Ovarian dysfunction, primarily attributed to endocrine disorders, manifests as menstrual irregularities and, in severe cases, infertility (1–5). In the last few decades, hormone replacement therapy (HRT) has been employed as an intervention for individuals grappling with ovarian dysfunction. Nevertheless, prolonged usage of HRT is associated with heightened risks of breast cancer, coronary heart disease, and venous thromboembolism. Furthermore, its efficacy in enhancing clinical pregnancy rates remains insubstantial (6, 7). Women of reproductive age frequently encounter the development of polycystic ovaries attributed to hormonal imbalances, resulting in delayed follicular development (8, 9). Additionally, stress and various factors affecting ovarian function can contribute to conditions such as diminished ovarian reserve (DOR), premature ovarian insufficiency (POI), and even premature ovarian failure (POF) (10). As women age, ovarian function naturally diminishes, leading to the onset of perimenopausal syndrome (PMS) and significantly impacting their daily lives (11, 12). The array of ovarian dysfunctions mentioned earlier constitutes factors associated with endocrine disruptions in women, resulting in compromised oocyte quality, diminished fertility, and premature aging.
Presently, acupuncture stands as a widely utilized intervention for addressing abnormal ovarian function, attributed to its well-tolerated side effects (13, 14). Acupuncture has been found to improve ovarian function, promote ovulation, relieve pain, ease emotional anxiety, and even delay diminished ovarian function (15–20). Extensive research has indicated that acupuncture not only enhances ovarian function and fosters ovulation but also provides relief from pain, mitigates emotional anxiety, and has the potential to postpone diminished ovarian function (21). Animal experiments and the subsequent integrated data analysis derived from these experiments can, to a certain extent, yield valuable a priori information. This information proves instrumental in guiding experimental or trial design and, in some cases, even informs clinical decision-making. The meta-analysis of preclinical animal studies plays a pivotal role in translational medicine, contributing to the development of more precise medical treatments (22, 23). While animal experiments offer valuable insights, their limitations include a low repetition rate of results. In an effort to delve further into the efficacy of acupuncture and minimize unnecessary attrition of animals in experiments, this study strategically categorized the PCOS animal model as a high-response group for ovarian function, while DOR, POI, POF, and PMS were collectively considered a low-response group for ovarian function. The aim was to investigate the effectiveness of acupuncture as a standalone intervention across different abnormal ovarian functions, providing a precise theoretical foundation for the acupuncture treatment of ovarian dysfunction diseases. Notably, this study protocol has been prospectively registered with Prospero (CRD42022316279).
2 Materials and methods 2.1 Search strategyThe search spanned from the inception of databases until November 2023, covering the Chinese National Knowledge Infrastructure (CNKI), Wanfang Data (WFDB), VIP Information Database, China Biology Medicine (CBM Disc), as well as international databases including PubMed, Embase, Cochrane Library, and Web of Science. Employing a combination of subject terms and free words, the search aimed to comprehensively capture relevant literature. Additionally, a manual search for references in pertinent literature and reviews was conducted (refer to Appendix 1) to ensure a thorough examination and avoidance of any potential omissions.
2.2 Inclusion criteriaThe research was considered eligible if it met the following criteria:
1. Female Sprague Dawley (SD) rats were utilized as animal models for PCOS, POF, POI, DOR, and PMS.
2. Implementation of either simple acupuncture or electroacupuncture as the intervention.
3. Formation of experimental groups comprising both the model and acupuncture cohorts, with the control group consisting solely of the model group. In instances where included articles featured more than two groups, only the acupuncture and model groups were retained.
4. Assessment encompassing at least one of the following outcome indicators: luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (T), estradiol (E2), anti-Müllerian hormone (AMH), LH/FSH ratio, ovarian weight (OW), ovarian volume (OV), and follicle number (FN). Notably, all hormone tests were conducted using serum samples.
2.3 Exclusion criteriaThe research that satisfied any of the following conditions are excluded:
1. Disruption of ovarian tissue morphology during the modeling process.
2. Utilization of acupuncture or electroacupuncture as a pre-treatment intervention before or during the construction of animal models.
3. In cases where data were duplicated across multiple instances, only the complete study was considered.
4. Studies lacking sufficient data were also excluded.
2.4 Selection of studiesTwo reviewers (W.H.J. and L.L.Y.) independently performed literature screening, data extraction, and quality assessment for the trials. In cases of disagreement, a third party (Z.Y.M.) was consulted, and a consensus was reached. Titles and abstracts were meticulously reviewed, with articles not meeting the eligibility criteria being excluded. In instances of missing data, attempts were made to acquire the necessary information by reaching out to the corresponding authors via email. For articles presenting data solely in image format, two independent authors (L.Y.) utilized the webplot digitizer (https://apps.automeris.io/wpd/) to extract mean difference (MD) and standard deviation (SD) values from the images. The extracted data were cross-checked, and any disagreements were resolved through consultation with a third party (J.L.X. and L.Y.).
The characteristics and general information were systematically extracted and organized in tabular form. The extracted data encompassed details such as authors, publication date, animal species, intervention methods, modeling techniques, and study outcomes.
2.5 Bias risk and quality evaluationThe chosen studies underwent evaluation based on the guidelines outlined in the Animal Research: Reporting of In Vivo Experiments (ARRIVE) protocol (24, 25). To gauge the quality of each study, a ratio of the obtained score to the maximum possible score was calculated. This process resulted in three distinct quality intervals: studies scoring between 0.8–1 were categorized as “excellent,” those with scores between 0.5–0.8 were deemed “average,” while scores below 0.5 were categorized as “poor” (21, 26, 27).
The risk of bias in the selected studies was assessed using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) tool (28). Each item within the tool was categorized as “yes,” “no,” or “unclear” to assign scores to the respective articles. Studies with at least one item identified as having a high risk of bias were classified as having an overall high risk of bias. Those with unclear risk of bias for at least one item were considered to be at an unclear risk of bias, while studies demonstrating low risk of bias across all items were rated as having a low risk of bias.
Two authors (Z.Y.M. and H.J.H.) independently conducted the quality and risk of bias assessments. Any discrepancies were resolved through discussion or, when necessary, by seeking the judgment of a third expert (L.Y.) until a consensus was reached.
2.6 Statistical analysisIn this study, serum sex hormone levels, ovarian weight, ovarian volume, and the number of follicles in both the disease and intervention groups were treated as continuous data in comparison to the control group. Standardized mean difference (SMD) was employed to evaluate the outcome indicators. The meta-analysis for each serum sex hormone level and ovarian morphology was conducted using Stata 15.0, with a confidence interval (CI) set at 95%.
If the P > 0.10 and I2 < 50%, indicating minimal heterogeneity among studies, the fixed-effects model is selected. Conversely, if these conditions are not met, the random-effects model is employed. Due to insufficient data on the main factors, we refrained from conducting subgroup analyses to investigate heterogeneity at this stage (29). However, we intend to perform future subgroup analyses, exploring variables such as different acupuncture protocols, shorter (≤2 weeks) or longer (>2 weeks) durations, and variations in animal model species.
For outcome indices with 10 or more included studies, we will conduct sensitivity analyses to explore the causes of heterogeneity. Additionally, Egger's method will be employed to test for publication bias. If the P < 0.05, indicating the presence of publication bias, appropriate measures, such as trimming or adjustments, will be considered to address the identified bias.
3 Results 3.1 Results of the searchA total of 1,093 publications were initially identified. Following rigorous selection criteria, 29 articles, encompassing a total of 623 rats, were ultimately included in the present meta-analysis. The distribution of these studies comprised 18 articles focused on PCOS, five on POF, three on PMS, zero on DOR and an additional three on PMS (Figure 1).
