For the influence of stimulation durations, there were no significant differences in tolerance and safety except for emotions like fatigued and energetic, indicating that tolerability and safety in this study remained stable regardless of the duration of stimulation. Since it was found that participants experienced a significant increase in fatigue and a significant decrease in feelings of being full of energy, the prolonged stimulation may have a negative impact on the emotional state of the participants, particularly in terms of fatigue. The findings suggests that subjects can adapt to tES stimulation with increasing time, which has significant implications for the design of long-term use of the device or repeated stimulation protocols. It also provides a reference for selecting experimental durations for future experimental methods and devices aiming to test tolerability and safety.
By analyzing the scores of the MoCA and SAS scales, it is evident that this experiment does not induce changes in mild cognitive impairment or anxiety state among the subjects. This indicates that both the experimental design and tES itself have no obvious harmful psychological effects on the participants. Furthermore, statistical analysis of VAS, SSR, and AEs suggests that tDCS brought about more changes in pain perception in subjects, while the vibration and other sensations brought about by tACS may be more influenced by the characteristics of the AC current waveform. However, there was no discernible difference between the two types of stimuli in terms of emotional responses and Stroop performance. Generally, the tolerability and safety of tDCS were found to be inferior to that of tACS in this study conditions.
The current study has some limitations. The study only targeted basic tES and did not investigate the potential effects of more stimulating parameters such as electrode position, duty cycle, and waveform on tolerance and safety. Limited by the total stimulus duration, this study did not include longer stimulus durations of more than 20 minutes per session. The sample size was small, and the subjects were mostly from universities, so future studies should consider selecting a larger and more diverse sample to examine differences across various subjects. Furthermore, the study was focused on the behavioral data, lacking biomarkers to further explore neurophysiological mechanisms.
5 ConclusionThe study demonstrates significant differences in tolerability and safety between tDCS and tACS when using preset gel particle electrodes, suggesting that the choice of stimulation type should be tailored to individual differences. There were no significant differences in tolerance and safety effects for different stimulation durations. It is recommended to select shorter durations when validating other transcranial electrical stimulation devices and treatment approaches to efficiently explore tolerability and safety.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statementThis study was approved by the Human Ethics Committee of the University of Science and Technology of China (IRB No. 2022KY275). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsCC: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Conceptualization, Software, Visualization. YP: Investigation, Software, Writing – review & editing. MZ: Software, Validation, Writing – review & editing. YL: Software, Writing – review & editing. MD: Validation, Writing – review & editing. MY: Writing – review & editing, Resources. TM: Data curation, Writing – review & editing. CW: Visualization, Writing – review & editing. YW: Writing – original draft, Methodology. XC: Formal analysis, Investigation, Writing – review & editing. GZ: Conceptualization, Data curation, Project administration, Writing – review & editing. XZ: Conceptualization, Methodology, Project administration, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from The National Natural Science Foundation of China (71942003, 32171080, 32100886, 32161143022, and 32200914), The Chinese National Programs for Brain Science and Brain-like Intelligence Technology (2021ZD0202101), the Global Select Project (DJK-LX-2022008) of the Institute of Health and Medicine, Hefei Comprehensive National Science Center, and Shanghai Key Laboratory of Brain-Machine Intelligence for Information Behavior.
AcknowledgmentsChatGPT-4o mini, a generative AI technology developed by OpenAI, was used to improve the readability and language of the article.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1441533/full#supplementary-material
References1. Mills KR, Murray NMF, Hess CW. Magnetic and electrical transcranial brain stimulation: physiological mechanisms and clinical applications. Neurosurgery. (1987) 20:164–8. doi: 10.1097/00006123-198701000-00033
PubMed Abstract | Crossref Full Text | Google Scholar
2. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. (2016) 127:1031e48. doi: 10.1016/j.clinph.2015.11.012
PubMed Abstract | Crossref Full Text | Google Scholar
3. Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimulation. (2008) 1:97–105. doi: 10.1016/j.brs.2007.10.001
PubMed Abstract | Crossref Full Text | Google Scholar
4. Kaelin-Lang A, Cohen LG. Enhancing the quality of studies using transcranial magnetic and electrical stimulation with a new computer-controlled system. J Neurosci Methods. (2000) 102:81–9. doi: 10.1016/S0165-0270(00)00284-3
PubMed Abstract | Crossref Full Text | Google Scholar
5. Been G, Ngo TT, Miller SM, Fitzgerald PB. The use of tDCS and cvs as methods of non-invasive brain stimulation. Brain Res Rev. (2007) 56:346–61. doi: 10.1016/j.brainresrev.2007.08.001
PubMed Abstract | Crossref Full Text | Google Scholar
