Medication safety has been receiving increasing attention since the World Health Organization’s (WHO) Global Patient Safety Challenge: Medication Without Harm initiative was introduced in 2017 (who.int/initiatives/medication-without-harm). Unsafe use of medicines accounts for nearly one per cent of global healthcare expenditure1,2. However, the concept of rational use of drugs is much older, with the WHO endorsing its significance in a resolution in 1986 and estimating that more than half the medicines are used inappropriately in 20023,4. As per the Organization for Economic Cooperation and Development (OECD) 2022 report5, the cost associated with preventable medication errors was 54 billion USD in OECD countries. More significantly, medication-related harm may be the cause of about 1/10th of hospitalizations and about 1/5th of patients admitted to hospitals experience medication-related harm5.
Some medicines are known to have a higher risk, requiring additional caution6,7. Moreover, this risk is variably distributed with certain patients more likely to experience medication-related harm8. Such groups include the elderly, paediatric, immune-compromised, renal and hepatic impaired patients, and patients at transition of care9-12. A systematic review by Hodkinson et al13 2020 found that three per cent of patients were exposed to preventable medication harm, with more than 25 per cent of this harm considered severe and avoidable medication-related harm occurring most commonly at the stage of prescription and monitoring of the medication use13.
Earlier studies have adequately described the burden of unsafe and irrational use of medicines globally13. There have been several publications from various parts of India estimating the burden of medication errors or irrational use of medicines arising from various policies and practices ranging between 20-60 per cent of family spending14-18. National Health Systems Resource Centre (NHSRC), India estimated that about 90 per cent of medicines consumed are inessential or irrational, and contribute to the huge out-of-pocket expenditure on health19. The Indian Council of Medical Research (ICMR), the apex medical research agency in the country, constituted a National Task Force (NTF) in 2022 to conduct research for developing possible solutions to improve safe and rational use of medicines (SRUM). A Technical Advisory Group (TAG) was constituted to advise the operations of the NTF. To ensure appropriate relevance of the planned research and facilitate effective use of resources, the TAG advised a prioritization exercise to identify solution-oriented research ideas that are relevant to India.
In this context, this study undertook a stakeholders’ consult to pool expert suggestions related to the topics relevant for undertaking research.
We considered various prioritization methods, including Delphi, Multi-Criteria Decision Analysis (MCDA), Child Health and Nutrition Research Initiative (CHNRI) and consultative approaches, among others. We found that the CHNRI method had a relatively greater transparent, replicable, systematic and structured approach that was suited to our objectives when compared to the other methods. Thus, for this study, the research prioritization exercise was adapted from the CHNRI methodology20. There have been a few such ‘setting-research-priorities’ exercises on improving medication safety, but the present work is the first from a low- and middle- income country (LMIC)21-23. This paper describes the process and results of this prioritization exercise meant to steer the course of the NTF. The objective was to identify research ideas in the field of SRUM through a survey of relevant stakeholders, and further to prioritise the research ideas using a pre-identified set of criteria.
Material & MethodsThis study was conducted jointly at the Division of Discovery and Development Research, Indian Council of Medical Research, from November 2022 to March 2023.
Operational definitionsThere are various definitions for ‘safe use of medicines’ (SUM) and ‘rational use of medicines’ (RUM), however, for the purpose of this study, the definitions adapted were to simplify the message for a broad range of stakeholders. SUM was defined for the purpose of this study as freedom from injury due to medical errors or preventable adverse events during the medication-use process24. RUM was defined as the process of appropriate use of medicines based on the patients’ need4.
Study methodologyChild Health and Nutrition Research Initiative (CHNRI) was the methodology adopted in this study. CHNRI is an established methodology for setting research priorities, helpful to funding agencies and policymakers to inform research gaps and guide resource allocation, and has been described in detail previously25,26. Further to prioritizing research ideas, the ideas received were classified into four major domains based on the question that an idea proposes and the general research design needed to answer the question: (i) ‘Description’-ideas that estimate the burden of a health condition/problem and its determinants and usually involve observational epidemiological methods; (ii) ‘Development’-ideas that involve testing a new/improve an existing intervention, usually through the design and conduct of clinical trials; (iii) ‘Delivery’-ideas that involve research aimed at delivering effective interventions to the people who need them, through implementation or operations research methods; and (iv) ‘Discovery’-ideas that generate novel interventions/new knowledge that can further be developed into potential interventions, usually involving laboratory-based studies. The classification of ideas into the four major domains was done as described by Rudan I to aid the elimination of low-value descriptive research ideas25.