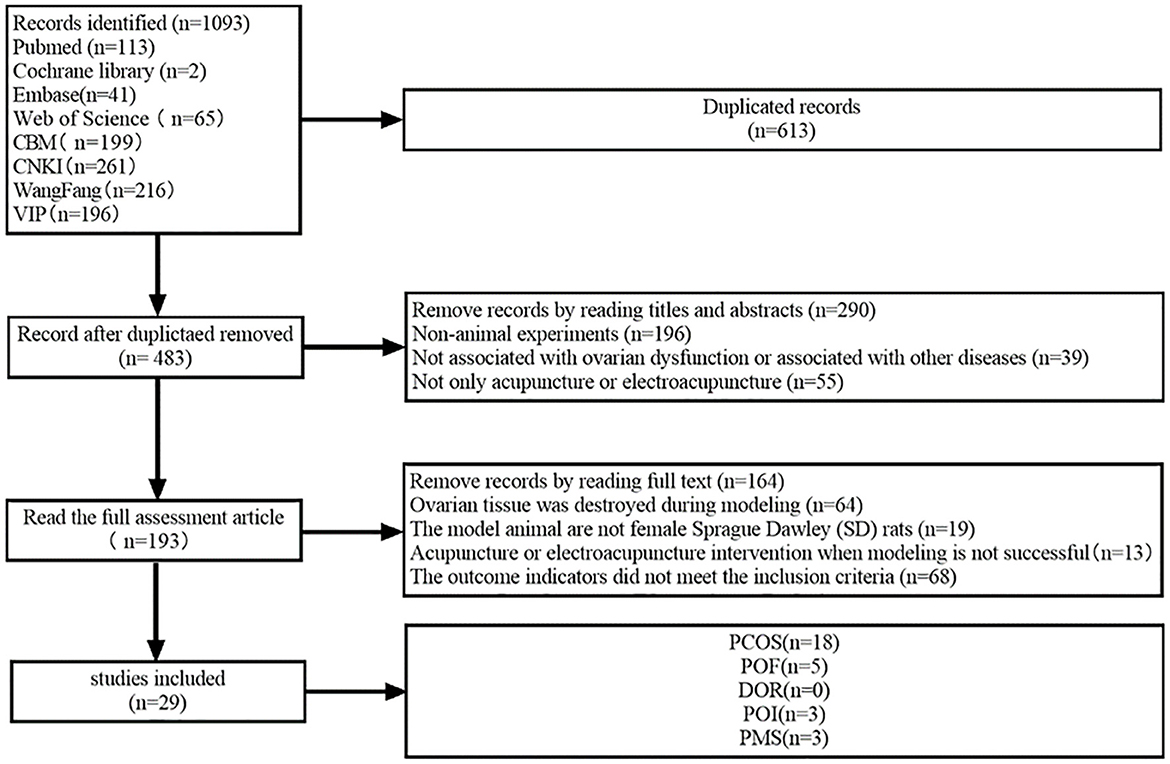
Figure 1. Flowchart of literature search.
3.2 Characteristics of included studiesTwelve studies employed manual acupuncture, while 17 studies utilized electroacupuncture as the intervention method. Ovarian function was assessed through the collection of specimens, encompassing both serum samples and ovarian tissue. Detailed baseline characteristics of the included studies are provided in Table 1.
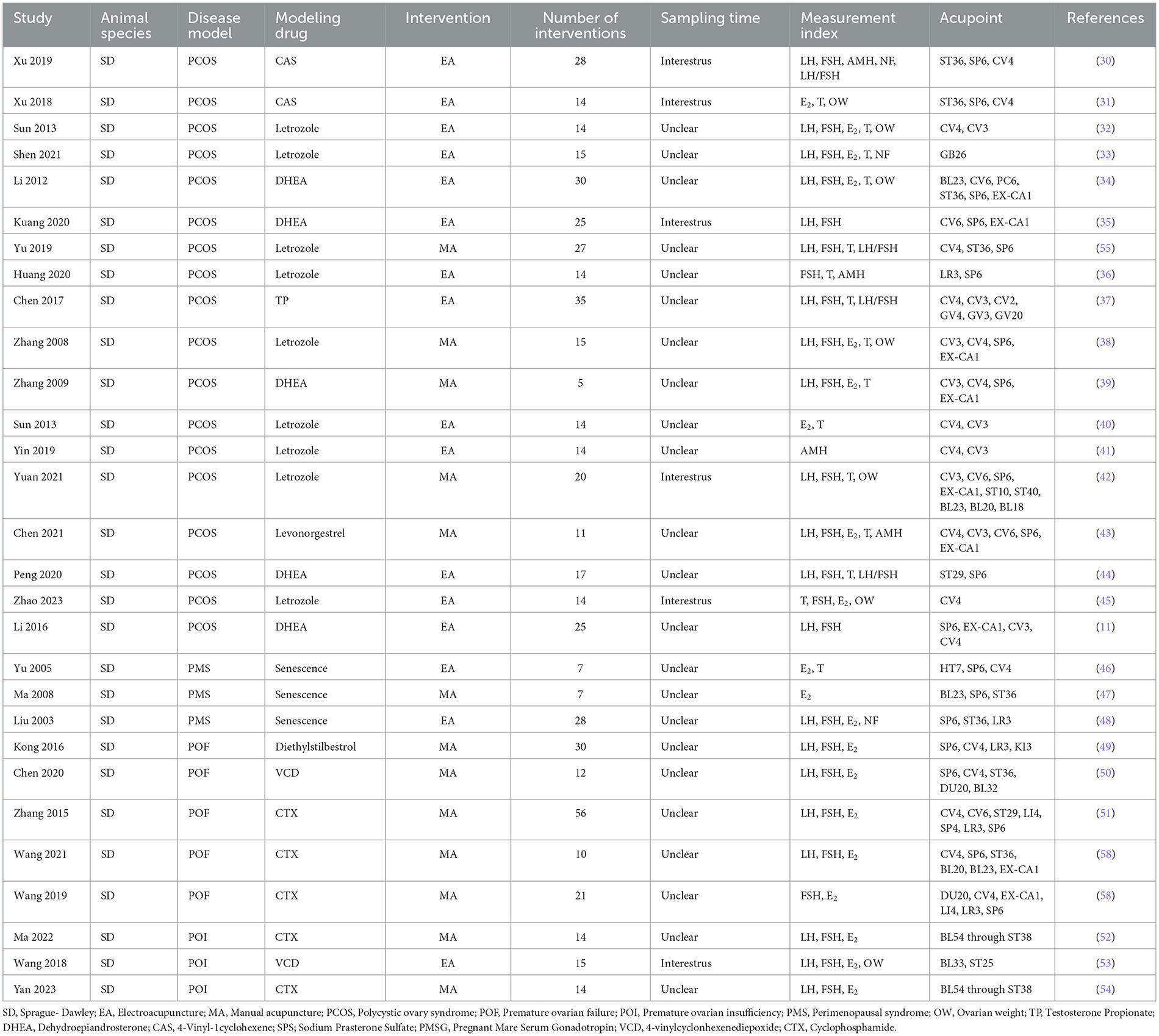
Table 1. General characteristics of the included studies.
