Thyroid hormone plays a critical role in early neurocognitive development, growth, and energy metabolism throughout childhood and adolescence. Thyroid dysfunction, including hyperthyroidism and hypothyroidism, is prevalent among adults and has been associated with increased morbidity and mortality related to cardiovascular disease (1). Additionally, hypothyroidism has been documented to correlate with an increased risk of various other conditions, including diabetes mellitus, obesity, metabolic syndrome, fatigue, and depression (2–5). This association holds true even among adolescents aged 12–18 years (6). Children with thyroid dysfunction, such as hyperthyroidism, require a period of surveillance or even prompt treatment, according to recent guidelines (7).
The national prevalence of thyroid dysfunction among adults in the has been extensively studied using data from the National Health and Nutrition Examination Survey (NHANES) conducted during various periods spanning 1988–1994, 1999–2002, and 2007–2012 (8, 9). Estimates from these surveys indicate that subclinical hypothyroidism affects approximately 4.3% and subclinical hyperthyroidism affects around 3.2% of the US adult population (9). The prevalence of thyroid dysfunction in children exhibits significant geographic variability (10, 11). For instance, notable differences exist in the prevalence of Graves’ disease between the United States and the United Kingdom (12, 13). However, there is limited research on the epidemiology of thyroid dysfunction among US adolescents using nationally representative data. A recent NHANES-based study estimated the prevalence of subclinical hypothyroidism among adolescents to be 2.0% (6), using an upper reference thyroid stimulating hormone (TSH) level of 4.5 mIU/L commonly employed in adult populations. Notably, studies comprehensively examining the prevalence of thyroid dysfunction applying age-adjusted normal reference ranges, are lacking. Furthermore, changes in dietary iodine intake, as evidenced by reduced urinary iodine concentration in children after 2001–2004, may have influenced the prevalence and characteristics of thyroid dysfunction in this demographic (14).
We aimed to estimate the overall prevalence of thyroid dysfunction, as well as the prevalence across key demographic subgroups, in a nationally representative cohort of US adolescents. We also aimed to identify risk factors contributing to thyroid dysfunction within this population.
Methods Study designThe NHANES, initiated in 1999 and conducted biennially, are a series of cross-sectional surveys executed by the National Center for Health Statistics of the Centers for Disease Control and Prevention (15). NHANES systematically samples noninstitutionalized individuals across the US, providing a population-based perspective. Detailed descriptions of its study design and methodologies are well-documented elsewhere (16). In brief, the NHANES employs interviews, physical examinations, and laboratory analyses to evaluate the health of the non-institutionalized civilian in the US. The survey protocols have received approval from the National Center for Health Statistics Ethics Review Board, and informed consent was obtained from all participants.
Our study utilized data from NHANES spanning the years 2001 to 2002 and 2007 to 2012, which provided information on measurements of TSH and free thyroxine (fT4). Notably, data from the 2003 to 2006 cycles were omitted due to the absence of reported thyroid hormone data during that period. The study population consisted of participants aged 12–18 years, with exclusion criteria for missing data or pregnancy.
Thyroid dysfunctionThyroid dysfunction was evaluated through the measurement of serum TSH and fT4 concentrations. In the NHANES data sets, serum TSH from participants was measured with a two-site (sandwich) immunoenzymatic assay, fT4 was measured with a 2-step enzyme immunoassay, and TPOAb and TgAb titers were measured with a sequential two-step immunoenzymatic “sandwich” assay. We adhered to the established reference ranges for children and adolescents: 0.6–5.8 mU/L for TSH and 0.8–1.9 μg/dL for fT4 (17–21). In the absence of antithyroid drugs or thyroid hormone therapy, subclinical hypothyroidism was defined by elevated TSH (>5.8 mIU/L) with normal fT4 levels, and subclinical hyperthyroidism by reduced TSH (<0.6 mIU/L) with normal fT4 levels. Overt hypothyroidism was identified as a TSH level > 5.8 mIU/L with fT4 below the normal range, and overt hyperthyroidism was indicated by a TSH level < 0.6 mIU/L with fT4 above the reference range. Participants on thyroid hormone or antithyroid medication were classified as having overt hypothyroidism or hyperthyroidism, respectively.