Study ParticipantsIn this study, the research priority-setting exercise was developed after setting up the advisory and management teams. The management team developed the protocol for the study and coordinated its execution, while the advisory team reviewed and recommended the protocol (details in Supplementary Figure). The advisory team and management teams did not participate in the identification of ideas. In the first survey, involving the identification of research ideas, the NTF invited solution-oriented ideas using a questionnaire from various stakeholders such as policymakers, clinicians, epidemiologists, pharmacists, public health experts, programme leaders, scientists, experts from scientific organizations, medical colleges and private health organizations. Professional networks having interest and expertise in SRUM were also invited to share ideas. These included the Indian Society for Rational Pharmacotherapeutics (ISRPT), ICMR and its 31 institutes, the Nursing Research Society of India (NRSI) and the International Society of Pharmacovigilance Medication Error special interest group. In addition, 160 global and Indian researchers who have published on SRUM were identified from MEDLINE®/PubMed®. To enable the involvement of beneficiaries, ideas were invited from Indian civil society or patient groups such as All India Drug Action Network (AIDAN), WHO Patients for Patient Safety Network – India chapter and Patient Academy for Innovation and Research, India. Finally, national stakeholders who received special invitations to the survey included the Indian Pharmacopoeia Commission (IPC), the Central Drugs Standards Control Organisation (CDSCO), the National Institute of Pharmaceutical Education and Research (NIPER), the National Medical Council, the Pharmacy Council of India, the Indian Nursing Council and NHSRC.
The survey form included a section on consent before the participants shared their inputs, to allow the management team to ensure participants’ consent for reporting their ideas for academic dissemination.
Through this method, we reached out to more than 5000 potential participants directly or through networks, associations, institutions or agencies. In addition to basic demographic information, each responder was invited to share anywhere between two and six ideas each, on potential solutions to improve the SUM and RUM. The ideas received were checked for duplicates, re-phrased where necessary, and classified into various sub-themes (Supplementary Table). Some ideas that did not include enough information for classification were grouped as ‘unclassifiable’. The ideas were then stratified into two domains – SUM and RUM. Some ideas that were suggested under the RUM category had to be re-appropriated to SUM, and vice-versa based on the definition adopted for the purpose of this study.
Scoring methodologyIn the second survey that involved scoring of research ideas based on pre-defined criteria, 33 Indian stakeholders with relevant subject expertise were identified by the NTF. In addition to providing necessary instructions on the scoring method, an online training session was also conducted to sensitize the scorers. The scoring was carried out using a transparent set of five criteria (Table I) for which four response options were available: 0 (research idea unlikely to meet the criterion/NO); 1 (research idea likely to meet the criterion/Yes); 0.5 (not sure if research idea can meet the criterion); and blank (if the expert felt insufficiently informed to judge)26. Each research idea received a score against each of the five criteria by each of the 27 experts. An intermediate score for each criterion was calculated by adding up all the non-blank answers (‘1’, ‘0’, or ‘0.5’) given by all 27 scorers. This score was then divided by the number of answers received (and converted into percentages). The calculation did not consider blanks in the numerator and denominator. This approach is suitable for handling missing responses because it acknowledges that not all experts will have enough knowledge about every research option to evaluate them against all criteria. The overall research priority score percentage (RPS%) assigned to each research question was an unweighted mean of all five criteria-specific scores calculated using MS Excel. The final output of the prioritization process was a list of research ideas or questions, ranked by their scores.
Table I. Various criteria utilized for evaluation of research ideas during survey 2
Criterion Description 1. Innovativeness In your opinion, will the research idea generate truly novel & non-existing knowledge? 2. Effectiveness In your opinion, is the research idea well-defined with clear objectives & more likely to yield solutions/answers (in <3 yr) to existing problems? 3. Translational value In your opinion, will the research idea lead to interventions that are sustainable & easily scalable across multiple settings? 4. Answerability In your opinion, will the research idea yield decisive & definitive solutions/answers (in <3 yr) to existing problems than the other ideas? 5. Applicability In your opinion, does this research idea have relevance to Indian context? ResultsIn this study, out of ⁓6000 potential participants contacted in the first survey, 190 (3.8%) participants responded with research ideas. Total of 441 ideas related to SUM and 411 ideas related to RUM were received in this study. Characteristics of responders to survey 1 are provided in Table II. A vast majority (70%) were clinicians, while around 12 per cent of respondents resided outside India. After excluding ideas which were duplicate or unclassifiable, there were finally 122 and 87 unique ideas, in the SUM and RUM domains, respectively (Fig. 1). Of the 209 ideas, 67 were from descriptive research, which was of low priority, while 126 and 16 were from the development and delivery domains, respectively. There were no ideas that were classified as ‘discovery’.