3.3 Quality assessmentA comprehensive evaluation of 21 items was conducted in accordance with the ARRIVE guidelines. Based on the quality score-to-maximum score ratio, items 18, 19, and 20 were deemed suboptimal. Meanwhile, items 2, 3, 4, 6, 7, 9, 10, 12, 14, 16, 17, and 21 were classified as having an average rating, while items 1, 5, 8, 9, 11, 13, and 15 demonstrated an excellent level of adherence. The final aggregate score, calculated as 0.65, was considered to be of an average quality. A detailed breakdown of the quality assessment for the identified studies is presented in Table 2.
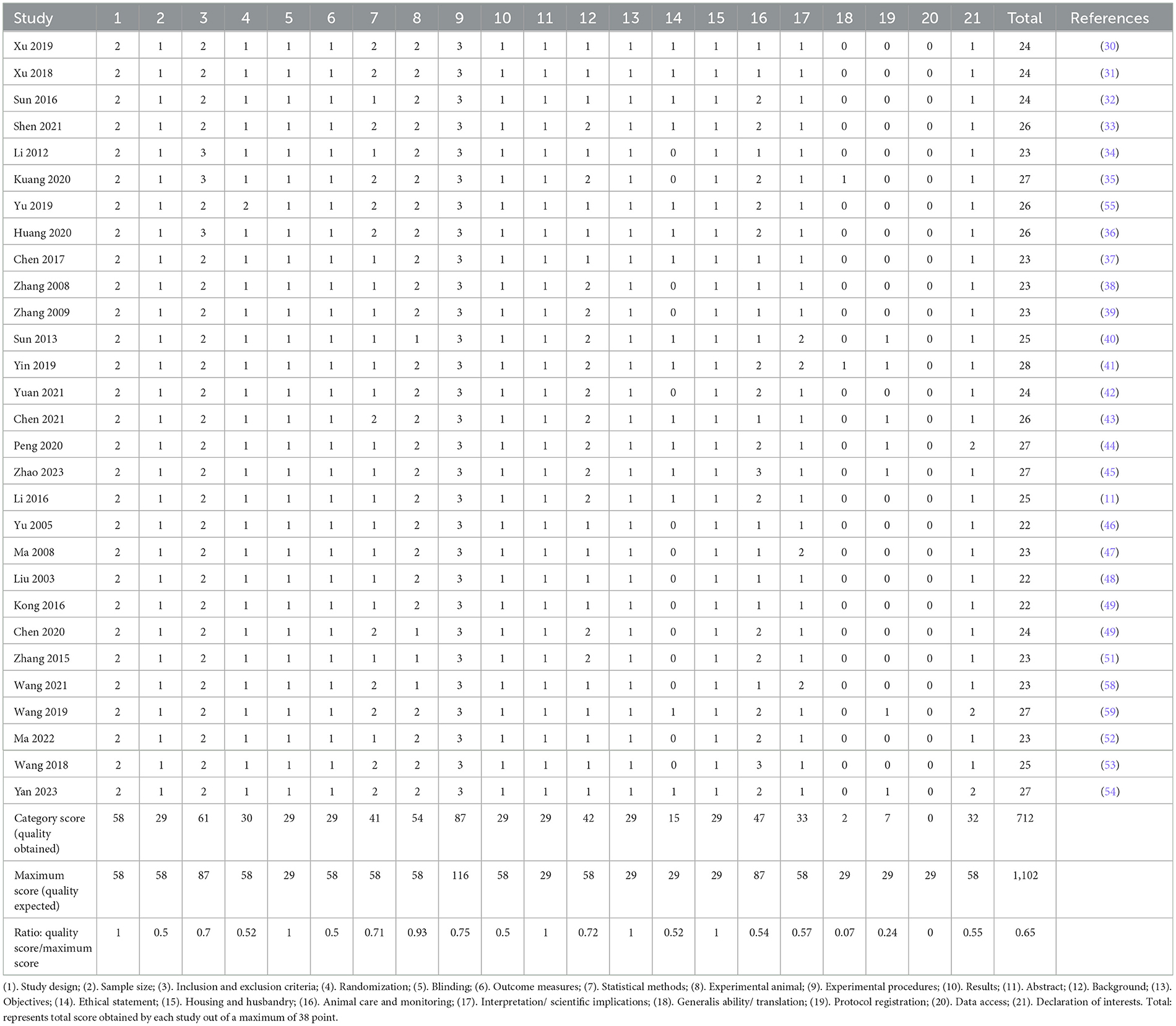
Table 2. Quality assessment according to the Animal Research Reporting In Vivo Experiment (ARRIVE) guidelines.
3.4 Risk of biasThe quality assessment was conducted using the SYRCLE risk assessment tool for animal experiments. The selected studies comprised seven with a low risk of sequence generation (30, 31, 42, 47, 50, 53, 55), eight with a low risk in baseline characteristics (35, 36, 43, 44, 52–55), and three with a low risk of random results (34, 37, 39). Additionally, all 29 studies exhibited a low risk in terms of incomplete result data, selective result reporting, and bias from other sources. A comprehensive overview of the risk of bias is provided in Table 3.
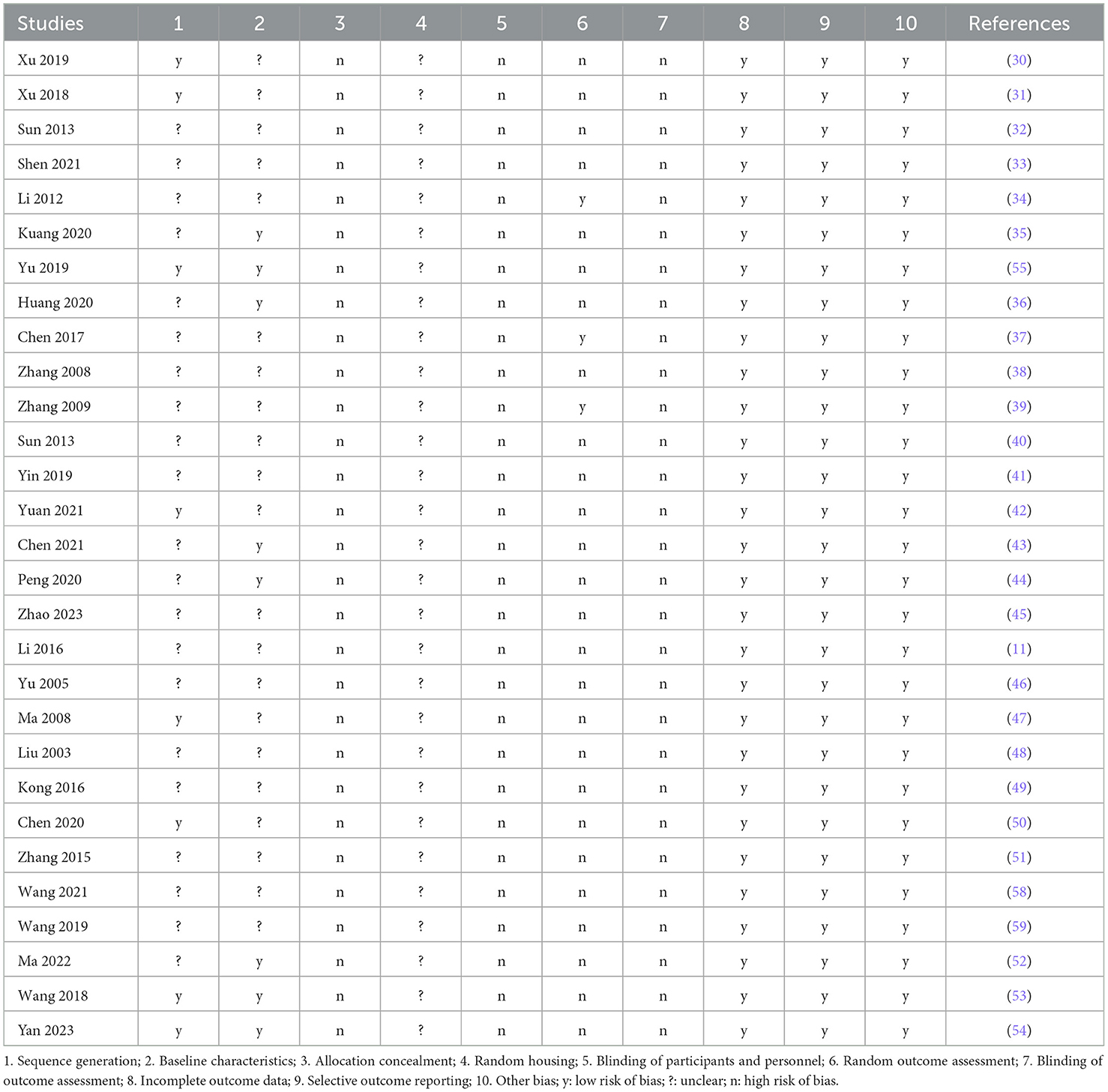
Table 3. Analysis of the risk of bias, based on SYRCLES methodology.
