There is significant epidemiological and clinical data to unequivocally support the notion that women experience more psychiatric problems, particularly mood and anxiety symptoms and sleep disorders, at some point in their lives than men (1–3). It is also known that for some women, this increased risk might be associated with reproductive events, such as the premenstrual or postpartum periods (4, 5), in which gonadal hormone levels drastically decrease, resulting in consequences on neuronal excitability, brain metabolism, and corticolimbic processing. The menopausal transition, also termed perimenopause, is defined as the phase in middle-age during which a woman transitions from a reproductive to a non-reproductive period. Perimenopause is characterized by sudden increases and decreases in estrogen levels linked to the aging of ovaries, and the transition to a non-reproductive life; this imbalance in the hormone milieu produces intense symptoms such as hot flushes, insomnia, irritability, and low libido (6, 7). Fluctuations in estrogen levels during perimenopause have also been linked to symptoms of depression, anxiety, and mild cognitive complaints, which are particularly relevant in a subset of middle-aged women with high sensitivity to extreme changes in estrogen levels, as they might suffer from psychiatric disorders that require treatment (4, 8, 9).
Natural menopause, that is, the last menstrual cycle, occurs between 45-53 years in most women. At this age, women transition out of the reproductive stage due to a drastic loss of ovulatory function and cessation of menses. As antral ovarian follicles are reduced, the levels of follicle-stimulating hormone (FSH) rise, denoting the beginning of the menopausal transition. A group of experts from the Stages of Reproductive Ageing Workshop (STRAW) proposed the menstruation pattern as a better indicator of the peri- and menopause stages, defining three stages as follows: a) the early transition to menopause characterized by the persistence of an irregular menstrual cycle, b) the late transition to menopause characterized by a period of amenorrhea greater than 60 days in the last year, and c) early post-menopause, defined as the interval of 12-24 months after the last menstruation (10). In terms of endocrine aging, FSH increases in early perimenopause and post-menopause with fluctuating levels; this hormone peaks before the last menstruation and is associated with late perimenopause. Perturbations in FSH release result in erratic levels of estradiol (E2, the most abundant and potent estrogen produced by ovaries) and progesterone, which gradually decline until relatively low and stable concentrations are achieved (10). Notably, rapid fluctuations in E2 levels may have implications for women’s mental health, influencing factors such as the presence of depressive symptoms during the transition to menopause (4).
The endocrine and neurobiological bases that underlie the physiological and neuropsychiatric symptoms associated with the transition to menopause have been investigated in animal models that mimic the long-term effects of the absence of ovarian hormones. Several aspects of natural and surgical menopause appear to be similar between humans and other mammalian species; however, other important characteristics may be species-specific, thus limiting translational research (11). However, humans and model animals show general similarities in hormone profiles across menopausal transitions, with both showing the termination of irregular cycles, declining fertility with age, changes in low- and high-density lipoprotein cholesterol, a decline in serum dehydroepiandrosterone, and alterations in temperature-regulation systems (12). In addition, animal models respond adequately to the beneficial effects of estrogen replacement therapy (ERT), showed improvements in the alterations of bone metabolism, lipid profiles, and cognitive changes. Interestingly, in the long term, an increase in irritability, cognitive alterations, deterioration of memory processes, as well as the onset of anxiety- and depression-like behaviors have been observed in model animals, all of which support their use in research to understand the impact of the long-term absence of steroid hormones in several organs, including the brain (13, 14). Thus, the use of animal models can help to elucidate the mechanisms involved in diseases and alterations during human menopause, contributing to solving health problems at this physiological stage in women. Additionally, these models allow us to understand the physiological, endocrine, and neural mechanisms associated with the menopausal transition, and to evaluate potential therapeutic strategies to restore such alterations, which could contribute to improving the quality of life of this group of women (15–17).
1.1 Menopause and depressionDepression is the primary cause of the disability in women worldwide producing a significant impairment of their normal functioning and quality of life (1). Global studies have reported that prevalence of depression in menopause women is 35.6% (95% CI: 32.0–39.2%), ranging from 33.9% (95% CI: 27.8–40.0%) in perimenopausal women to 34.9% (95% CI: 30.7–39.1%) in postmenopausal women. These results come from studies with a high degree of heterogeneity, highlighting that higher rates of depression have been detected in the studies with poorer quality and smaller sample size, which is a limitation for interpretation of the findings (18). While multicentric cross-sectional studies have shown contradictory results regarding the relationship between menopausal status and higher rates of depression, longitudinal studies in women aged 40-55 years old have identified relationships between the naturally occurring endocrine changes in the menopausal transition with mood disturbances. Increased vulnerability to depressive symptoms has previously been reported in perimenopause with odds ratios ranging from 1.33 to 1.79 in women with a history of symptoms; this observation was replicated in women who previously had mood complaints (19, 20), suggesting that menopause may reduce the ability of women to cope with stressors, leading them to experience a higher depression symptom burden. Interestingly, structured evaluations applied to detect depression as a syndrome in a small group of premenopausal women found that the rate of depression doubled when women reached perimenopause, and tripled in postmenopause, indicating that subsets of women may suffer from more complex forms of depressive symptoms (8, 21). Additionally, surgical menopause elicited by oophorectomy has been associated with a higher risk of depression (22–24), as well as augmented levels of anxiety (25), which are linked to an abrupt reduction in sex steroid levels. Different studies have found that the patient’s age at the time of surgery, the type of surgery (uni- or bilateral), and additional extirpation of the uterus all modulate the risk of depressive symptoms. Thus, women who were younger at the time of surgery (25), in addition to women older than 51 years (26), were both found to exhibit a higher risk of affective symptoms, while an increased risk for depression (syndromic) and complaints in general health are registered in women subjected to the extirpation of the uterus and ovaries (27).
Symptoms of the climacteric period that are common among menopausal women may contribute to discomfort and recurrence of depressive symptoms (28, 29), such as hot flashes (found at a rate of 60 to 89%) (5), or sleep alterations (experienced by 23 to 42% of menopausal women) (30, 31). Psychological features in patients, such as anxiety traits and neuroticism, may also contribute to an increased vulnerability to depressive symptoms during perimenopause (32), suggesting an interaction between psychological and biological variables in resilience or vulnerability to developing depressive symptoms during menopause.
1.1.1 Hypotheses to explain depression symptoms or mood disorders in menopauseSeveral hypotheses have been proposed to explain the increase in depression symptoms or mood disorders in menopause. At the neurological level, peri- and post-menopause have both been associated with lower gray matter volume in several cortical regions and subcortical structures (such as the hippocampus, amygdala, and thalamus) that regulate emotions, concurrent with a widespread loss of white matter in the major tracts interconnecting the cerebral cortex and subcortical regions, as well as hypometabolism in the parieto-temporal cortices (33). Volumetric changes were further reported in a meta-analytic review in which post-menopausal women (age 50-70 years old) had lower hippocampal and amygdala volumes relative to premenopausal women or men (34).