Table II. Characteristics of participants in Survey 1 and Survey 2
Responders/Potential participants Identification of ideas (Survey 1) Scoring of ideas (Survey 2) 190/∼5000 (3.8%) 27/33 (81.8%) Mean age (yr) 47 ± 0.55 46 ± 1.22 Gender (%)Male: 52
Female:48
Male: 88
Female: 22
Type of stakeholder (%)Clinicians (70.5)
Pharmacist (14.7)
Others (8.4)
Patient group/ civil society (2.1)
Policymaker (2.6)
Nurses (1.6)
Clinicians (59.3)
Pharmacist (11.1)
Policymaker (11.1)
Nurses (11.1)
Others (7.4)
Patient group/ civil society (0)
Country distributionIndia: 88.4%
Others:11.6% (4 responders from United Kingdom, 3 from United States, Portugal and Colombia, & 1 from Saudi Arabia, Netherlands, Canada, New Zealand, Switzerland, Argentina, Northern Ireland, Mexico & United Arab Emirates
India: 100% Experience working in LMIC settings (%) 88.9 100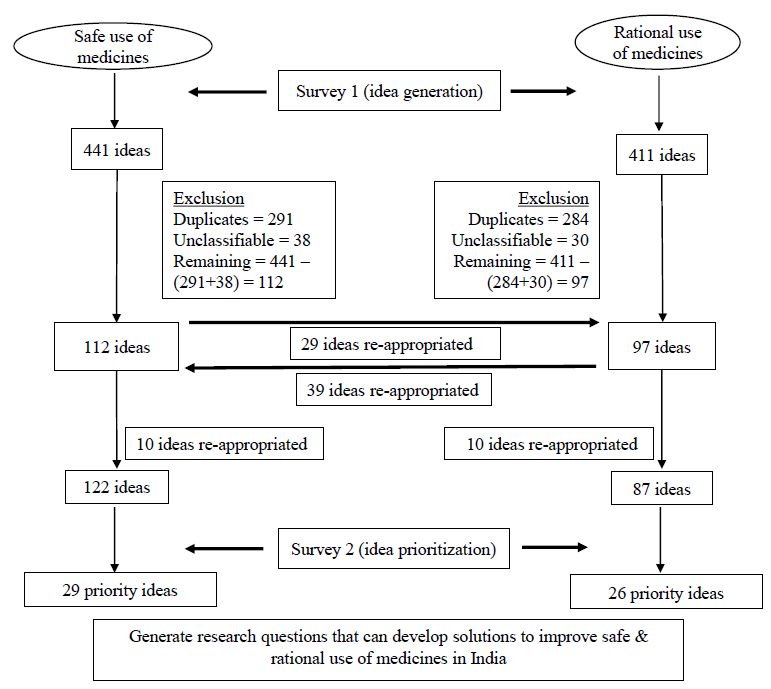
Export to PPT
Of the 33 stakeholders approached in survey 2, scores for 209 ideas from 27 stakeholders were received. The 15 ideas for SUM (Table III) and RUM (Table IV) that scored the highest RPS per cent are presented along with their criteria-wise scores. All 209 ideas that were scored in survey 2 are available in the supplementary material (Supplementary Materials 1 and 2).