3.5 Effect of acupuncture or electroacupuncture on ovarian function 3.5.1 Polycystic ovarian syndromeThe serum levels of LH, FSH, T, E2, LH/FSH ratio and AMH were subjected to analysis across the selected studies (30–40, 42–45, 55–57). The findings indicated the following: in comparison to the model group, the LH level demonstrated a significant decrease in the acupuncture group [acupuncture group n = 191, model group n = 190, I2 = 84.40%, SMD = −1.90 (95% CI −2.60 to −1.19); Z = 10.76, P = 0.000]. Similarly, the T level exhibited a significant reduction in the acupuncture group [acupuncture group n = 154, model group n = 153, I2 = 86.94%, SMD = −3.14 (95% CI −4.10 to −2.18); Z = 14.77, P = 0.023], and the ratio also showed a significant decrease in the acupuncture group [acupuncture group n = 49, model group n = 49, I2 = 86.94%, SMD = −3.13 (95% CI −4.93 to −1.33); Z = 3.41, P = 0.001]. Conversely, the E2 level [acupuncture group n = 153, model group n = 152, I2 = 94.47%, SMD = 0.85 (95% CI −0.64 to 2.33); Z = 0.88, P = 0.40], the FSH level [acupuncture group n = 149, model group n = 148, I2 = 91.59%, SMD = −0.67 (95% CI −1.60 to 0.27); Z = −1.05, P = 0.31], and the AMH level [acupuncture group n = 40, model group n = 40, I2 = 92.06%, SMD = 0.32 (95% CI −1.57 to 2.21); Z = 0.28, P = 0.80] did not exhibit a significant difference in the acupuncture group. The corresponding forest plots for each outcome measurement are illustrated in Figures 2A–F.
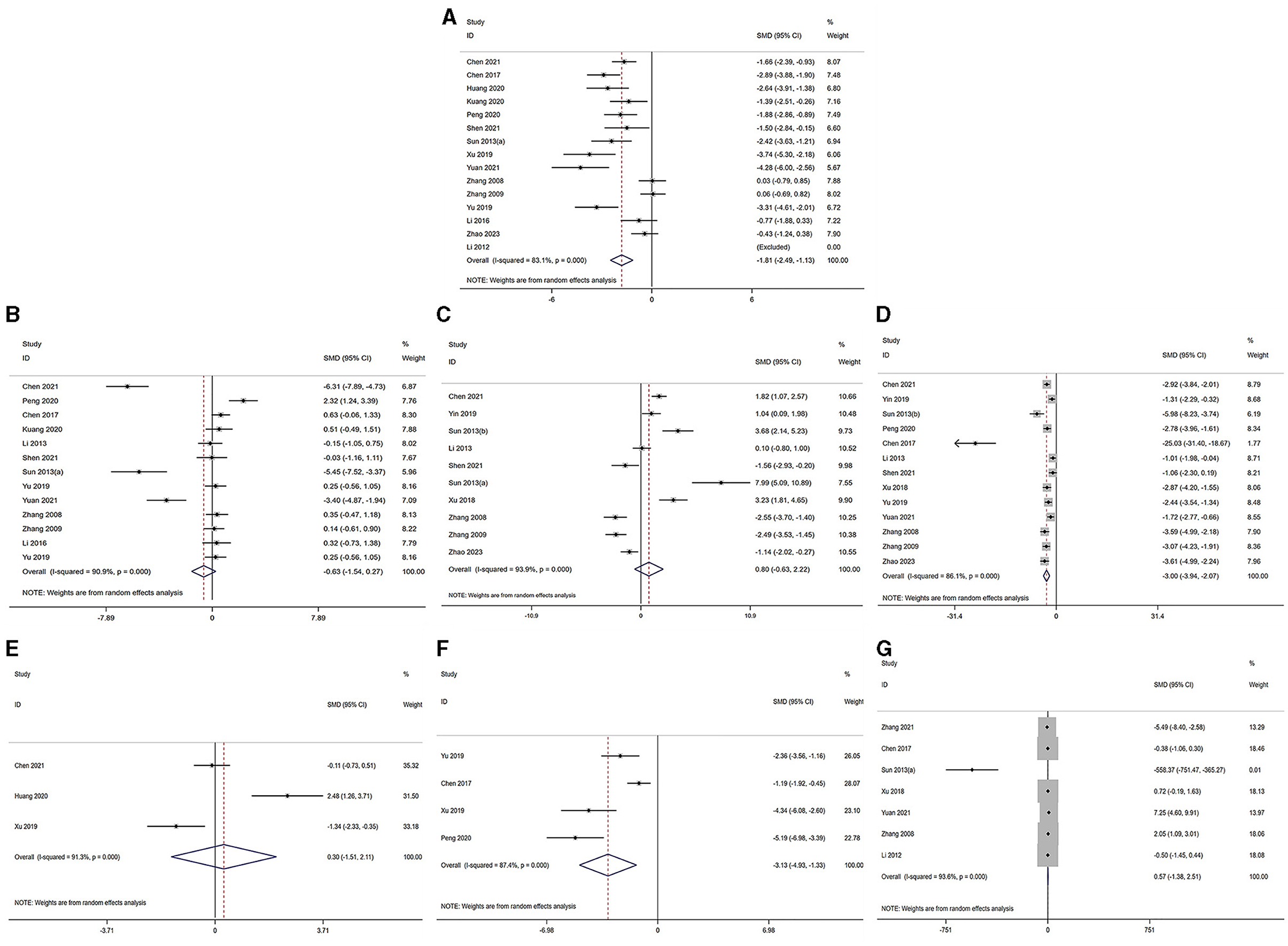
Figure 2. Forest plot of comparison acupuncture vs. control in model of PCOS. The statistical method is random effect. (A) Forest map of LH; (B) Forest map of FSH; (C) Forest map of E2; (D) Forest map of T; (E) Forest map of AMH; (F) Forest map of LH/FSH; (G) Forest map of OW. LH, luteinizing hormone; FSH, follicle-stimulating hormone; T, testosterone; E2, estradiol; AMH, Anti-Müllerian hormone; OW, Ovarian weight.
The analysis of ovarian weight, as reported in studies (31, 32, 34, 37, 38, 42, 45), revealed that in the acupuncture group, there was no significant difference in ovarian weight [acupuncture group n = 82, model group n = 81, I2 = 91.6%, SMD = 0.76 (95% CI −1.25 to 2.77); Z = 0.21, P = 0.838] when compared to the model group. Further details pertaining to the ovarian morphology in PCOS are presented in Figure 2G.