Variations in estrogen levels across perimenopause have been considered as factors influencing the etiology of mood symptoms (35). Estrogens play critical roles in modulating brain homeostasis, neural plasticity, and neuroprotection. In the model of classic actions of estrogens, these steroids were found to bind to the estrogen cytoplasmic receptors of subtypes α (ER-α) and β (ER-β), which are transported to the cell nucleus to stimulate transcription, producing slow effects in different tissues. However, estrogens also exert short-term effects mediated by non-traditional rapid/non-genomic/membrane-initiated estrogen signaling in different cell types, a mechanism mediated by calcium release and protein kinases (36). Estrogens have further been found to be involved in brain remodeling through the modulation spine dendritic sprouting in areas predominantly associated with cognition, anxiety, and mood, such as hippocampal formation, cerebral cortex, lateral septum and amygdala (37–39), among others. These steroids also participate in the synthesis, release, and metabolism of neurotransmitters such as monoamines, glutamate, acetylcholine, and GABA, and thus modulate neuronal excitability. As a neuroprotector, E2 has been proven to reduce NMDA excitotoxicity by influencing rapid actions mediated by membrane receptors, as well as by increasing the transcription of anti-apoptotic proteins by traditional intracellular mechanisms (40). These oscillations in the concentrations of steroids and neuropeptides impact serotonin and noradrenaline pathways which underlie mood disorders in woman in the menopausal transition or post-menopause; conversely, hormonal therapy in perimenopausal transition decreases the transport and catabolism of serotonin (41), and protects glucose metabolism in brain regions such as the hippocampus, entorhinal cortex, medial temporal cortex and posterior cingulate which have shown deterioration in women without hormonal therapy (42).
In support of the estrogen hypothesis, studies have reported that a longer duration of exposure to natural estrogens from the menarche to menopause transition was significantly associated with a reduced risk of depression in perimenopausal women, while a similar result was found in women with a history of prolonged use of oral contraceptives (43). Furthermore, women with a history of perimenopausal depression who were treated with E2 for three weeks, subsequently switching to placebo treatment, experienced an increase in the severity of depression symptoms; however, women with a history of depression that remains in the E2 arm of the study, as well as women without a history of depression in the arm with placebo, remained asymptomatic (44).
1.1.2 Use of hormone replaced therapy to relieve depressive symptomsIn recent years, the life expectancy of the population has increased, while psychiatric symptoms have been found to greatly decrease the quality of life of women; as such, it is necessary to develop treatments that relieve depression. Women experiencing different health issues during menopause may favor estrogenic hormone replacement therapy (HRT); however, in terms of mood regulation or depression symptoms, double-blind clinical trials have provided limited evidence regarding the efficacy of HRT in reducing affective symptoms during menopause (45). However, HRT seems to be more effective at reducing depressive symptoms in women in the early transition to menopause than in those in the late transition (46), thus strengthening the notion of an opportunity window for therapeutic intervention. Evidence collected from prior clinical studies have shown that methodological differences in aspects such as the inclusion of participants with or without a depression diagnosis (versus women with depression symptoms), or conducting evaluations at different stages of perimenopause, may contribute to a positive HRT response. Considering that the peri- and post-menopause stages are associated with varying release of estrogens and gonadotrophin levels in such a period, and individuality in the response to hormones, it is not surprising to find heterogeneity in the results of HRT with estrogens on depression symptoms in menopause.
Traditional HRT may include either a single estrogen, a combination of estrogens, or a combination of estrogens plus a progestogen. This treatment has been recognized by some clinical guides as a useful and effective therapy for the control of depressive symptoms (with the limitations described above), or as an adjuvant in the management of depression with antidepressant drugs (47). New treatments for depression and anxiety symptoms during menopause include synthetic estrogens, phytoestrogens, and molecules derived from plants used in folk medicine, such as flavonoids. The primary assessment of the efficacy and safety of these therapeutic approaches, as well as the elucidation of their mechanisms of action to induce antidepressant-like effects, have predominantly been investigated in different animal models of menopause.
2 Animal models for the study of HRT and depressionIn most mammalian species, females experience natural reproductive senescence in mid- to late-life; however, most species’ lifespans do not surpass their reproductive years. However, currently, human females live one-third of their lives after passing beyond the reproductive stage (48). Some aspects of reproductive senescence are not readily accessible to humans; thus, animal models are important tools for studying events occurring in human menopause at multiple levels, including the organ, system, cellular, molecular, and genomic levels. The endocrine and neuroendocrine aspects of these model should resemble those of human menopause to ensure translational validity. This will allow the knowledge found in animal models to be translated to humans, and ensures the relevancy of the study to the biological basis of human menopause and its therapeutic interventions, including the discovery of new agents for HRT (49).
The use of non-human primates as animal models of menopause is particularly useful because of their genetic, physiological, behavioral, and reproductive similarities to humans; however, these models are costly, and the study of reproductive senescence in this model may require many years due to their long lifespan. In this regard, rodents such as mice and rats have a short lifespan of two to three-years, making them useful models to study menopause, since rodent ovaries deteriorate with age. Rodent models have been used to evaluate the effects of gonadal hormones on various body systems, including developmental and cognitive processes (48). Furthermore, the neurobiological features of rodents make them excellent models for studying the expression of stress-evoked responses, depressive-like behaviors (i.e., despair and anhedonia), and other signs that often develop comorbid with depression, such as anxiety and alert responses.
There are 3 types of menopausal models: intact aging (natural menopause), surgical menopause or ovariectomy, and accelerated ovarian failure (Figure 1). The choice of the animal model generally depends on the study objectives.