6. Santarnecchi E, Muller T, Rossi S, Sarkar A, Polizzotto NR, Rossi A, et al. Individual differences and specificity of prefrontal gamma frequency-tACS on fluid intelligence capabilities. Cortex. (2016) 75:33–43. doi: 10.1016/j.cortex.2015.11.003
PubMed Abstract | Crossref Full Text | Google Scholar
7. Wischnewski M, Alekseichuk I, Opitz A. Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cogn Sci. (2023) 27:189–205. doi: 10.1016/j.tics.2022.11.013
PubMed Abstract | Crossref Full Text | Google Scholar
8. Soleimani G, Kuplicki R, Camchong J, Opitz A, Paulus MP, Lim KO, et al. Are we really targeting and stimulating DLPFC by placing transcranial electrical stimulation (tES) electrodes over F3/F4? Hum Brain Mapp. (2023) 44:6275–87. doi: 10.1002/hbm.26492
PubMed Abstract | Crossref Full Text | Google Scholar
9. Wallace D, Cooper NR, Paulmann S, Fitzgerald PB, Russo R. Perceived comfort and blinding efficacy in randomised sham-controlled transcranial direct current stimulation (tDCS) trials at 2mA in young and older healthy adults. PloS One. (2016) 11:e0149703. doi: 10.1371/journal.pone.0149703
PubMed Abstract | Crossref Full Text | Google Scholar
10. Kasten FH, Duecker K, Maack MC, Meiser A, Herrmann CS. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat Commun. (2019) 10:5427. doi: 10.1038/s41467-019-13417-6
PubMed Abstract | Crossref Full Text | Google Scholar
11. Paneri B, Adair D, Thomas C, Khadka N, Patel V, Tyler WJ, et al. Tolerability of repeated application of transcranial electrical stimulation with limited outputs to healthy subjects. Brain Stimulation. (2016) 9:740–54. doi: 10.1016/j.brs.2016.05.008
PubMed Abstract | Crossref Full Text | Google Scholar
12. Gbadeyan O, Steinhauser M, Mcmahon K, Meinzer M. Safety, tolerability, blinding efficacy and behavioural effects of a novel mri-compatible, high-definition tDCS set-up. Brain Stimulation. (2016) 9:547–54. doi: 10.1016/j.brs.2016.03.018
PubMed Abstract | Crossref Full Text | Google Scholar
14. Liu AL, Vöröslakos M, Kronberg G, Henin S, Krause MR, Huang Y, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. (2018) 9:5092. doi: 10.1038/s41467-018-07233-7
PubMed Abstract | Crossref Full Text | Google Scholar
17. Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimulation. (2013) 6:424–32. doi: 10.1016/j.brs.2012.04.011
PubMed Abstract | Crossref Full Text | Google Scholar
18. Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche M. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol-London. (2013) 591:1987–2000. doi: 10.1113/jphysiol.2012.249730
PubMed Abstract | Crossref Full Text | Google Scholar
19. Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol-London. (2004) 557:175–90. doi: 10.1113/jphysiol.2003.055772
PubMed Abstract | Crossref Full Text | Google Scholar
21. Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. J Neurosci Methods. (2010) 190:188–97. doi: 10.1016/j.jneumeth.2010.05.007
PubMed Abstract | Crossref Full Text | Google Scholar
22. Turi Z, Ambrus GG, Ho K-A, Sengupta T, Paulus W, Antal A. When size matters: large electrodes induce greater stimulation-related cutaneous discomfort than smaller electrodes at equivalent current density. Brain Stimulation. (2014) 7:460–7. doi: 10.1016/j.brs.2014.01.059
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wu XT, Zheng L, Jiang L, Huang XS, Liu YY, Xing LH, et al. A dry electrode cap and its application in a steady-state visual evoked potential-based brain-computer interface. Electronics. (2019) 8:1080. doi: 10.3390/electronics8101080
Crossref Full Text | Google Scholar
24. Gao KP, Liu JQ, Yang HJ, Liao LL, Jiang CP, Zhao N, et al. A novel bristle-shaped semi-dry electrode with low contact impedance and ease of use features for EEG signal measurements. IEEE Trans Biomed Engineering. (2020) 67:750–61. doi: 10.1109/TBME.2019.2920711
PubMed Abstract | Crossref Full Text | Google Scholar
25. Tallgren P, Vanhatalo S, Kaila K, Voipio J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clin Neurophysiol. (2005) 116:799–806. doi: 10.1016/j.clinph.2004.10.001
PubMed Abstract | Crossref Full Text | Google Scholar
26. Guger C, Krausz G, Allison BZ, Edlinger G. Comparison of dry and gel based electrodes for P300 brain-computer interfaces. Front Neurosci. (2012) 6:60. doi: 10.3389/fnins.2012.00060
PubMed Abstract | Crossref Full Text | Google Scholar
27. Liu J, Lin S, Li W, Zhao Y, Liu D, He Z, et al. Ten-hour stable noninvasive brain-computer interface realized by semidry hydrogel-based electrodes. Research. (2022) 2022:9830457. doi: 10.34133/2022/9830457
PubMed Abstract | Crossref Full Text | Google Scholar
28. Shen GC, Zheng KY, Jiang CP, Shao SH, Zhao N, Liu JQ. A gelatin-based hydrogel electrode with high moisturizing ability for wearable EEG recording. IEEE Sensors J. (2023) 23:25689–97. doi: 10.1109/JSEN.2023.3317538
Crossref Full Text | Google Scholar
29. Hu MD, Ren J, Pan Y, Cheng LP, Xu X, Tan CL, et al. Scaled elastic hydrogel interfaces for brain electrophysiology. Advanced Funct Mater. (2024). doi: 10.1002/adfm.202407926
Crossref Full Text | Google Scholar
30. Khadka N, Borges H, Zannou AL, Jang J, Kim B, Lee K, et al. Dry tDCS: tolerability of a novel multilayer hydrogel composite non-adhesive electrode for transcranial direct current stimulation. Brain Stimulation. (2018) 11:1044–53. doi: 10.1016/j.brs.2018.07.049
PubMed Abstract | Crossref Full Text | Google Scholar
31. Valter Y, Shahabuddin S, McDonald N, Roberts B, Soussou W, Thomas C, et al. Feasibility of Direct Current stimulation through hair using a dry electrode: potential for transcranial Direct Current Stimulation (tDCS) application. IEEE Eng Med Biol Soc Conf Proc. (2021) 43:1584–7. doi: 10.1109/EMBC46164.2021.9630579
留言 (0)