3.5.2 Decreased ovarian functionBased on the disease characteristics, we classified POI, POF, and PMS into the category of decreased ovarian function. The serum levels of LH, FSH, and E2 were analyzed across studies (46, 47, 49–54, 58–60). The outcomes are as follows: Serum E2 level [acupuncture group n = 113, model group n = 113, I2 = 78.00%, SMD = 1.87 (95% CI 1.16–2.58); Z = 3.33, P = 0.008] demonstrated a significant increase compared to the model group. LH level [acupuncture group n = 82, model group n = 82, I2 = 94.14%, SMD = −1.81 (95% CI −3.65 to 0.02); Z = −1.75, P = 0.124] and FSH (acupuncture group n = 96, model group n = 96, I2 = 92.61%, SMD = −2.30 [95% CI −3.78 to 0.81]; Z = −2.19, P = 0.06) in the acupuncture group were not significantly different from the model group. These findings are visually represented in Figure 3.
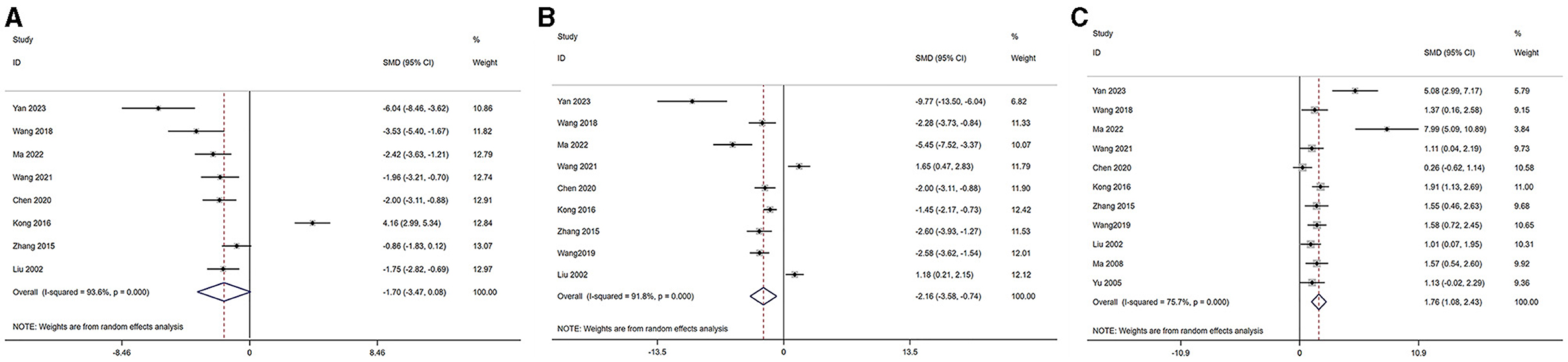
Figure 3. Forest plot of comparison: acupuncture vs. control in model of decreased ovarian function. The statistical method is random effect. (A) Forest map of LH; (B) Forest map of FSH; (C) Forest map of E2. LH, luteinizing hormone; FSH, follicle-stimulating hormone; T, testosterone; E2, estradiol.
4 Heterogeneity and bias analysis 4.1 Heterogeneity analysisIn this study, a sensitivity analysis was conducted for each outcome index that included more than 10 pieces of literature. The analysis encompassed 15 articles on LH analysis, 13 articles on FSH analysis, 10 articles on E2 analysis, 13 articles on T analysis in PCOS, and 11 articles on E2 analysis in decreased ovarian function (Figure 4). Remarkably, the omission of Chen's study in 2021 (43) significantly influenced the stability of results in the E2 analysis within the context of PCOS. This impact could potentially be attributed to variations in the modeling drugs employed in this particular study, introducing heterogeneity in the outcomes. Furthermore, the heterogeneity of other results persisted, as evidenced by the continued detection using Egger's test.
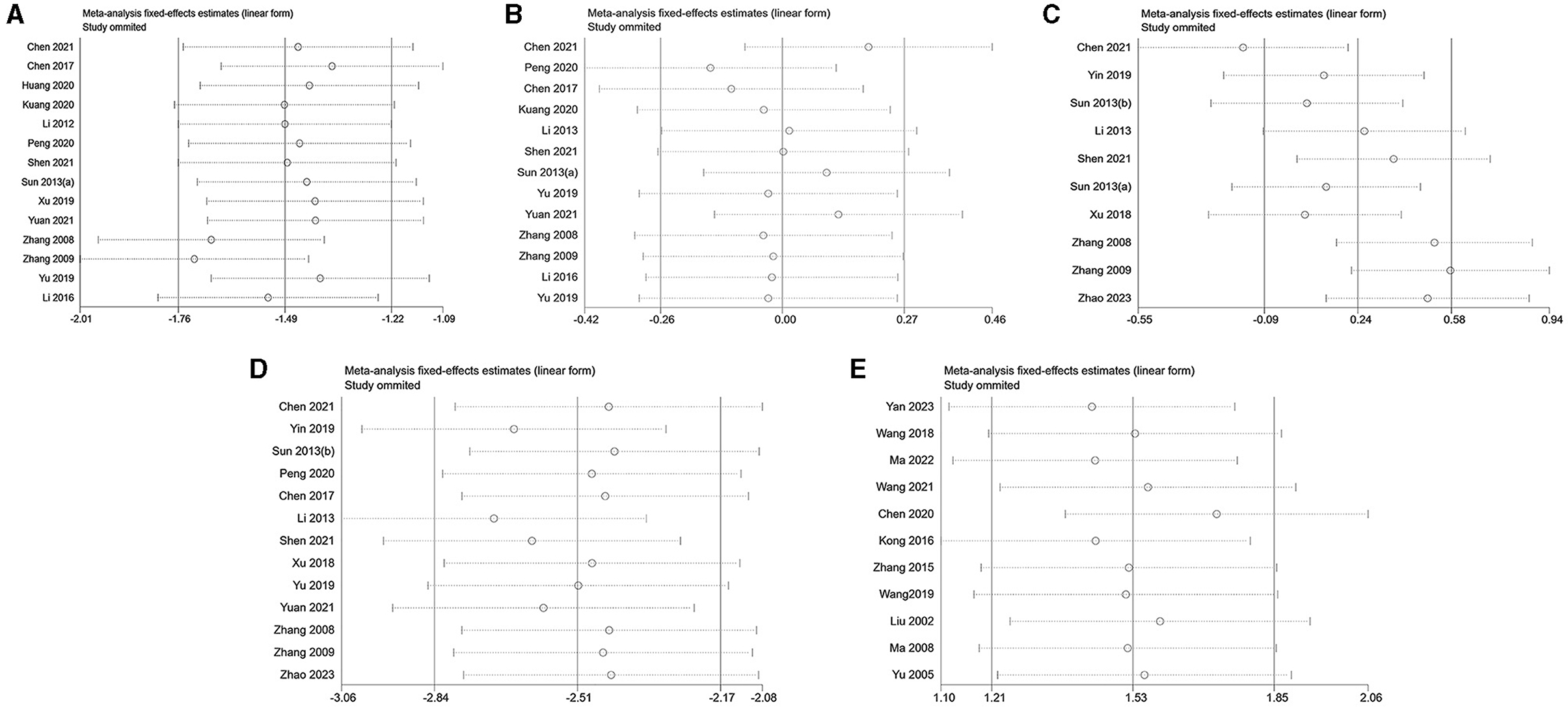
Figure 4. Sensitivity analysis. (A) LH of PCOS; (B) FSH of PCOS; (C) E2 of PCOS; (D) T of PCOS; (E) E2 of decreased ovarian function.