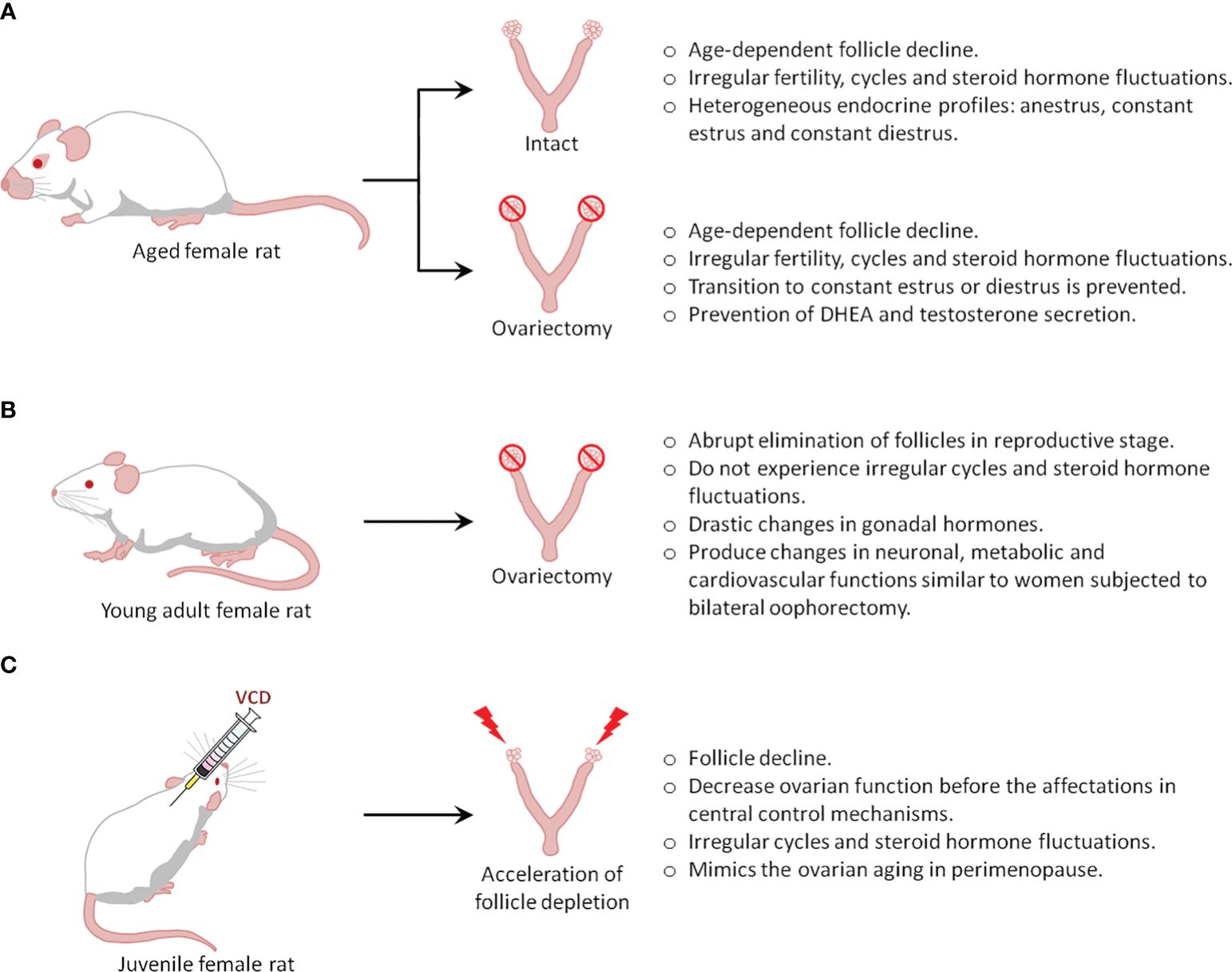
Figure 1 Types of menopause rat models.
2.1 The intact aging rat model (natural reproductive senescence)Humans and rats both follow similar stages of reproductive senescence, transitioning from regular cycling to irregular cycling to acyclicity. Further, they share multiple features and endocrine changes, including a) a decline in follicles, b) irregular cycling and steroid hormone fluctuations, and c) irregular fertility (50). In rodents, the length of their estrous cycle starts to become irregular at 8 months of age, indicating reproductive senescence, while at the age of 21 months, rats do not show cyclic changes in serum E2, progesterone, luteinizing hormone (LH), and FSH (51). The reliability of these changes in laboratory rodents and similar hormonal fluctuations in middle-aged rodents and women provides a well-defined model of irregular cycles of human menopause (49).
One drawback of this model is that rodents do not present true menopause, which is characterized by low to undetectable estrogen levels. Instead, they undergo reproductive senescence, referred to as ‘estropause’, which is instead characterized by low but persistent estrogen levels. Indeed, most aging rodents (60-70%) show a spontaneous transition to a constant estrus, characterized by sustained levels of E2 and low levels of progesterone that can last 10-100 days (50, 51). Thus, aged animals (21 months) showed well-developed ovarian follicles, but not corpora lutea, and further show higher levels of hypothalamic GnRH, pituitary FSH, and prolactin compared to estrus in 3-month-old cycling rats. Furthermore, pituitary LH is significantly lower in old rats (52). The remaining 30-40% of aging rodents transition from irregular cycling to anestrus characterized by consistently low levels of E2, progesterone, LH, and FSH (50, 51, 53); as such, these animals may represent a better model of the human perimenopause to menopause transition. Although all rodents enter a state of anestrus eventually, the period of constant estrus and anestrus may induce substantial differences in the rat brain (49). Additionally, a pseudo-gestational state (permanent diestrus) has also been described in aging rodents, as the ovaries of these animals develop corpora lutea, which may be transitory, or last for the rest of the rodent’s lifespan. Endocrinologically, this stage is characterized by low E2 and FSH levels, but the considerable production of progesterone (51, 53), a hormone that also induces permanent changes in the brain of rodents.
Overall, it is clear that rodent aging is associated with heterogeneous endocrine profiles, the least predominant being a transition to anestrus, which better resembles the human perimenopause-to-menopause transition. To increase the number of animals in anestrus, one common strategy is to ovariectomize rodents at an age when irregular cycles begin to develop, which prevents the establishment of constant estrus and diestrus in aged animals. However, this strategy introduces another variable that would differentiate the model from human menopause, caused by the loss of the ovaries, which are known to secrete hormones, including testosterone and dehydroepiandrosterone (DHEA), in menopausal women (54, 55).
2.1.1 Differences between human and rodent senescenceDifferences in reproductive senescence between humans and rodents have been determined based on neuroendocrine dissimilarities (Table 1). As women age, follicular reserves and serum estrogen and progesterone levels gradually decrease. In contrast, in aging rats, as their ovaries retain follicles throughout their lifespan, their estrogen levels do not decrease to undetectable levels; thus, the persistent estrus state is caused by a slightly elevated estrogen level, while the high progesterone levels in persistent diestrus occur secondary to increased corpora lutea activity (53). These data support the idea that the primary mechanism that ultimately results in reproductive senescence in women is ovarian follicle depletion, whereas in rats, it is an alteration in the hypothalamic-pituitary-gonadal (HPG) axis (9).