A narrower TSH range of 0.4–4.5 mU/L was employed for sensitivity analysis (6). Thyroglobulin antibody (TgAb) or thyroid peroxidase antibody (TPOAb) positivity was defined as per NHANES standards (22). Due to the unavailability of thyroid disease history in participants under 20 years in NHANES, a disease-free population was delineated as those without antithyroid or thyroid hormone prescriptions.
Statistical analysisStatistical analyses were performed using Stata (version 16.0, StataCorp) and R (version 3.5.2, R Foundation), utilizing NHANES weights for nationally representative estimates (23). Categorical variables were presented as proportions (95% confidence interval [CI]), and continuous variables as means (95% CI). Prevalence differences between NHANES cycles were assessed for significance using an approximation of the Wald test, checking if one estimate’s central value fell within the other’s 95% CI (24). After confirming no temporal trends across cycles, data from all NHANES cycles were combined to improve precision and minimize sampling error, as per analytic guidelines (23).
Prevalence was calculated for both the overall and disease-free populations. The Taylor series (linearization) method estimated standard errors, while the Korn and Graubard method determined 95% CIs for prevalence (25). Subgroup analyses, based on sex, race/ethnicity, and TPOAb and TgAb status, examined prevalence within the overall population. Multivariate logistic regression models identified risk factors for thyroid dysfunction, with statistical significance set at a two-sided p-value of < 0.05.