4.2 Publication bias analysisEgger's test was employed to examine the bias within the included literature, encompassing 15 types of literature in LH analysis, 13 types of literature in FSH analysis, 13 types of literature in T analysis in PCOS, and 11 articles on E2 analysis in decreased ovarian function. The findings reveal that a P < 0.05 for LH and E2 indicates the presence of publication bias, necessitating supplementary correction methods such as the trim-and-fill technique. Conversely, the analysis of FSH and T indicated a relatively low likelihood of publication bias, as summarized in Table 4.
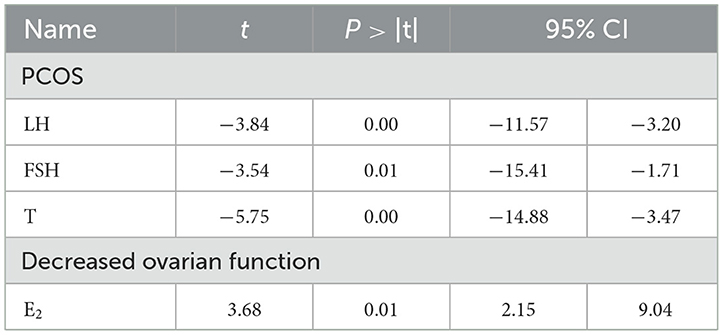
Table 4. Egger's test.
4.3 Trim and filling methodIn Figure 5, no additional data for LH, FSH, T in PCOS, and E2 in decreased ovarian function are apparent. The analysis indicates that the data for LH in PCOS and E2 in decreased ovarian function have undergone no filling study, suggesting the stability of the results.
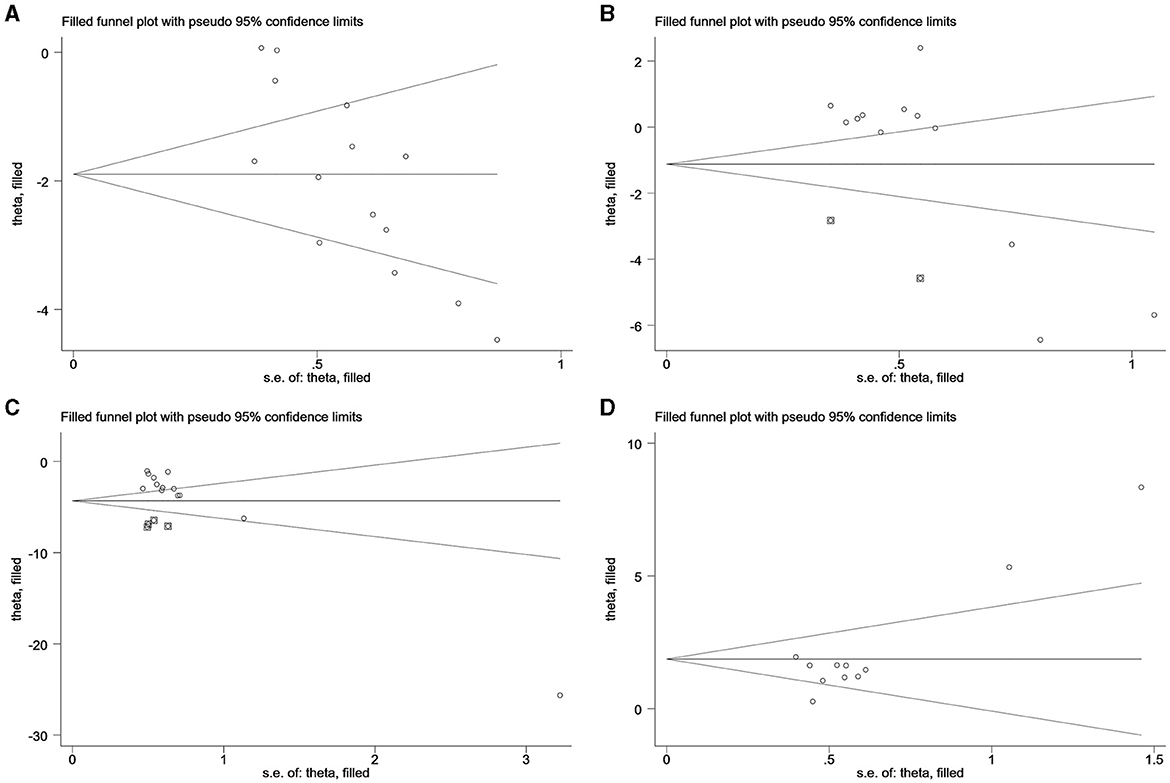
Figure 5. Trim and filling method. (A) LH of PCOS; (B) FSH of PCOS; (C) T of PCOS; (D) E2 of decreased ovarian function.
For LH analysis, two rounds of LINEAR iterations were performed with no additional articles identified, resulting in Q = 83.59, P = 0.00 < 0.05. This signifies the stability of the original results. Subsequently, a random-effects model was employed, yielding a combined result of IOR = 0.15 for the effect indicators, and a 95% CI of (0.07–0.30).
Similarly, the analysis of E2 underwent two rounds of LINEAR iterations with no additional articles added, resulting in Q = 45.56, P = 0.00 < 0.05, indicating the stability of the original results. The subsequent use of a random-effects model produced a combined effect indicator of IOR = 6.47, with a 95% CI of (3.18–13.16).
Notably, two points in the FSH plot and four points in the T plot, indicated as squares, are suggested as potential effect sizes for future inclusion. Combining the above forest plots ensures the symmetry of the funnel plots, eliminating publication bias by consistently incorporating articles with similar results from corresponding studies expressed in the plots.
5 DiscussionIn this comprehensive meta-analysis, our objective was to address the effectiveness of acupuncture in ameliorating ovarian dysfunction, drawing insights from the collective data of 623 rats across 29 articles. The study outcomes revealed that acupuncture led to a noteworthy reduction in serum LH, T and LF/FSH ratio levels in rats modeled with PCOS. Additionally, acupuncture exhibited a significant increase in serum E2 levels in rats modeled with decreased ovarian function. Intriguingly, despite these observed effects, acupuncture did not yield a significant alteration in ovarian volume in the context of PCOS.