Table III. Top 15 research ideas in the SUM domain based on the scores from 27 experts
Rank Research question Innovativeness score Effectiveness score Translational value score Answerability score Applicability score Overall score (RPS%)Criterion score calculated as: (sum of scores given by 27 scorers/count of scores given by 27 scorers)* 100
Maximum possible count for each criterion=135 (if all 5 criteria scored by all 27 scorers)
RPS calculated as unweighted mean of 5 criterion scores
1 Cost-effective interventions for antibiotic stewardship to promote safe prescriptions and usage 77.9 90 78.8 92 98 87 2 Estimating the extent and pattern of polypharmacy in geriatric population In India and interventions to promote safe use of drugs 67.3 87.9 88 88 90.4 84 3 Drug take-back programme in India: Policymaking, regulations, and implementation. 81.3 85.6 85.4 81.3 84 84 4 Methods for finding adverse events due to irrational use of drugs and QALY lost 82 88.5 76 81.3 90 84 5 Burden of medication errors at patients’ homes and interventions to improve them 72.1 84.3 87.5 73.9 95.8 83 6 Fixed drug combinations as an intervention in improving medication adherence and clinical outcome in cardiac multi-morbidity 74 84 80 84 93.8 83 7 Effectiveness of personal health cards/Pill cards with list of medications (in generic name) in improving medication safety in geriatric population at primary health centres and digitalization of the same with periodic updates 68.1 86.5 81.1 90.4 86.5 83 8 Impact of hospital-based drug safety alerts on the prescribing of drugs 71.1 85.6 86.5 83.2 88.2 83 9 Methods to promote rational use of antibiotic utilization in ICU in a tertiary care teaching hospital 54 90 84.6 91.7 93.8 83 10 Preventability of ADRs and drug-drug interactions in ICU patients on multiple antimicrobial agents 69.2 87 80.8 82.6 94.2 83 11 Importance of risk communication on the prevention of medication errors during transitions of care in tertiary hospitals 72.2 81.5 78.8 82.3 93.5 82 12 Effectiveness of a “How to take your Medicines desk” in improving patients’ knowledge regarding drug administration, fixed drug combinations, over-the-counter, and antibiotic resistance for improving drug safety 66 92 84 76 90 82 13 Developing guidelines for OTC medications and self-administration to ensure safe use of medicines and educating patients and pharmacists on OTC 60.3 86.5 85.2 80.8 90.4 81 14 Drug-induced liver injury and/or failure specifically focusing on DILI or liver failure due to alternative system of medicine. 72.7 82.6 87 71.7 89.1 81 15 Developing or strengthening pharmacovigilance services and patient reporting of ADR 61.5 87 80.8 76.9 94.2 80Table IV. Top 15 research ideas in the RUM domain based on the scores from 27 experts
Rank Research question Innovativeness score Effectiveness score Translational value score Answerability score Applicability score Overallscore (RPS%)Criterion score calculated as: (sum of scores given by 27 scorers/count of scores given by 27 scorers)* 100
Maximum possible count for each criterion=135 (if all 5 criteria scored by all 27 scorers)
RPS calculated as unweighted mean of 5 criterion scores
1 Mobile app-based antimicrobial stewardship intervention to align prescriptions with local antibiotic policy/STG and to assess its effectiveness to improve rational use of antimicrobials as well as user feedback using structured questionnaire. 78.8 98.1 84.6 88.1 94.2 89 2 To develop diagnostic marker of antibiotic resistance 86.5 88 88 70.1 94 85 3 Intervention study to measure the impact of behavioural intervention in prescribing antibiotics in children in primary care setting 76.1 84.1 82.6 80.4 84.8 82 4 Educational intervention to improve rational use of medicines by training all prescribers and final year medical students 59.6 90.4 87.1 86.5 88.5 82 5 Antimicrobial switch as a stewardship intervention in tertiary medical facilities 73.4 83.5 88.1 68.4 85 80 6 Study on economic impact due to irrational use of antibiotics 75 75 82.7 75.9 92.3 80 7 A multi-method tool will be developed to assess patient medication adherence for common diseases occurring in our region and measures to be taken to improve it which will help in promoting rational use of medicines 72.3 80.8 80 78 84 79 8 Steps to avoid antibiotic use in viral infections 46 84 90 82 90.1 78 9 Interventions to prescribe narrow spectrum first-line antibiotic when it is sufficient instead of starting with broad spectrum antibiotics 54.3 79.2 80.4 93.5 84.8 78 10 Interventions to avoid drug substitution in community pharmacies 62.8 77.5 79.2 78.6 90.5 78 11 Studying the extent of use of left over medicine at home by general public to determine its irrational use 74.1 82 73.9 74.2 84 78 12 Steps to improve compliance of prescriptions to the Hospital Antibiotic Policy for Specific Infections. 