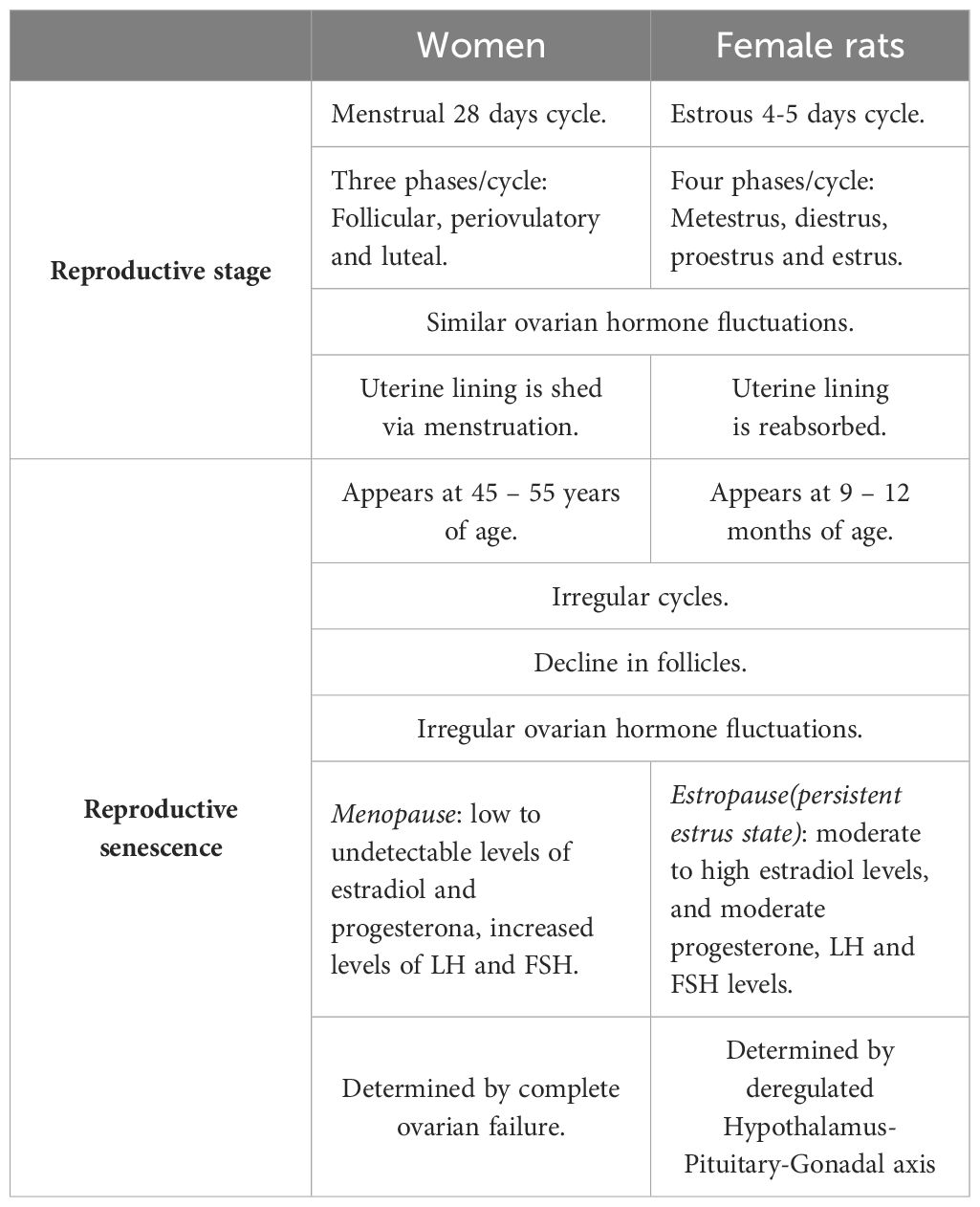
Table 1 Key differences between rodent and human reproductive senescence.
Although the basic mechanism responsible for rodent reproductive senescence differs from that of human menopause, the aging rodent model can still be used to provide insights into the general mechanisms or consequences of menopause.
2.2 Studies based on aged rat as a menopause model for the study of depressionAged rats may be an ideal menopausal model to study the role of ovarian hormones in the pathogenesis of depression and the mechanisms underlying the antidepressant efficacy of drugs and hormones. However, intact aged rats have been scarcely used for this purpose because they show complex endocrine regulation, resulting in the development several types of endocrine profiles, as stated above. Thus, to prevent these different profiles, ovariectomized aged rats are more frequently used.
2.2.1 Studies based on gonadally-intact aged female ratThe few published studies using gonadally intact aged females have provided limited information regarding the relationship between ovarian hormones, menopause, and depression symptoms; instead, these animals have been used to study the novel mechanisms of anxiolytic or antidepressant actions of drugs or E2. For example, one study showed that anxiety and depression behavior in aged females (26 m.o.) were reduced by treatment with a G protein-coupled estrogen receptor (GPER) agonist (G-1) which also could increase intracellular ER-α and ER-β (56). Additionally, one study evaluated the anxiolytic and antidepressant effects of Cannabidiol in middle-aged intact rats (13 months old) exposed to social isolation, finding that this drug reduced depressive and anxiety behaviors induced by the stressor (57). Although corticosterone has typically been associated with depressive behaviors, recent data have suggested a putative antidepressant effect of corticosterone (58). In this study, corticosterone was found to exert mild antidepressant effects in aged intact female rats. These data indicate an improvement in anxiolytic and antidepressant behaviors; however, the mechanisms are not directly related to endocrine changes associated with menopause.
In contrast to the anxiolytic and antidepressant effects observed in intact aged rats, one study found no effects of E2 and prolame (a synthetic estrogen) on anxious or depressive behavior in gonadally- intact female rats coursing with irregular estrous cycles (14 months); however, when the aged rats were ovariectomized, prolame exerted anxiolytic and antidepressant actions (59). Thus, to better understand the role of these hormones in depression and its treatment, it would be useful to compare the endocrine statuses of all animals. In this regard, one study evaluating the influence of age (reproductive senescence) on the efficacy of fluoxetine in young, middle-aged, and senescent rats in the metestrus/diestrus phase of their estrous cycle found that as female rats aged, the antidepressant effect of fluoxetine was reduced (60), suggesting that age-related endocrine changes may reduce the efficacy of fluoxetine to alleviate depressive behavior. Classic neuroendocrine studies have demonstrated that the transition from the reproductive to non-reproductive stages in rats alters the diurnal rhythm of neurotransmitter release from the mesencephalic nuclei (61, 62), suggesting that the estropause transition is associated with a generalized alteration in neurotransmitter systems, such as those relevant to the actions of antidepressant drugs.
2.2.2 Studies in ovariectomized aged female ratsConsidering the endocrine complexity of aging females, ovariectomized aged rats are most frequently used to study the role of gonadal hormones in depressive-like behaviors, while several hypotheses regarding the relationship between hormones (mainly E2), depression, and the perimenopause-to-menopause transition have been tested.
2.2.2.1 Ovariectomy induces depressive-like behaviors in aged ratsTo examine the effects of long-term ovarian hormone deprivation on the development of depressive-like behaviors, Sprague-Dawley rats were ovariectomized at 5 months of age, four months later they were subjected to 6 weeks of chronic unpredictable stress and tested in the forced swimming test (FST, an animal model of depression) at 10 months of age. Overall, this treatment increased depressive-like behavior in the FST and also induced an anhedonic state. Long-term ovariectomy impaired the negative feedback of the hypothalamus-pituitary-adrenal (HPA) axis (57). These results suggest that ovarian hormones are relevant for assessing the resilience to chronic unpredictable stress in aged female rats, and that E2 restitution in ovariectomized aged rats may confer resilience to stress and reduce depressive- or anxiety-like behaviors.
2.2.2.2 E2 produces antidepressant-like effects in aged ratsPrior research has shown that chronic E2 treatment (over 10 weeks), as well as vitamin D treatment, induces antidepressant effects in ovariectomized middle-aged rats (12 months); both compounds upregulate each other’s receptors and show neuroprotective effects (63). In the chronic mild stress paradigm, an animal model of anhedonia, a single injection of estradiol valerate produced antidepressant-like actions in ovariectomized middle-aged rats evaluated 3 weeks after surgery (64). It has also been observed that ethinyl-estradiol (a synthetic analog of E2) induces an antidepressant effect in middle-aged rats exposed to FST one week after ovariectomy (63). In contrast with these studies, data from our laboratory indicated that subacute (3 days) or chronic (26 days) E2 treatment failed to induce antidepressant effects in middle-aged rats exposed to FST three weeks after ovariectomy; however, in these studies, treatment with the synthetic estrogen prolame effectively reduced depressive behavior (59, 65), indicating that the antidepressant effects of estrogens may depend on the type of estrogen.