Absolutely, our statement provides a concise and accurate summary of the crucial roles played by LH and FSH in ovarian function. These hormones, secreted by the pituitary gland, are instrumental in orchestrating the complex processes involved in the development of follicles and oocytes within the ovaries. FSH is essential for promoting the maturation of follicles, working in tandem with LH to facilitate ovulation. LH, in turn, acts synergistically with FSH, particularly during the late follicular stage, to promote the development and optimal functioning of follicles. The regulation of the hypothalamus-pituitary-ovary axis (HPOA) by LH and FSH is a key aspect of maintaining ovarian function. This regulatory mechanism plays a central role in promoting follicular development and finely tuning the secretion of estrogen, thereby contributing significantly to the overall regulation of the female reproductive system (61). Our statement aptly captures the interconnected nature of sex hormone regulation and how acupuncture can impact these dynamics. T serves as a crucial precursor in the biosynthesis of E2 and exerts a negative feedback regulatory effect on the hypothalamus-pituitary axis (62, 63). LH plays a significant role in the regulation of T synthesis, and therefore, the effects of acupuncture on T align with its impact on LH. For PCOS, characterized by increased LH secretion, elevated LH/FSH ratio, and elevated T levels in some patients, research findings indicate that both acupuncture and electroacupuncture can significantly reduce serum LH, T, and LH/FSH levels. This suggests that acupuncture holds promise in effectively addressing sex hormone level disorders in PCOS and potentially restoring abnormal ovarian function. E2 is a key component in follicular development and maturation, exerts both positive and negative feedback effects on the hypothalamus (64, 65). Additionally, E2 plays a decisive role in maintaining ovarian function and mitigating ovarian aging (66). This highlights the potential of acupuncture to influence a range of sex hormones, contributing to the restoration of hormonal balance and, consequently, improved ovarian function. The study outcomes reveal that both acupuncture and electroacupuncture interventions have the potential to enhance serum E2 levels across various degrees of ovarian hypoplasia, thereby contributing to the alleviation of the ovarian hypoplasia process. However, the observation of no significant difference in ovarian weight between the acupuncture group and the model group in PCOS suggests the need for cautious interpretation. This lack of significance may be associated with the relatively short duration of acupuncture treatment in animal experiments and the limited number of articles included in this study (67). It underscores the importance of considering these factors and highlights the necessity of supplementing relevant outcome indicators in future studies to provide a more comprehensive understanding of the impact of acupuncture on ovarian weight in PCOS models.
In the current study, it was observed that only six of the included articles proposed the collection of serum samples during the inter-estrous period in model animals (30, 31, 35, 42, 45, 53). However, a sensitivity analysis was conducted to assess the impact of excluding any of the articles that sampled during the interestrus period from LH, FSH, and T analyses. Remarkably, the results indicated that such exclusions had no significant effect on the overall meta-analysis results. Given the similarity between the animal estrous cycle and the human menstrual period, it is noteworthy that there are substantial changes in serum sex hormones across different estrous cycles (68). This factor may exert an influence on the outcomes of our study (69). Follicular development exhibits a close association with the level of E2, especially in patients with PCOS during the anogenital follicular phase. Here, an increase in E2 may play a role in suppressing oocyte meiosis, potentially resulting in abnormal oocyte maturation (70). In the context of the early stages of PMS, a reduction in LH and T levels indicates a decline in ovarian function. It is crucial to recognize that hormonal variations at different points in time can have diverse effects on ovarian function and follicular development. Therefore, when investigating alterations in sex hormones, the changes in the motility cycle emerge as a significant factor that cannot be overlooked.
This meta-analysis puts forth the proposition that acupuncture exhibits the capacity to reduce elevated hormones, elevate lowered hormones, and normalize disordered hormone levels in the treatment of dysfunctional ovarian diseases. Hence, our inference is that the regulation of ovarian dysfunction through acupuncture or electroacupuncture primarily occurs via the modulation of serum sex hormones.
Additionally, previous studies have elucidated the multifaceted impact of acupuncture or electroacupuncture on ovarian function. These effects encompass diverse mechanisms, such as modulating protein content within ovarian tissue, altering sex hormone receptor activity, modifying gene expression associated with reproductive factors, influencing metabolic factors, and modulating various signaling pathways (41, 48, 71–78), and alteration of the expression of various proteins in the hypothalamus (79, 80). For example, most PCOS patients are usually accompanied by insulin resistance (IR), and lipocalin can increase insulin sensitivity and promote glucose metabolism, which is a key signaling factor in the regulation of glucose-lipid metabolism, and plays an important role in regulating the modulation of reproductive and metabolic disorders (81–85). Studies have demonstrated that acupuncture can ameliorate glycolipid metabolism in PCOS by downregulating lipocalin receptor expression, upregulating lipocalin protein expression, and activating the AMP-activated protein kinase (AMPK) pathway (86–88). POF, POI, and PMS are characterized by reductions in ovarian volume and cell numbers. However, acupuncture has been shown to increase granulosa cell and follicle numbers by activating the Phosphoinositide 3-kinase/Protein Kinase B (PI3K/AKT) signaling pathway, thereby improving ovarian function and fertility (59, 89). In conclusion, the confirmed regulatory effect of acupuncture or electroacupuncture on ovarian function provides a novel therapeutic perspective for the treatment of diseases associated with ovarian function (Figure 6).
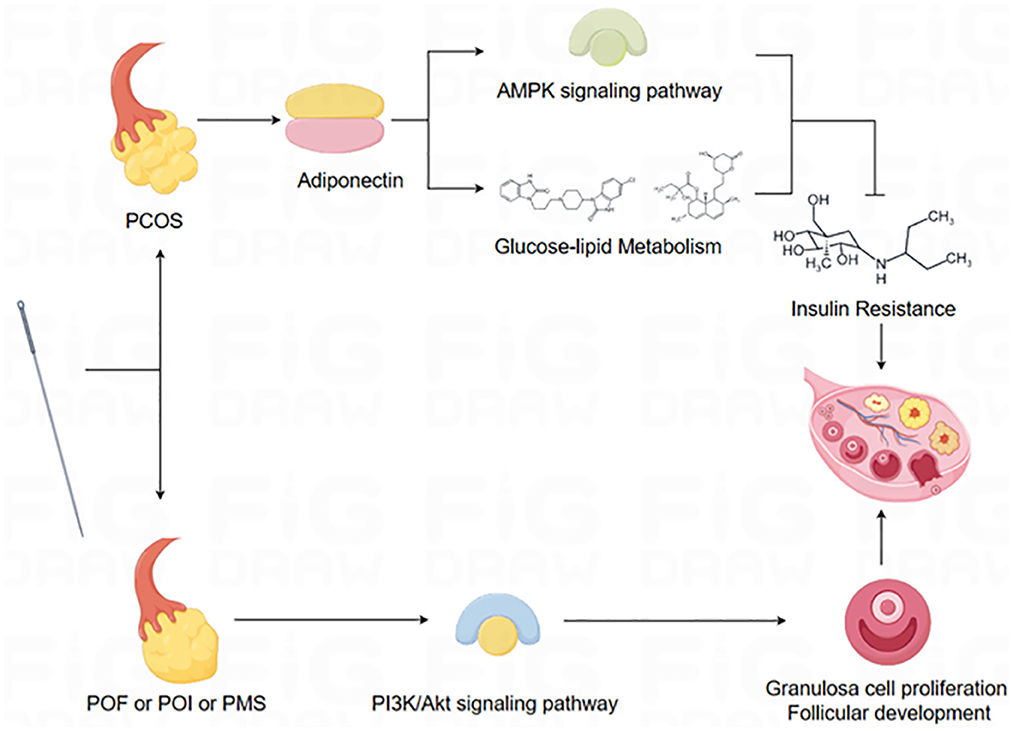
Figure 6. Schematic diagram of the mechanism of action of acupuncture.
6 Strength and limitationsThe study did not undertake a comprehensive analysis of all indices. Given the inclusion of diverse diseases and the inconsistent changes observed in serum sex hormones and ovarian morphology across these conditions, the effects of each hormone were individually accounted for. This approach allowed for a nuanced explanation of hormonal changes across different diseases.
A notable portion of the articles did not account for the impact of different estrous cycles on outcomes during serum sample extraction. Considering the relevance of estrous cycle recovery in animal models as an important indicator of acupuncture response in diseases related to ovarian dysfunction, future studies should meticulously consider the fluctuation of sex hormone levels across different estrous cycles. Researchers are advised to choose outcome indicators judiciously to more accurately reflect acupuncture efficacy.