40 88 80.8 86 92 77 13 Feasibility and fidelity testing of prescription audit using Artificial Intelligence & Machine Learning 92 77.1 68.4 67.7 79.2 77 14 Impact of patient education about medications at transitions of care to improve reconciliation 65.2 78.3 80.4 80.4 79.5 77 15 Rational use of medicines in pregnant women in ambulatory setting 61.5 78.8 74.2 80.8 88.5 77 Supplementary Materials 1 Supplementary Materials 2In the SUM domain, the top three research topics focused on testing cost-effective strategies for the improvement of antimicrobial stewardship, safe use of polypharmacy in geriatric patients, and drug take-back policy interventions. In the RUM domain, the top three topics were on testing mobile application-based antimicrobial stewardship interventions, development of diagnostics for antimicrobial resistance, and behavioural interventions to improve rational prescription of antimicrobials in children. Across both domains, 9 out of 30 (30%) topics were related to antimicrobial resistance interventions and stewardship; 5 out of 30 (16%) ideas were related to educational/communication/behavioural interventions, while 3 out of 30 (10%) topics were related to policy/technological interventions. Ideas which had elements of both safe and rational use, were classified into safe or rational domains based on the proposed outcomes. On considering the level of interventions, 7 (23%) topics focused on household/community/primary-care level interventions.In the domain of safe use, the RPS of the top 15 ideas were relatively more clustered, with a range of 87-80, compared to the domain of rational use, where the RPS ranged from 89 to 77. Across both domains, when we analysed individual criterion scores, on an average, ‘innovativeness’ received lower scores while ‘effectiveness’ and ‘applicability’ received higher scores.
DiscussionThis research priority-setting exercise was undertaken to help steer the focus of research for the SRUM NTF by identifying and prioritizing a set of research ideas to address issues of unsafe and irrational use of medicines in India. Through this study the aim was to make the data available openly for relevant stakeholders in India to identify and develop interventions to improve safe and rational use of medicines. To our knowledge, this is the first such work from an LMIC and the first such work globally on RUM. This is also the first such work that focuses exclusively on solutions that can improve SRUM. Moreover, this research priority-setting exercise is based on the CHNRI methodology, which is a well-documented systematic process. The main advantage of this methodology is that it is transparent, objective, and replicable. The CHNRI methodology allows policymakers and funding agencies to understand the strengths as well as the weaknesses of every research priority while evaluating against predefined criteria.
The research ideas prioritized in both domains of SRUM reflect real-world challenges faced by a wide range of stakeholders such as clinicians, pharmacologists, policymakers, and researchers. Most of the top 15 ideas scored higher for their applicability and effectiveness, thus reflecting a preference for ideas with higher chances of implementation. Some of these ideas included general solutions to improve SRUM, while a few had a narrow focus on specific solutions addressing a particular issue. While antimicrobial stewardship is identified by policymakers as one of the high-priority health programmes in India, antimicrobial resistance is considered a public health problem27. One of the topics which scored high priority – development of diagnostics for antimicrobial resistance – was recommended to be dropped by the advisory team to avoid duplication of research efforts. Safe use of polypharmacy among the elderly population emerged as a priority in our analysis. It is a key consideration for India, as the country is progressively facing a rapid demographic and epidemiological transition with a significant proportion of elderly suffering from chronic morbidities and on various medications28. Communication/behavioural interventions were also scored high, underlining the crucial role of knowledge and practices of health workers and beneficiaries. Patient medication safety training and training courses for healthcare professionals were also among the top research priorities identified by the WHO exercise18, thus highlighting that these topics are of universal concern.
In our study, testing the efficacy of technological interventions was also one of the top priorities. The top research ideas for both safe and rational use of medicines address issues that need the involvement of multiple types of stakeholders. Some of the proposed interventions were targeted towards medical doctors, some were targeted at pharmacists, while few were targeted at patients, indicating the importance of multiple stakeholders in ensuring SRUM. Further, many of the top ideas involved interventions that require some degree of systems-level changes, such as health cards/pill cards, fixed-drug combinations for cardiovascular diseases and promoting rational use of antibiotics in intensive care units, among others, thus re-iterating that interventions to improve SRUM effectively will have a greater likelihood of success with the involvement of all levels of the healthcare systems in India. We would like to state here that preparing a list of the top 15 ideas in each domain was based on an arbitrary cut-off and the ideas ranked immediately below the top 15 did not differ greatly in their RPS from the earlier ideas. Further, many of the lower-ranked ideas may have contextual relevance and can be taken up in consultation with stakeholders. The finding that the scores of the top 15 ideas were relatively closely spaced (87-80) in the domain of safe use of medicines are similar to those by the original authors of the CHNRI method29, wherein they state that the collective opinion of experts regarding research ideas, expressed through categorical responses, maximises relatively quickly in terms of identifying the ideas that have the most support. This can occur simply due to the relative nature of scoring in the CHNRI process; alternatively, it can mean that a certain set of ideas were assigned higher values due to a shared belief regarding the perceived benefits of researching these ideas.