Results Participant characteristicsOur analysis encompassed 2,182 individuals, representing approximately 12.97 million US adolescents. The group had a weighted mean age of 15.1 ± 0.06 years, with males constituting 51.4%. Racial composition included 61.1% White people, 13.8% Black people, and 12.3% Mexican Americans. TPOAb and TgAb positivity were found in 5.7 and 9.7% of participants, respectively. Participants with hypothyroidism, compared to euthyroid individuals, were predominantly female, had a greater proportion of White people, and showed higher TPOAb and TgAb positivity rates. In contrast, hyperthyroid participants, also more likely to be female, had higher representation among Black people and Mexican Americans, though TPOAb or TgAb positivity rates did not differ significantly from the euthyroid group (Supplementary Table S1).
Prevalence of thyroid dysfunctionIn the overall population, the prevalence of hypothyroidism was 1.4% (95% CI, 0.6–2.7%), comprising 0.41% subclinical and 1.03% overt hypothyroidism (Table 1). In the disease-free population, these figures were 0.64% for overall hypothyroidism, 0.41% for subclinical and 0.23% for overt hypothyroidism.
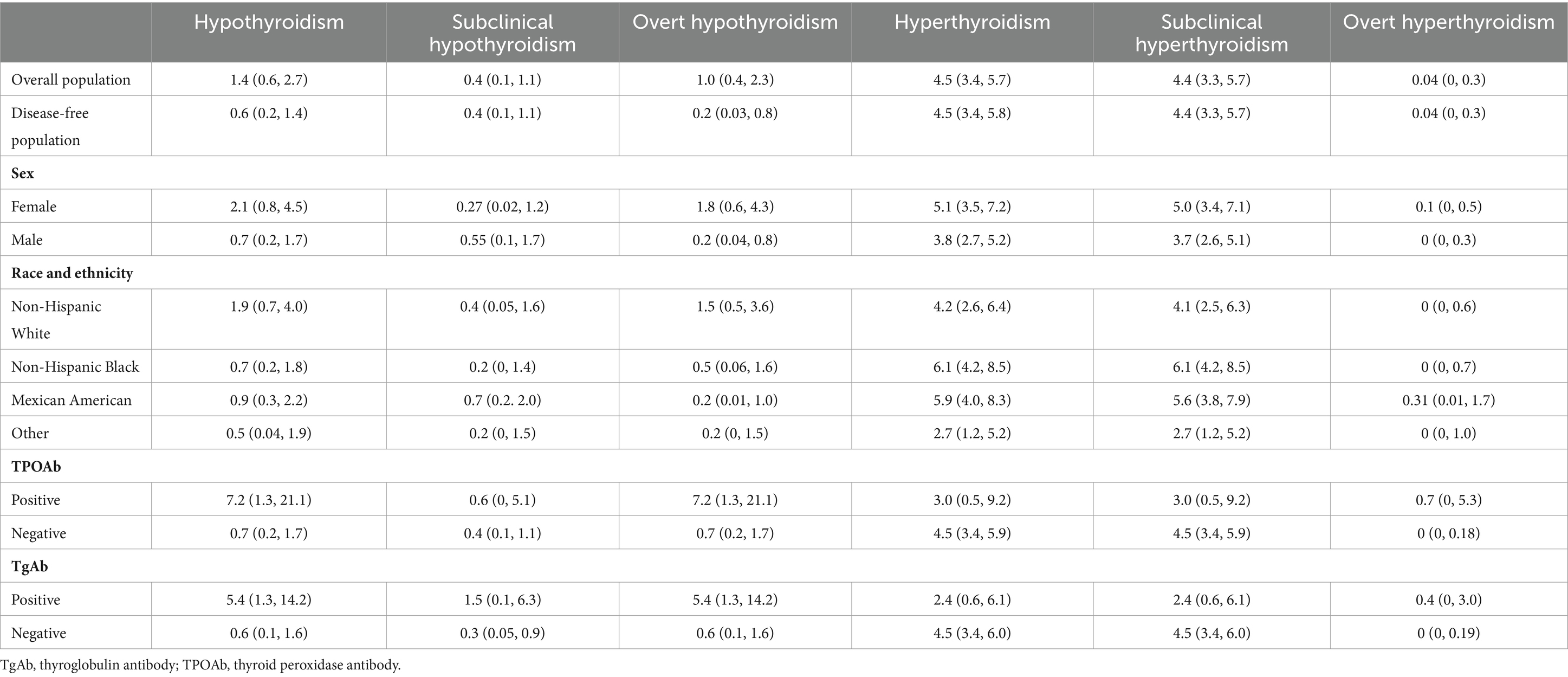
Table 1. Prevalence of thyroid dysfunction among US adolescents aged 12–18 years.