The reliance on specific outcome indicators to gauge the integrative modulatory effects of acupuncture on disorders of abnormal ovarian function may potentially constrain the comprehensive interpretation of acupuncture efficacy. And since changes in serum sex hormones are a common indicator for assessing the efficacy of acupuncture in clinical trials, we likewise chose serum sex hormones as an observational indicator of efficacy in our study. However, in future research endeavors, we should take full advantage of the opportunity provided by animal experiments to incorporate additional outcome indicators. This approach would better summarize the multifaceted effects of acupuncture on ovarian function. For example, parameters such as protein content, gene expression and signaling pathway alterations must be studied in depth.
The predominance of studies focused on PCOS in this analysis may restrict the generalizability of the findings to other ovarian dysfunction diseases. Nevertheless, it is essential to highlight that this study marks the first endeavor to evaluate the effects of acupuncture or electroacupuncture on improving animal models of ovarian dysfunction across various diseases, providing insights into the underlying mechanisms of acupuncture or electroacupuncture. The aim of this study was to comprehensively observe the efficacy of acupuncture therapy on ovarian dysfunction diseases, and the previous analysis of acupuncture and electroacupuncture by subgroups did not appear to have a significant impact on the analytical results, so this study did not describe the subgroup analysis of acupuncture and electroacupuncture. And due to the limited number of articles we screened, especially POF, PMS and POI, subgroup analysis of acupuncture and electroacupuncture was difficult to achieve, so future studies should further include more articles to further analyze and analyze the efficacy of acupuncture and electroacupuncture.
Finally, this study observed the effects of acupuncture on various ovarian dysfunctions through animal experiments. In future research, we will focus on observing whether acupuncture has similar therapeutic effects in clinical studies, which will require further in-depth analysis and exploration.
7 ConclusionThis meta-analysis underscores that acupuncture treatment has the potential to enhance ovarian function in PCOS, POF, POI, and PMS model rats by effectively regulating serum sex hormones. The findings of this review provide a valuable foundation for future research into the therapeutic effects of acupuncture or electroacupuncture. It is anticipated that this comprehensive review will serve as a noteworthy reference in this evolving field of research, encouraging further exploration and insights into the application of acupuncture in the context of ovarian dysfunction.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributionsYZ: Writing – review & editing, Writing – original draft. YL: Writing – review & editing. LL: Writing – review & editing. JH: Writing – review & editing. HW: Writing – review & editing. LJ: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received financial support from several sources, including grants from the National Natural Science Foundation of China (Nos. 82105028 and 82074549), the Xinglin Scholar Research Promotion Project of Chengdu University of Traditional Chinese Medicine (No. OJRC2022009), the Hundred Talents Program of the Hospital of Chengdu University of Traditional Chinese Medicine (No. 22-Q29), and the Young Elite Scientists Sponsorship Program by CACM (No. CACM-2023-QNRC2-B03).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1348884/full#supplementary-material
References6. Wang Y, Huang R, Li X, Zhu Q, Liao Y, Tao T, et al. High concentration of chemerin caused by ovarian hyperandrogenism may lead to poor IvF outcome in polycystic ovary syndrome: a pilot study. Gynecol Endocrinol. (2019) 35:1072–7. doi: 10.1080/09513590.2019.1622087
PubMed Abstract | Crossref Full Text | Google Scholar
7. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women principal results from the women's health initiative randomized controlled trial. JAMA. (2002) 288:321–33. doi: 10.1001/jama.288.3.321
PubMed Abstract | Crossref Full Text | Google Scholar
8. Li Y, Chen C, Ma Y, Xiao J, Luo G, Li Y, et al. Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. (2019) 228:167–75. doi: 10.1016/j.lfs.2019.04.046
PubMed Abstract | Crossref Full Text | Google Scholar
9. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of pcos as functional ovarian hyperandrogenism revisited. Endocr Rev. (2016) 37:467–520. doi: 10.1210/er.2015-1104
PubMed Abstract | Crossref Full Text | Google Scholar
10. Liu H, Jiang C, La B, Cao M, Ning S, Zhou J, et al. Human amnion-derived mesenchymal stem cells improved the reproductive function of age-related diminished ovarian reserve in mice through Ampk/Foxo3a signaling pathway. Stem Cell Res Ther. (2021) 12:317. doi: 10.1186/s13287-021-02382-x
PubMed Abstract | Crossref Full Text | Google Scholar
11. Li RX, Ma M, Xiao XR, Xu Y, Chen XY Li B. Perimenopausal syndrome and mood disorders in perimenopause: prevalence, severity, relationships, and risk factors. Medicine. (2016) 95:e4466. doi: 10.1097/MD.0000000000004466
PubMed Abstract | Crossref Full Text | Google Scholar
12. Lan J, Wu C, Liang W, Shen J, Zhuo Z, Hu L, et al. An effective treatment of perimenopausal syndrome by combining two traditional prescriptions of chinese botanical drugs. Front Pharmacol. (2021) 12:744409. doi: 10.3389/fphar.2021.744409
PubMed Abstract | Crossref Full Text | Google Scholar
14. Wang RR, Su MH, Liu LY, Lai YY, Guo XL, Gan D, et al. Systematic review of acupuncture to improve ovarian function in women with poor ovarian response. Front Endocrinol. (2023) 14:1028853. doi: 10.3389/fendo.2023.1028853
PubMed Abstract | Crossref Full Text | Google Scholar
15. Liu Y, Wu L, Yao S, Wu C, Lu L, Yi W. Acupuncture for infertile women without undergoing assisted reproductive techniques (ART) a systematic review and meta-analysis. Medicine. (2019) 98:e16463. doi: 10.1097/MD.0000000000016463
PubMed Abstract | Crossref Full Text | Google Scholar
16. Ye Y, Zhou CC, Hu HQ, Fukuzawa I, Zhang HL. Underlying mechanisms of acupuncture therapy on polycystic ovary syndrome: evidences from animal and clinical studies. Front Endocrinol. (2022) 13:1035929. doi: 10.3389/fendo.2022.1035929
PubMed Abstract | Crossref Full Text | Google Scholar
17. Xi D, Chen B, Tao H, Xu Y, Chen G. The risk of depressive and anxiety symptoms in women with premature ovarian insufficiency: a systematic review and meta-analysis. Arch Womens Ment Health. (2023) 26:1–10. doi: 10.1007/s00737-022-01289-7
PubMed Abstract | Crossref Full Text | Google Scholar
18. Smith CA, de Lacey S, Chapman M, Ratcliffe J, Norman RJ, Johnson NP, et al. The effects of acupuncture on the secondary outcomes of anxiety and quality of life for women undergoing IVF: a randomized controlled trial. Acta Obstet Gynecol Scand. (2019) 98:460–9. doi: 10.1111/aogs.13528
PubMed Abstract | Crossref Full Text | Google Scholar
19. Xia J-f, Inagaki Y, Zhang J-f, Wang L, Song P-p. Chinese medicine as complementary therapy for female infertility. Chin J Integr Med. (2017) 23:245–52. doi: 10.1007/s11655-016-2510-5
PubMed Abstract | Crossref Full Text | Google Scholar
21. Xiao LY, Li Z, Du YZ, Shi HY, Yang SQ, Zhang YX, et al. Acupuncture for hypertension in animal models: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2021) 2021:8171636. doi: 10.1155/2021/8171636
PubMed Abstract | Crossref Full Text | Google Scholar
22. Ritskes-Hoitinga M, Leenaars M, Avey M, Rovers M, Scholten R. Systematic reviews of preclinical animal studies can make significant contributions to health care and more transparent translational medicine. Cochrane Database Syst Rev. (2014) 2014:ED000078. doi: 10.1002/14651858.ED000078
留言 (0)