2.2.2.3 Antidepressant-like effect of estrogen restitution depends on timing of administrationIt has been suggested that, the antidepressant-like effects of estrogens observed in the ovariectomized aged female rat model may depend on the time of treatment initiation. Indeed, there is evidence to suggest that ethinyl-estradiol induces an antidepressant effect in ovariectomized middle-aged rats (15 months) exposed to the FST when administered at 1 week after ovariectomy, but not after 3 weeks, suggesting that the administration of ethinyl-estradiol is a crucial factor that contributes to its antidepressant-like effect (63). The relevance of the time of hormone treatment after ovariectomy in middle-aged rats has also been studied by other groups; one study compared the effect of long-term versus short-term post-ovariectomy E2 replacement and found that, in middle-aged rats (20 months), one month of treatment immediately after ovariectomy was more effective at reducing depressive behavior in the FST than E2 treatment five months after surgery, suggesting that a delay in hormone treatment reduces their favorable effects (66). The importance of the delay in receiving an estrogen treatment was recently studied in our laboratory, where experiments were conducted in middle-aged ovariectomized rats; in this study, the antidepressant effect of E2 or prolame (sub-acute treatment) was evaluated 3, 8, 16 or 24 weeks after ovariectomy, with results showing that E2 did not induce an effect at any post ovariectomy time, in contrast, prolame had antidepressant effect only 3 weeks after ovariectomy (63). In agreement with this finding, another study found that ovariectomized middle-aged rats (12 months old) that received an E2 capsule immediately after ovariectomy did not show any reduction in depressive behavior when evaluated in the FST after two weeks of treatment (67).
2.2.2.4 E2 synergizes with antidepressant drugs to alleviate depressive-like behaviorsSome researchers have hypothesized that there is a synergistic relationship between E2 and antidepressant drugs in the alleviation of depression-like behavior. Indeed, one study reported that ethinyl-estradiol synergized with citalopram (at non-effective doses) to induce antidepressant effects in ovariectomized middle-aged rats (15 months) one week after ovariectomy, as measured in the FST (63). Another study showed that the combination of ineffective doses of fluoxetine (1.25 mg/kg) and E2 (2.5 ug/rat) reduced depressive behavior in aged rats (12-14 m.o.) exposed to the FST, furthermore, the chronic treatment with the same fluoxetine and E2 doses reversed the anhedonic state in middle-aged rats exposed to the chronic mild stress paradigm (68). In contrast, a single injection of a low dose of estradiol valerate (1 mg/kg) failed to potentiate or shorten the latency of action of low doses of chronic citalopram (5 mg/kg) (64). These data suggest that estrogen presentation and/or the type of SSRI may influence the synergistic relationship between estrogens and SSRI to induce antidepressant effects in middle-aged female rats.
2.3 Ovariectomy as a model of surgical menopauseNatural menopause is a clinical term that indicates the end of the reproductive period in women; however, a similar physiological state can be induced by bilateral oophorectomy, which is commonly referred to as surgical menopause. Both types of menopause in women can cause low plasma and brain concentrations of E2, progesterone, and other steroid hormones, as well as a marked elevation of FSH concentrations. These changes can affect specific brain neurotransmitter systems and peripheral physiological processes, negatively affecting the quality of life (42). The long-term absence of steroid hormones is associated with physiological changes that predispose individuals to mood disorders such as anxiety and depression, among others (42). In natural menopause, such changes occur gradually, and require a long time to stabilize. However, in surgical menopause, they are established over a shorter period, thus influencing symptom severity (42, 69, 70).
In experimental animals, ovariectomy is commonly used to evaluate the effects of gonadal hormonal deprivation, mainly estrogens, and subsequent exogenous hormone treatments on the brain and periphery. Rodent anatomy differs from that of humans; for example, rodents have a bifurcated uterus called the uterine horn (48), and the ovaries are located at the posterolateral poles of the kidneys, each attached by a mesovarium to the dorsal body wall of the abdominal cavity (71). The selection of the method for ovariectomy is essential for the success of the surgery, particularly when the number of animals is small, and the duration of the experiment is short. In anesthetized rats, the ovaries can be removed by three methods, as described below:
1. A single midline dorsal skin incision, 3 cm long, is made between the middle of the back and the base of the tail. This allows access to the peritoneal cavity, in which the ovary can be observed surrounded by fat. Blood vessels must be ligated to prevent bleeding, after which the connection between the Fallopian tube and the uterine horn is cut, the ovary is removed, and the incision is sutured;.
2. A single ventral transverse incision is made on the middle part of the abdomen, further a small transverse peritoneal incision of 0.4 to 0.6 cm is made with surgical scalpel blade on the middle part of the abdomen slightly to the right, immediately adjacent to the second right nipple of the rat. After accessing the peritoneal cavity, the adipose tissue can be pulled until the right uterine tube and the ovary surrounded by a variable amount of fat can be identified and exteriorized by gentle retraction. This procedure is then repeated for the left ovary through the same incision. The wound is then closed in two layers (muscle and skin) using sterile sutures, the peritoneum and muscle with an absorbable suture, and the skin with a non-absorbable suture;.
3. A double dorsolateral incision is made approximately 1 cm long above the ovaries, with the use of dissecting scissors. The skin is cut, allowing access to the dorsal muscles and peritoneal cavity. Subsequently, the operation is performed in the same manner as modality 1. The muscle incisions do not require suturing, and skin wounds can be closed bilaterally with a single catgut suture (72, 73). Full recovery from ovariectomy can be achieved within one week. The reported advantages of transverse incisions in abdominal surgery include less pain and a low incidence of hernia formation (72–74).
Experimental interventions occur either at the time of ovariectomy or commence once E2 has reached a low to undetectable level in the plasma, which typically occurs within 1-2 weeks. Thus, estrogen can be administered after ovariectomy, alone or in combination with other ovarian hormones, with variations in the type of estrogen administered, dose, route of administration, and treatment duration, while controlling for interactions with endogenous steroid hormones since the ovaries have been removed (48).