For hyperthyroidism, the prevalence was 4.5% (95% CI, 3.4–5.7%), with 4.4% subclinical and 0.04% overt hyperthyroidism (Table 1). This prevalence was mirrored in the disease-free population. From 2001–2002 to 2011–2012, subclinical hyperthyroidism remained consistent at 4.99% vs. 5.13% in the overall cohort (Figure 1).

Figure 1. Trends in prevalence of thyroid dysfunction among US adolescents aged 12–18 years, 2001–2002 to 2011–2012.
The proportion of TPOAb and TgAb positivity in the overall population were 5.8% (95% CI, 4.3–7.6%) and 9.8% (95% CI, 7.9–11.9%), respectively (Table 2). These rates were slightly lower in the disease-free population, at 5.4 and 9.3%, respectively (Table 2). All TPOAb-positive adolescents were also TgAb-positive, whereas 60.5% of those with TgAb positivity had TPOAb positivity (Table 2).
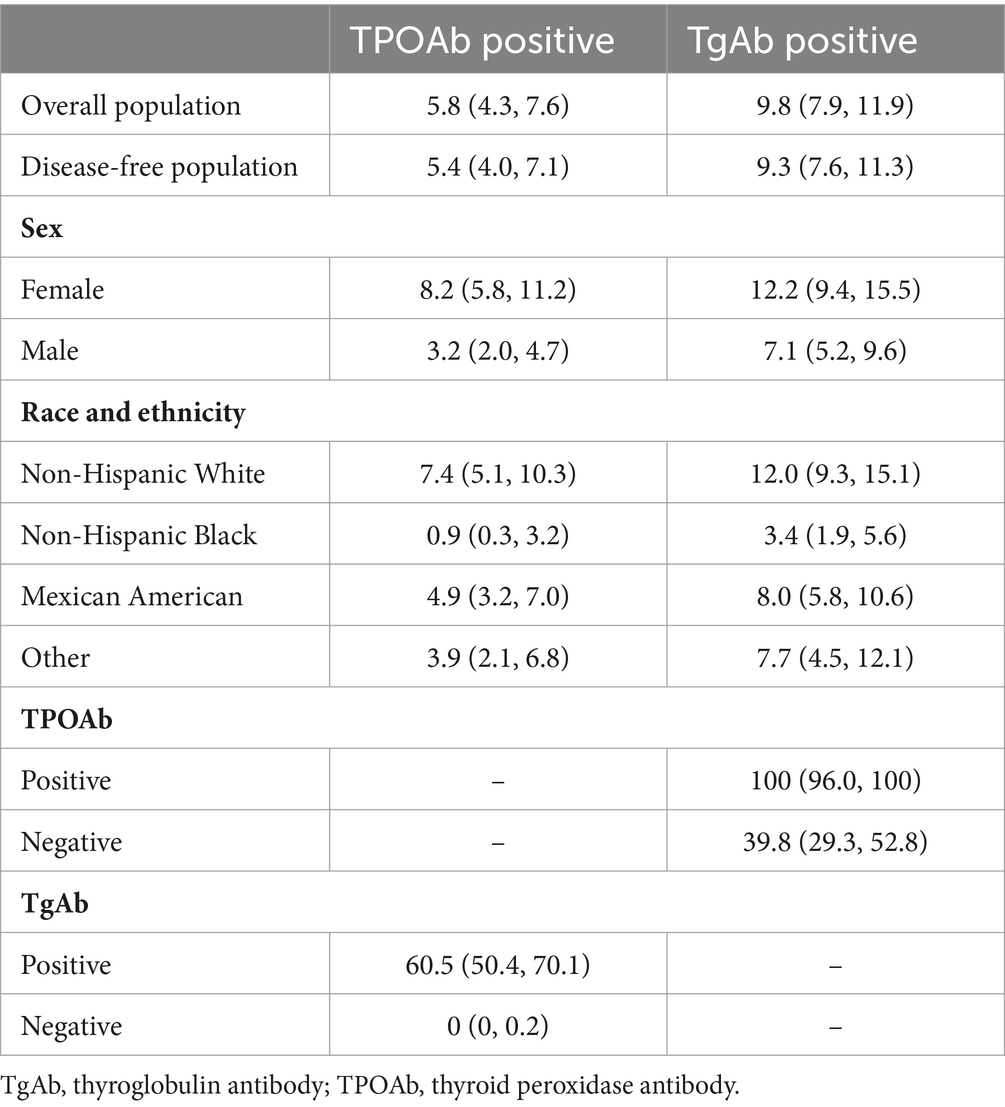
Table 2. Prevalence of antibody abnormality among US adolescents aged 12–18 years.
Using a TSH cutoff of 0.4–4.5 mU/L, the prevalence of subclinical and overt hypothyroidism was 1.7 and 0.92%, respectively, while subclinical and overt hyperthyroidism were 2.28 and 0.04%, respectively (Supplementary Figure S1).
Risk factors for thyroid dysfunctionAdolescents testing positive for TgAb were at a sixfold increased risk of hypothyroidism compared to negative counterparts (OR 6.0, 95% CI 1.19–30.7, p = 0.031), but no significant differences were noted in hypothyroidism prevalence based on age, gender, race, ethnicity, or TPOAb status (Table 3).