These results were then discussed in detail with the advisory team before obtaining inputs during separate meetings from the competent authority of ICMR and other relevant Indian stakeholders (including ISRPT, NRSI, AIDAN, IPC, CDSCO, NIPER, Pharmacy Council of India, Indian Nursing Council and NHSRC). All stakeholders were in general agreement with the prioritized list of research ideas and no changes were required in the list of top 15 research ideas in both SUM and RUM domains.
It is acknowledged that there could be other ways of classifying the ideas received than to group them into different themes. One such method is to group different ideas based on where they fall in the pharmaceutical value chain, highlighting every important link between the manufacturer and the patient30. In this classification, interventions such as improved product labelling will fall under manufacture or regulations, while antimicrobial stewardship will fall under prescribers/pharmacists. Some of the themes that we did not consider relevant for the purpose of this study included intellectual property, substandard medicines, development of new diagnostics/drugs, etc.
Identification of research ideas was carried out by inviting national and international experts identified through publications or relevant networks. Of these, there were only 22 non-Indian responders and 88.9 per cent of all responders had experience working in the LMIC setting. The response rate to survey one, which included the identification of research ideas, was only 3.8 per cent despite two reminders, and this may reflect the need for creating interest and awareness in the domain. Other reasons why the response may have been low were that we reached out to experts through professional networks rather than as individuals, and the response period was only two wks. However, from the responders, we received 209 unique research ideas across the two domains, which is a substantial number. However, we admit that some relevant research ideas may not have been accounted for in this list. Scoring of research ideas, i.e., survey 2, was done only by Indian scorers who could determine whether the idea was contextually relevant for India. Having said this, we understand that India is a country with a heterogeneous health system and substantial inequity in healthcare utilization. With such wide-ranging scenarios, the conclusions of the study may still not be generalizable to all Indian healthcare settings. By engaging with various relevant networks to gather ideas, a serious attempt was made to ensure regional representation and lower selection bias. One of the major limitations of the CHNRI process is that it is driven by expert opinion, which can lead to a bias in the scoring, based on the scorer`s value judgement. Other limitations in this study include those that are inherently associated with the CHNRI methodology, such as the risk of not having achieved saturation of ideas, the possibility of scoring ideas by persons with inadequate expertise in the subject, and poor quality of responses received. In this study, we attempted to mitigate the risk of missing out on important research ideas or inappropriate ranking of ideas as per the prioritization exercise, by discussing the ranked ideas with the TAG members and other relevant national stakeholders. These discussions with subject experts provided some level of independent validity to the prioritization results.
The findings of the research priority-setting exercise will help the ongoing SRUM efforts by highlighting opportunities for research. This may further help translate the priority ideas into research topics and further research questions to answer pressing problems. Suitable partners will be identified to evaluate some of these research questions to eventually help formulate policy recommendations. An interesting finding is that many of the priority ideas found in this study aligned with those of global priority, e.g., safe disposal practices31 and enhanced pharmacovigilance have been highlighted as areas of action by WHO1. Furthermore, the ideas related to the rational use found in this study such as interdisciplinary collaborations, and pharmacy workflow improvements are core components included by WHO to promote rational use of medicines3. Patient engagement, which underlines many of the top research ideas found in this study, also underlines many of the top research priorities reported by the WHO priority exercise on research into safe use of medicines18. Our findings can initiate various discussions at the national/regional level, which will ultimately help policymakers and practitioners guide their decision-making. Eventually, the NTF is expected to identify policy, technological, or educational interventions that can improve SRUM. Thus, there is a scope in the future for implementing shared solutions based on mutual learning across countries.
Overall, this study has identified and prioritized possible solutions to improve SRUM in India. Further work will entail working closely with development partners in India to translate the identified research ideas into research questions and develop and test solutions that are policy-ready and can be adopted by health systems in India.
留言 (0)