2.3.1 Ovariectomy induces depressive-like behaviors in young adult ratsPreclinical studies have shown that ovariectomy in the medium- and long-term reduces the plasma and brain concentrations of steroid hormones and other molecules, which can negatively impact brain function (75, 76). Similar to endocrine changes in women, FSH levels increase with time following ovariectomy in mice, and are higher at 4 weeks than at 1-2 weeks (77). In contrast, progesterone and E2 levels are lower at 4 weeks than at 1-2 weeks post-ovariectomy and reach undetectable levels at 3 and 15 months post-ovariectomy, respectively (77, 78). Notably, these hormonal changes have been associated with an increase in anxiety- and depression-like behaviors, which depend on the time elapsed after ovariectomy, a phenomenon termed the ‘post-ovariectomy timeframe’. Anxiety-like behavior was higher in rats at 12 weeks than at 3 weeks post-ovariectomy (79); interestingly, no significant changes on anxiety-like behavior with respect to rats in proestrus-estrus and metestrus-diestrus phases were observed 1-week post-ovariectomy; however, 3 weeks post-ovariectomy, high anxiety-like behavior was detected in comparison with that of rats in the proestrus-estrus phase. Similar levels of anxiety-like behavior were detected in rats at 6, 9, 12, and 15 weeks post-ovariectomy (80), which coincided with a reduction in E2 and progesterone levels. Moreover, in the FST, an increase in total time of immobility was detected 2 weeks post-ovariectomy (81), which is considered to indicate a depressive-like state; however, this behavior was higher at 6 weeks post-ovariectomy in rats in the proestrus-estrus and metestrus-diestrus phases, and was maintained at high but similar levels in rats at 9, 12, and 15 weeks post-ovariectomy (14, 80). Anxiety- and depression-like behaviors are associated with a reduction in the number of Fos-immunoreactive cells in the lateral septal nucleus, a brain structure involved in the physiopathology of affective disorders, as well as the therapeutic effects of antidepressant drugs (82). It is important to mention that changes in Fos immunoreactivity in lateral septum cells and anxiety- and depression-like behaviors associated with long-term ovariectomy in rats were reversed by E2 treatment (80), highlighting the role of these limbic structures (and possibly their connections) in the regulation of stress-linked affective-like behaviors in individuals with depression associated with sex hormones.
2.3.2 E2 produces antidepressive-like effect in ovariectomized young ratsAs ovariectomy induces drastic changes in neuronal, metabolic, and cardiovascular functions that emulate those shown by women subjected to bilateral oophorectomy in our laboratory and many others, we have used the ovariectomy model in young adult female rodents to understand the effects of ERT on depression-like behaviors and their underlying mechanisms (65, 83, 84). In investigations of the antidepressant-like effects of hormonal agents, researchers have used different tests to reveal behavioral and neurochemical disturbances related to clinical depression. In this regard, FST mice and rats eventually develop passive behavior (immobility) that reflects despair. Hormones and drugs with antidepressant-like activity show reduced immobility and increased active behavior (14, 85, 86). Ovariectomy increases depressive behavior in young adult rats; however, E2 replacement at physiological levels can reduce this behavior (87). Furthermore, studies using FST have confirmed that the antidepressant effects of estrogen depend on the type of estrogen, age, endocrine condition, and the initiation time of estrogen replacement after the estrogenic decline (88), termed the post-ovariectomy time frame. Clinical and preclinical studies have determined that the variability in the response to ERT may be partly due to a critical period or window of opportunity during which ERT must be started to alleviate depressed mood (89, 90).
2.3.3 Antidepressant effect of estrogen depends on timing of administration in young adultEstrada-Camarena et al. (91) found that the immobility behavior observed in young adult ovariectomized rats was reduced by acute E2 treatment when applied one or three weeks after surgery, but not after 12 weeks. In contrast, an acute treatment with the synthetic compound 17α-ethynyl-estradiol induced antidepressant-like actions even 12 weeks after ovariectomy, suggesting that the physicochemical features and bioavailability of each estrogen may modify their effectiveness as antidepressant drugs in a particular time frame of hormone deprivation (92). According to this notion, results obtained from our laboratory using a synthetic estrogen program have indicated its potential to reduce despair usefulness timeframes in which E2 is not further effective, particularly in middle-aged rats (59, 65). Together, these findings show that the antidepressant-like effects of estrogens in the model of surgical menopause are modulated by variables such as age and post-ovariectomy time frame, and the therapeutic potential of estrogens such as ethinyl-estradiol and prolame has been revealed in studies on the time frame of ovariectomy.
In this regard, the window of opportunity is related to the effects of estrogen on the structure and function of brain regions that regulate mood. One study carried out on rats ovariectomized with long hormone deprivation frames at 9- and 15-months showed increased dendritic spine density in the CA1 in response to E2, whereas longer hormone deprivation frames (19 months) did not (93). The loss of E2 efficacy in the 19-month post-ovariectomy group could be attributed to the duration of E2 deprivation rather than age, as old rats ovariectomized at 20 months of age responded to E2 replacement one month after ovariectomy when the rats were 21 months old (93). While structural changes in the CA1 region can be generalized to mood regulation, the behavioral results in the FST with E2 are contradictory (65, 66). This situation opens the opportunity to explore different aspects related to the HRT treatment of depression in menopause, such as the interplay between the post-ovariectomy time frame and different estrogen treatments, the metabolism of synthetic estrogens with potential as HRTs, and the role of additional brain areas or circuits involved in menopausal depression.
Recent studies using an ovariectomized rat model have been conducted to evaluate other substances with promising effects, thus expanding the therapeutic possibilities for alleviating depression in women (Table 2). In these studies, ovariectomized rodents treated with antidepressant drugs such as maprotiline, hormones such as progesterone and allopregnanolone, phytoestrogens like puerarin and genistein, dietary isoflavones, and flavonoids such as chrysin showed reduced depressive-like behavior (95–99) in the FST. Interestingly, at 4-weeks post-ovariectomy, rats treated with curcumin (100 mg/kg/4 weeks), a secondary metabolite extracted from Curcuma longa, showed reductions in the production of inflammatory cytokines and reestablished concentrations of dopamine, serotonin, and noradrenaline in brain structures related to depression (100). These beneficial effects were similar to those produced by E2 (100 μg/kg) and the antidepressant drug fluoxetine (20 mg/kg). Similar effects have been reported in 8–12 weeks post-ovariectomy rats treated with the flavonoid chrysin (1 mg/kg), which reduced the total time of immobility in the FST, similar to 1 mg/kg progesterone and 1 mg/kg allopregnanolone (96). These data show the utility of long-term ovariectomy on understanding the neurobiological basis underlying the long-term absence of ovarian hormones produced by ovariectomy and the identification of potential therapeutic substances for the treatment of depression symptoms associated with surgical menopause (14).