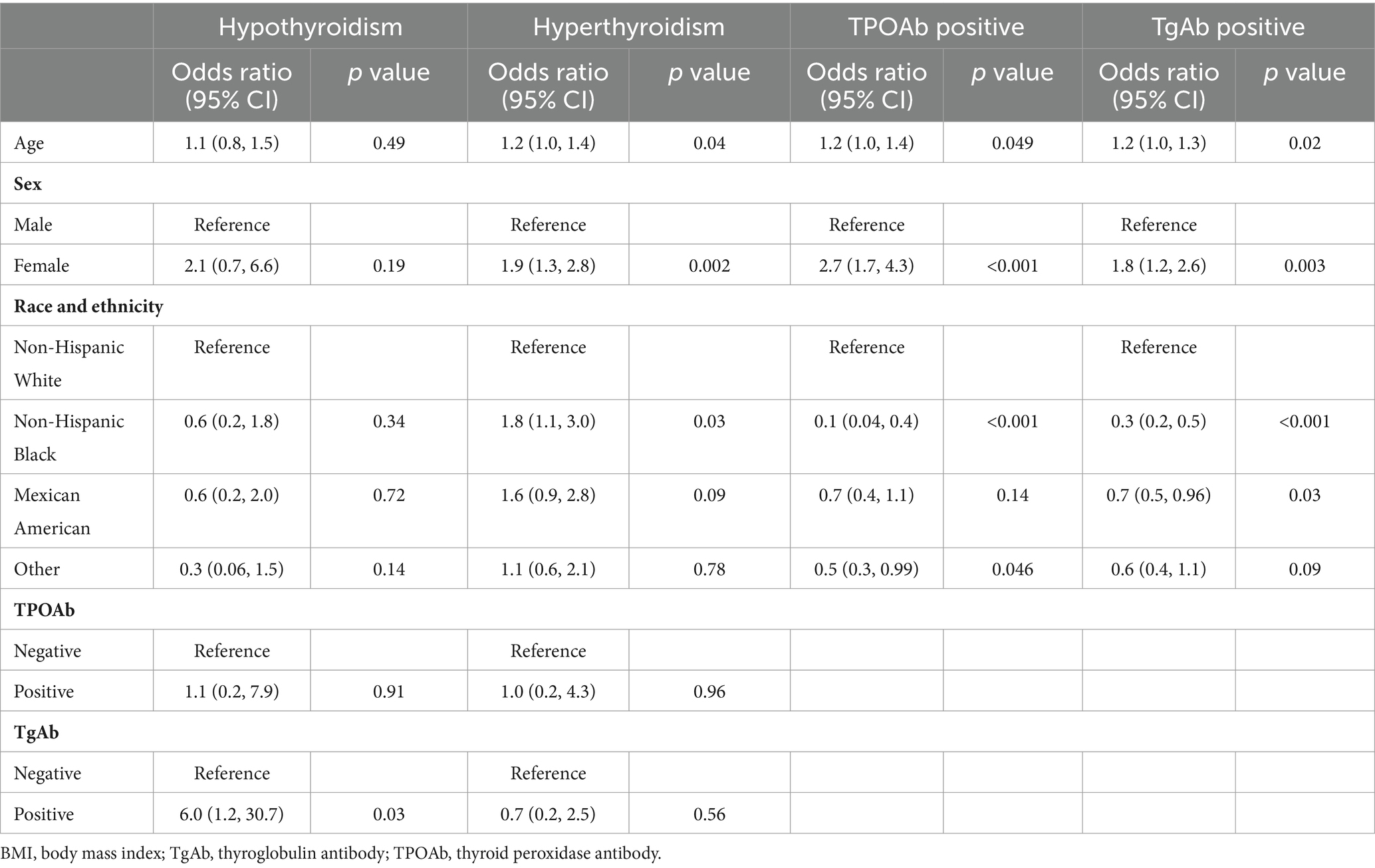
Table 3. Factors associated with thyroid dysfunction and antibody abnormality among US adolescents aged 12–18 years.
Hyperthyroidism prevalence was significantly higher in adolescents as age grows (OR 1.17, 95% CI 1.01–1.35, p = 0.035), in female adolescents compared to males (OR 1.89, 95% CI 1.27–2.83, p = 0.002), and among Black people compared to White people (OR 1.78, 95% CI 1.07–2.99, p = 0.028). TPOAb or TgAb status did not significantly affect hyperthyroidism prevalence.
Female adolescents and adolescents with an older age were more likely to be positive for TPOAb and TgAb. Black and Mexican Americans had a lower risk of TPOAb and TgAb positivity than White Americans (Table 3).
DiscussionOur study represents the first nationally representative investigation in the United States aimed at determining the prevalence of thyroid dysfunction among adolescents. Benefiting from a substantial participant pool, we showed that subclinical hyperthyroidism was the most common form of thyroid dysfunction, affecting 4.4% of the population, with 104 adolescents exhibiting this condition. Conversely, the prevalence of hypothyroid dysfunction was relatively low, impacting <1.0% of adolescents. Overt hyperthyroidism was identified as an rare occurrence among adolescents.