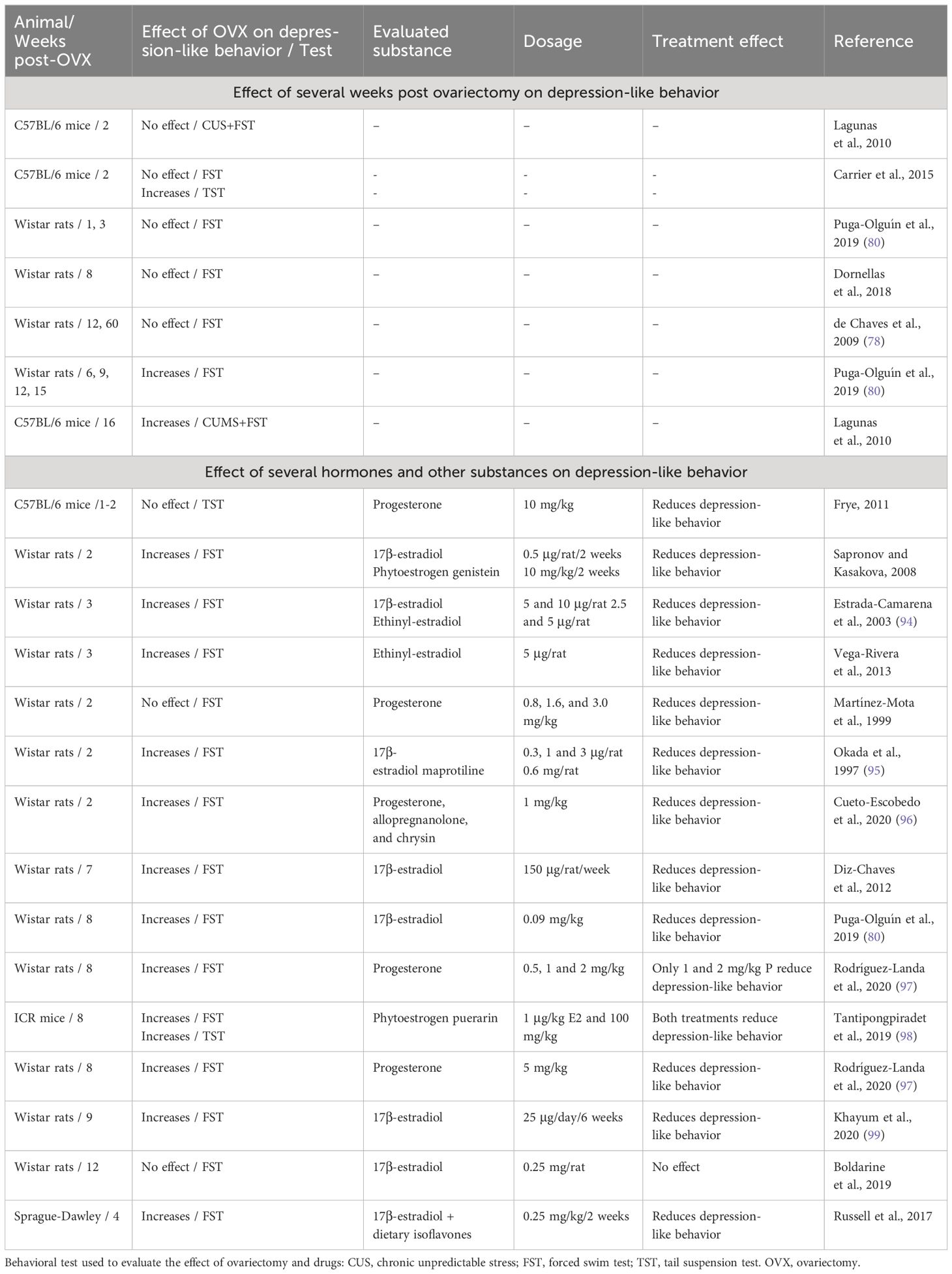
Table 2 Effects of timing post-ovariectomy and some pharmacological treatments on depression-like behaviors in mice and rats.
Although ovariectomy appears to be the easiest model for conducting studies in rodents, this evidence demonstrates that this condition courses with dynamic changes at the molecular, cellular, and structural levels that modify the behavior and response to different stimuli. Knowledge of these processes is critical for the selection of a specific post-ovariectomy timeframe according to the study objectives. Evidence suggests that such a process may reveal or veil the effects of treatments, such as restitution with estrogens. In addition, the ovariectomy model has some limitations because the majority of women during the transition to menopause preserve their reproductive system intact, which must be considered when carrying out translational research. Second, the most common model of ovariectomy is in young adult animals, which usually exhibit regular estrous cycles before extirpation of the ovaries, suggesting important differences in the hormonal profile and regulation of the HPG axis compared to aged gonadally intact females. Finally, the age at which ovariectomy is performed and the period of hormonal deprivation (post-ovariectomy time) in rodents must be considered, as these factors may influence the sensitivity to hormonal treatment.
2.4 Accelerated ovarian failure as a model of menopauseAlthough they show great utility, the aged rat and ovariectomy models were unable to replicate all of the features of the transition to menopause experienced by middle-aged women. For example, the aged rat model does not allow the dissociation of the alterations related to the transition from those due to aging, whereas the ovariectomy model prevents females from gradually reducing ovarian function, as it favors the abrupt depletion of estrogens. This situation has generated an urgency to develop more adequate models that emulate the neuroendocrine changes and health risks linked to perimenopause. Certain classes of substances have been shown to produce early ovarian failure in women (101). One such compound is 4-vinyl cyclohexene diepoxide (VCD), a metabolite of 4-vinyl cyclohexene (VHC), used in the industrial production of diepoxides and epoxy resins. VCD is an organic volatile compound with alkylating properties that appears to be more potent than its parent compound in producing ovotoxic effects, suggesting that this metabolite is the active form of VHC (101). Two long-term studies have identified that both toxicants induced ovarian and uterine atrophy, accompanied by the loss of ovarian follicles and corpora lutea in female mice, indicating that infertility may occur as a result of the rapid exhaustion of primordial follicles. Notably, these studies identified that rats are sensitive to VCD, but not to VHC, which suggests that differences between species are probably associated with their metabolism (102).
It has previously been shown that VCD favors atresia of the primordial and primary follicles through the activation of apoptotic pathways involving the Bcl-2 family and caspase-3, as well as the mitogen activated protein kinase family (103, 104). Furthermore, studies using culture of ovaries of rats at 4PND, which are rich in primordial and primary follicles, identified that the KITLG neurotrophic factor reduced the ovotoxicity of VCD, which binds to its oocyte-associated receptor KIT, stimulating downstream signaling, whose function is oocyte growth and follicle survival. In vitro assays have further demonstrated that VCD directly inhibits autophosphorylation of the KIT receptor located on the plasma membrane of the oocyte, interfering with this signaling and producing atresia (105, 106).
2.4.1 Ovarian failure induced by VCD in female rodentsThe most common treatment for ovarian failure in mice and rats is the intraperitoneal administration of VCD at doses ranging from to 80-160 mg/kg, over at least 15 days (107, 108). Depending on the dose and duration of VCD treatment, lengthening of the estrous cycle may be observed, accompanied by fluctuating levels of E2, which, after a certain time, tend to decrease, while the levels of LH/FSH increase (109, 110). Interestingly, ovarian failure is elicited before an increase in gonadotropins can detected, confirming that the primary target of VCD is the ovaries (104, 108). A VCD-induced menopause model in rats has been recently explored, with longitudinal studies identifying an endocrine pattern similar to that experienced in women in perimenopausal transition. To summarize, at 100 days after treatment, VCD produced low levels of progesterone and anti-Mullerian hormone, reduced levels of androgens, no changes or increased levels of E2, and no changes in FSH and corticosterone levels (111). Changes in gonadal and pituitary hormones related to follicle depletion may account for the abnormalities in the behavior of rats evaluated using different tests of depressive-like behavior.
Studies in juvenile (usually postnatal day [PND] 28) female mice and rats have demonstrated that repeated doses of VCD administered over a longer period are necessary to induce the complete loss of reproductive capacity; females showed a significant reduction in primordial and primary follicles after treatment for 10 days, while all primordial and primary follicles were lost after a treatment of 20 days (107). In turn, doses administered over long periods also significantly reduced the onset of ovarian failure from 135 days post-treatment to 52 days post-treatment (107). This generates a significant reduction of time in the establishment of a menopause-like stage, relative to natural decline of ovarian function in middle-aged females. In addition, the identification of different stages of menopause (pre-, peri-, and post-menopause) in the model of VCD-induced ovarian failure would allow the characterization of neurobehavioral disturbances and their associated mechanisms at specific points in time, which is a frequent limitation of studies enrolling women. A possible restriction in the interpretation of the results is that natural estropause occurs at older ages, mainly in rats; hence, the earlier onset of ovarian failure induced by VCD appears to be indicative of the induction of premature or precocious menopause. Interestingly, recent reports have described that VCD can also induce ovarian failure in adult and middle-aged females (48, 109, 112), which strengthens the validity of VCD administration in modeling menopause.
2.4.2 VCD-induced ovarian failure is related to depressive-like behaviors in rodentsIn support of the notion of the existence of perimenopausal stage in the VCD model, mice treated with VCD developed abnormalities in the locomotion in the open field test, showing reductions in the time spent in the center and the distance traveled in the open field, which is indicative of high levels of anxiety. These females also showed high levels of corticosterone after exposure to the test and relative to the controls without VCD (113), revealing a facilitation in the HPA axis in peri-estropausal females in challenging conditions. Another study was designed to evaluate the time course of neurobehavioral disturbances in mice at 20, 35, and 52 days after treatment with VCD. A time-response effect was detected in the expression of depression-like behavior in the tail suspension test; females had higher levels of immobility for 35 days post VCD treatment, an effect that was maintained at 52 days post VCD treatment. In turn, high anxiety levels in the plus maze test were observed at 20 days post-VCD treatment relative to the control, gradually reducing to lower levels at 52 days post-treatment. Alterations in sleep were also observed in VCD-treated females, showing a reduction in REM sleep during the inactive and active periods. In these females, a remarkable reduction in the percentage of time spent in proestrus manifested mainly at 52 days post-VCD treatment, together with a decrease in ovarian and uterine indices (110). Similar to mice, a transversal study in rats treated with VCD showed that females exhibited a lower number of entries into the open arms of the elevated plus maze relative to controls, and increased time in the corner of an open field test, expressed in a consistent manner, and increased anxiety-like behavior in different tests (112, 114). Altogether, these findings strengthen the idea that VCD model rodents display set of signs that emulate menopausal mood symptoms.
Healthy cognitive systems are required to face daily life events. Hence, alterations in cognition may represent a challenge for women transitioning to menopause, increasing the risk of developing affective disorders. One study designed to induce follicular failure in middle-aged rats showed that females experienced an impairment of working memory that started in the early transition, which was amplified in mid- and post-follicular depletion. This observation attributed to reproductive perturbations rather than aging, as untreated age-matched controls did not exhibit cognitive failures (49). Longitudinal evaluation of these females revealed that in the post-follicular condition, middle-aged rats retained the impairment of working memory relative to younger VCD-treated animals, suggesting additive effects between age and ovarian failure on hippocampal-dependent memories. Interestingly, cognitive flexibility is not affected in younger or older females (49), indicating that some cognitive domains are more affected by the interruption of ovarian function than others. The results also implied that the different stages of transition to a non-reproductive stage had an impact on the severity of neurobehavioral disturbances.
With due caution, these abovementioned signs observed in VCD-treated females may support the notion of a causal relationship between the cessation of ovarian function and the cognitive, neurobehavioral, and mood symptoms experienced by women undergoing menopause.
2.4.3 Effect of E2 in the depressive-like behaviors induced by VCDStudies in rats have shown that HRT can reduce depression-like signs in females with ovarian failure. For example, middle-aged VCD rats (11-12 months-old) were treated for 21 days with E2 plus levonorgestrel, with results showing that this treatment regimen improved the entry of females to the center of an open field, indicating a reduction in anxiety, while combinations of E2 plus levonorgestrel and E2 plus progesterone were effective in reducing immobility behavior in the FST, denoting antidepressant-like effects (114). In addition, the cognitive evaluation of middle-aged VCD rats showed divergent results related to the E2 treatment schedule. Tonic administration of E2 via an osmotic pump over 12 days improved learning in 11-to 12-month-old rats (115); however, daily E2 injections were ineffective at improving learning in the water maze (114). The combination of E2 and levonorgestrel, but not E2 plus progesterone, was found to improve learning relative to vehicle, E2, progesterone, or levonorgestrel alone (114). These results indicate the important additive effects of estradiol and levonorgestrel in the treatment of depressive-like signs and cognitive symptoms in menopause. Levonorgestrel, a synthetic progestin, is a 19-nortestosterone derivative used in emergency contraception. Similar to progesterone, levonorgestrel exerts a significant androgenic activity (116), which may account for the behavioral differences detected in middle-aged females with ovarian failure. Altogether, these findings suggest that some hormone combinations used in menopausal hormone restitution may be more successful in managing affective symptoms in women with follicle-depleted ovaries than in those with surgical menopause.
2.4.4 Effect of E2 in brain targets in the model of VCDE2 interacts with the serotonergic system through several pathways to modulate depression (117). In the VCD model, ovarian failure was found to be related to the reduced expression of ER-β mRNA and tryptophan hydroxylase (TPH) immunoreactivity in the dorsal raphe nucleus (DRN), as well as low levels of serotonin in dorsal hippocampus, which were increased with a treatment of E2 (21 days, pellet implant) (118). One study further analyzed the changes in the CA1 hippocampal region in VCD-treated mice, which showed a reduced number of spines and terminals expressing ER-α with respect to the controls treated with the vehicle (119). ER-α is critical in the rapid regulation of NMDA receptors to facilitate neuroplasticity and neuroadaptation to environmental changes. Indeed, one study demonstrated that VCD reduced the serum levels of E2 and progesterone in follicle-depleted female mice, as well as serotonin synthesis in the DRN and the number of fibers arriving at the basolateral amygdala (120). These changes were accompanied by the facilitation of long-term potentiation mediated by glutamate release in the amygdala, which was restored to normal levels following treatment with E2 (120). It would be interesting to investigate the effect of a combination of E2 with progesterone and levonorgestrel, or even the effect of new estrogens proposed to regulate depression in menopause, serotonin levels, and ER in brain structures related to depression and mood regulation. Finally, questions regarding whether different estrogens in combination with antidepressant drugs are effective in reducing depression in females with accelerated ovarian failure remain unanswered.
3 DiscussionPreclinical studies have shown that the antidepressant-like effects of different estrogen treatments may vary depending on the animal model of menopause used in the investigation. The most consistent findings on the therapeutic potential of estrogens in depression have been found in the ovariectomized rat model, with E2, estradiol valerate, ethinyl estradiol, and prolame (63, 68, 84, 94). These effects were found independent of female age, as rats ranging from 2.5 to 15 months of age showed a response to estrogens including a reduction in despair or anhedonia. Preclinical studies have further suggested that factors such a
留言 (0)