Our findings revealed a notable disparity in the prevalence of hyperthyroidism among different racial and ethnic groups. Non-Hispanic Black people exhibited approximately 1.8 times the likelihood of developing hyperthyroidism compared to non-Hispanic White people. However, no significant difference was observed in the prevalence of hypothyroidism among Black people, Mexican Americans, and White people. White people were more likely to test positive for TPOAb and TgAb, respectively, in comparison to Black people. It is worth noting that, to the best of our knowledge, no previous study has specifically addressed this issue of racial and ethnic differences in the prevalence of thyroid dysfunction in a nationally representative adolescent population in the US. In a population-based study conducted by McLeod et al., utilizing the NHANES database, it was demonstrated that Black people had a higher likelihood of having prevalent thyrotoxicosis than White people, which aligns with our findings. It is important to highlight that the McLeod study encompassed a broader age range, including adolescents and adults aged 12–49 years, whereas our study focused exclusively on adolescents (26).
In our analysis, we observed that female had a 1.9 times higher likelihood of developing hyperthyroidism compared to male. Furthermore, female were approximately 1.8–2.7 times more likely to test positive for TPOAb and TgAb. However, no significant difference in the risk of hypothyroidism was observed between female and male. It is worth noting that studies investigating sex differences in the risk of thyroid dysfunction are scarce in the existing literature, and none have been conducted on a national scale within the US. Interestingly, our findings align with a nationwide study conducted in France, which also reported a higher prevalence of Graves’ disease, the main form of hyperthyroidism in children and adolescents, among girls compared to boys. This study also revealed a significant interaction between age and sex, indicating that as age increases, the ratio of affected girls to boys also increases (27). Similarly, in our study, we observed a higher risk of hyperthyroidism as age advanced among adolescents. However, no such association was found for hypothyroidism.
An interesting finding from our study was the higher prevalence of TgAb positivity compared to TPOAb positivity among adolescents, and that all adolescents who tested positive for TPOAb were also positive for TgAb. In a NHANES III analysis, the overall population showed a prevalence of 13.0 and 11.5% for TPOAb and TgAb positivity, respectively (28). However, among individuals with positive TPOAb, 54.5% also had positive TgAb. These findings highlight distinct profiles of TPOAb and TgAb between adolescents and adults. It is evident that both TPOAb and TgAb serve as indicators of thyroid autoimmunity, albeit with different specific applications in clinical practice. The differences observed in their prevalence and co-occurrence suggest the need for future studies to unravel the underlying mechanisms driving these distinctions.
Considering the relatively low incidence of thyroid dysfunction in adolescents, it may be justified to forego routine thyroid screening in the general pediatric population, instead focusing on specific conditions and disease contexts. While population-based studies have documented the association between subclinical hypothyroidism and cardiometabolic risk factors in young individuals (6), such a relationship with hyperthyroidism has not been established. Future longitudinal investigations are necessary to ascertain whether subclinical hyperthyroidism may elevate the risk for these cardiometabolic factors, potentially leading to adverse cardiometabolic outcomes.
This study’s limitations encompass the limited number of cases for overt hyperthyroidism, lack of follow-up of thyroid function in the included individuals from the NHANES datasets, lack of information regarding puberty, lack of information on supplement intake which can affect thyroid function, absence of information on the etiology of thyroid dysfunction, along with the absence of geographic data and data from the most recent decade.
ConclusionIn conclusion, our study provides crucial insights into the prevalence of thyroid dysfunction among U.S. adolescents, highlighting subclinical hyperthyroidism as the most common form. Significant racial and gender disparities in hyperthyroidism prevalence were observed, with non-Hispanic Black people and females showing higher risks. These findings underscore the need for ongoing research to better understand and manage thyroid dysfunction in adolescents, considering evolving dietary and environmental factors.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by National Center for Health Statistics Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsJC: Investigation, Writing – original draft, Formal analysis. LZ: Conceptualization, Writing – review & editing, Investigation. XZ: Formal analysis, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Methodology, Supervision.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was partly supported by Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Nanjing University School of Medicine (2022-381 YXZX-NFM-02). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1366485/full#supplementary-material
References1. Inoue, K, Ritz, B, Brent, GA, Ebrahimi, R, Rhee, CM, and Leung, AM. Association of subclinical hypothyroidism and cardiovascular disease with mortality. JAMA Netw Open. (2020) 3:e1920745. doi: 10.1001/jamanetworkopen.2019.20745
Crossref Full Text | Google Scholar
2. Chang, CH, Yeh, YC, Caffrey, JL, Shih, SR, Chuang, LM, and Tu, YK. Metabolic syndrome is associated with an increased incidence of subclinical hypothyroidism - a cohort study. Sci Rep. (2017) 7:6754. doi: 10.1038/s41598-017-07004-2
PubMed Abstract | Crossref Full Text | Google Scholar
3. Knudsen, N, Laurberg, P, Rasmussen, LB, Bulow, I, Perrild, H, Ovesen, L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. (2005) 90:4019–24. doi: 10.1210/jc.2004-2225
PubMed Abstract | Crossref Full Text | Google Scholar
4. Gronich, N, Deftereos, SN, Lavi, I, Persidis, AS, Abernethy, DR, and Rennert, G. Hypothyroidism is a risk factor for new-onset diabetes: a cohort study. Diabetes Care. (2015) 38:1657–64. doi: 10.2337/dc14-2515
PubMed Abstract | Crossref Full Text | Google Scholar
5. Louwerens, M, Appelhof, BC, Verloop, H, Medici, M, Peeters, RP, Visser, TJ, et al. Fatigue and fatigue-related symptoms in patients treated for different causes of hypothyroidism. Eur J Endocrinol. (2012) 167:809–15. doi: 10.1530/EJE-12-0501
PubMed Abstract | Crossref Full Text | Google Scholar
6. Chen, X, Deng, S, Sena, C, Zhou, C, and Thaker, VV. Relationship of TSH levels with Cardiometabolic risk factors in US youth and reference percentiles for thyroid function. J Clin Endocrinol Metab. (2021) 106:e1221–30. doi: 10.1210/clinem/dgaa900
PubMed Abstract | Crossref Full Text | Google Scholar
7. Mooij, CF, Cheetham, TD, Verburg, FA, Eckstein, A, Pearce, SH, Leger, J, et al. European thyroid association guideline for the management of pediatric Graves' disease. Eur Thyroid J. (2022) 11:11. doi: 10.1530/ETJ-21-0073
PubMed Abstract | Crossref Full Text | Google Scholar
8. Hollowell, JG, Staehling, NW, Flanders, WD, Hannon, WH, Gunter, EW, Spencer, CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab. (2002) 87:489–99. doi: 10.1210/jcem.87.2.8182
PubMed Abstract | Crossref Full Text | Google Scholar
9. Zhang, X, Wang, Y, Wang, H, and Zhang, X. Trends in prevalence of thyroid dysfunction and its associations with mortality among US participants, 1988-2012. J Clin Endocrinol Metab. (2023) 109:e657–66. doi: 10.1210/clinem/dgad558
Crossref Full Text | Google Scholar
10. Rodanaki, M, Lodefalk, M, Forssell, K, Arvidsson, CG, Forssberg, M, and Aman, J. The incidence of childhood thyrotoxicosis is increasing in both girls and boys in Sweden. Horm Res Paediatr. (2019) 91:195–202. doi: 10.1159/000500265
PubMed Abstract | Crossref Full Text | Google Scholar
11. Wong, GW, and Cheng, PS. Increasing incidence of childhood Graves' disease in Hong Kong: a follow-up study. Clin Endocrinol. (2001) 54:547–50. doi: 10.1046/j.1365-2265.2001.01252.x
PubMed Abstract | Crossref Full Text | Google Scholar
12. Williamson, S, and Greene, SA. Incidence of thyrotoxicosis in childhood: a national population based study in the UK and Ireland. Clin Endocrinol. (2010) 72:358–63. doi: 10.1111/j.1365-2265.2009.03717.x
PubMed Abstract | Crossref Full Text | Google Scholar
13. Rivkees, SA, and Mattison, DR. Propylthiouracil (PTU) Hepatoxicity in children and recommendations for discontinuation of use. Int J Pediatr Endocrinol. (2009) 2009:132041. doi: 10.1186/1687-9856-2009-132041
Crossref Full Text | Google Scholar
14. Zhang, K, Cheng, J, Yu, J, Chen, Y, Shi, X, Zhu, C, et al. Trends in iodine status among U.S. children and adults: a cross-sectional analysis of National Health and nutrition examination survey data from 2001-2004 to 2017-2020. Thyroid. (2022) 32:962–71. doi: 10.1089/thy.2022.0103
PubMed Abstract | Crossref Full Text | Google Scholar
15. Curtin, LR, Mohadjer, LK, Dohrmann, SM, Montaquila, JM, Kruszan-Moran, D, Mirel, LB, et al. The National Health and nutrition examination survey: sample design, 1999-2006. Vital Health Stat 2. (2012) 15:1–39.
PubMed Abstract | Google Scholar
17. Marks, AG, and LaFranchi, SH. Thyroid function testing. 1st ed. New York: Springer (2010).
18. Nelson, JC, Clark, SJ, Borut, DL, Tomei, RT, and Carlton, EI. Age-related changes in serum free thyroxine during childhood and adolescence. J Pediatr. (1993) 123:899–905. doi: 10.1016/S0022-3476(05)80385-3
PubMed Abstract | Crossref Full Text | Google Scholar
19. Strich, D, Edri, S, and Gillis, D. Current normal values for TSH and FT3 in children are too low: evidence from over 11,000 samples. J Pediatr Endocrinol Metab. (2012) 25:245–8. doi: 10.1515/jpem-2011-0494
PubMed Abstract | Crossref Full Text | Google Scholar
20. Lem, AJ, de Rijke, YB, van Toor, H, de Ridder, MA, Visser, TJ, and Hokken-Koelega, AC. Serum thyroid hormone levels in healthy children from birth to adulthood and in short children born small for gestational age. J Clin Endocrinol Metab. (2012) 97:3170–8. doi: 10.1210/jc.2012-1759
PubMed Abstract | Crossref Full Text | Google Scholar
21. Brown, ML, Quinonez, LG, Staffa, SJ, DiNardo, JA, and Wassner, AJ. Relationship of preoperative thyroid dysfunction to clinical outcomes in pediatric cardiac surgery. J Clin Endocrinol Metab. (2021) 106:e2129–36. doi: 10.1210/clinem/dgab040
PubMed Abstract | Crossref Full Text | Google Scholar
22. Yehuda, M, Wang, CH, Pak, Y, Chiu, KC, and Gianoukakis, AG. Parity and risk of thyroid autoimmunity based on the NHANES (2001-2002, 2007-2008, 2009-2010, and 2011-2012). J Clin Endocrinol Metab. (2017) 102:3437–42. doi: 10.1210/jc.2017-00290
PubMed Abstract | Crossref Full Text | Google Scholar
23. Johnson, CL, Paulose-Ram, R, Ogden, CL, Carroll, MD, Kruszon-Moran, D, Dohrmann, SM, et al. National Health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2. (2013) 161:1–24.
PubMed Abstract | Google Scholar
24. O'Hearn, M, Lauren, BN, Wong, JB, Kim, DD, and Mozaffarian, D. Trends and disparities in cardiometabolic health among U.S. adults, 1999-2018. J Am Coll Cardiol. (2022) 80:138–51. doi: 10.1016/j.jacc.2022.04.046
PubMed Abstract | Crossref Full Text | Google Scholar
25. Parker, JD, Talih, M, Malec, DJ, Beresovsky, V, Carroll, M, Gonzalez, JF, et al. National Center for Health Statistics data presentation standards for proportions. Vital Health Stat 2. (2017) 175:1–22.
26. McLeod, DS, Cooper, DS, Ladenson, PW, Whiteman, DC, and Jordan, SJ. Race/ethnicity and the prevalence of thyrotoxicosis in young Americans. Thyroid. (2015) 25:621–8. doi: 10.1089/thy.2014.0504
PubMed Abstract | Crossref Full Text | Google Scholar
27. Simon, M, Rigou, A, Le Moal, J, Zeghnoun, A, Le Tertre, A, De Crouy-Chanel, P, et al. Epidemiology of childhood hyperthyroidism in France: a Nationwide population-based study. J Clin Endocrinol Metab. (2018) 103:2980–7. doi: 10.1210/jc.2018-00273
PubMed Abstract | Crossref Full Text | Google Scholar
28. Dwivedi, SN, Kalaria, T, and Buch, H. Thyroid autoantibodies. J Clin Pathol. (2023) 76:19–28. doi: 10.1136/jcp-2022-208290
留言 (0)