RNA therapy refers to the treatment or prevention of diseases using RNA-based molecules. The first siRNA-based treatment was approved by the Food and Drug Administration (FDA) in 2018 (Figure 1; Kim et al., 2019; Kim, 2022). Since the advent of RNA interference (RNAi), significant progressions has been made in understanding and applying gene silencing mechanisms and treating human diseases (Saw and Song, 2020). Different classes of small RNAs are categorized based on various aspects like the origin, structure, related effector proteins, and biological roles. Based on the coding potential, RNA molecules are categorized into coding RNAs and noncoding RNAs (ncRNAs). ncRNAs are a group of RNAs that do not encode functional proteins, including long noncoding RNAs (lncRNAs), small interfering RNAs (siRNAs), microRNAs (miRNAs), and piwi interactors (piRNAs) are known. SiRNAs, microRNAs, and piRNAs exist only in eukaryotes cells. However, Argonaute proteins, in addition to their eukaryotic silencing function, can also be found in scattered bacterial and archaeal species (Ghildiyal and Zamore, 2009; Kaikkonen et al., 2011). SiRNAs and miRNAs are the most widely distributed phylogenetically. The precursors of these two molecules are double-stranded and both of them can be considered as targets for the treatment of many different diseases, including cancers (Bader et al., 2011; Tabernero et al., 2013; Schultheis et al., 2014), and infections (DeVincenzo et al., 2010; Chandra et al., 2012; Tribolet et al., 2020). On the other hand, piRNAs are animal specific small silencing RNAs. Unlike siRNAs (20–23 nucleotide RNA duplex with 2 nucleotides 3′overhang) and miRNAs (19–25 nucleotide RNA duplex with 2 nucleotides 3′overhang), piRNAs are processed from long single-stranded (24–30 nt) precursor transcripts and do not require Dicer enzyme for processing (Cai et al., 2022). In addition, piRNAs have 2′-O-methyl modified 3′ and direct PIWI-clade proteins. But siRNAs and miRNAs bind to members of the Ago clade of Argonaute proteins (Faehnle and Joshua-Tor, 2007).
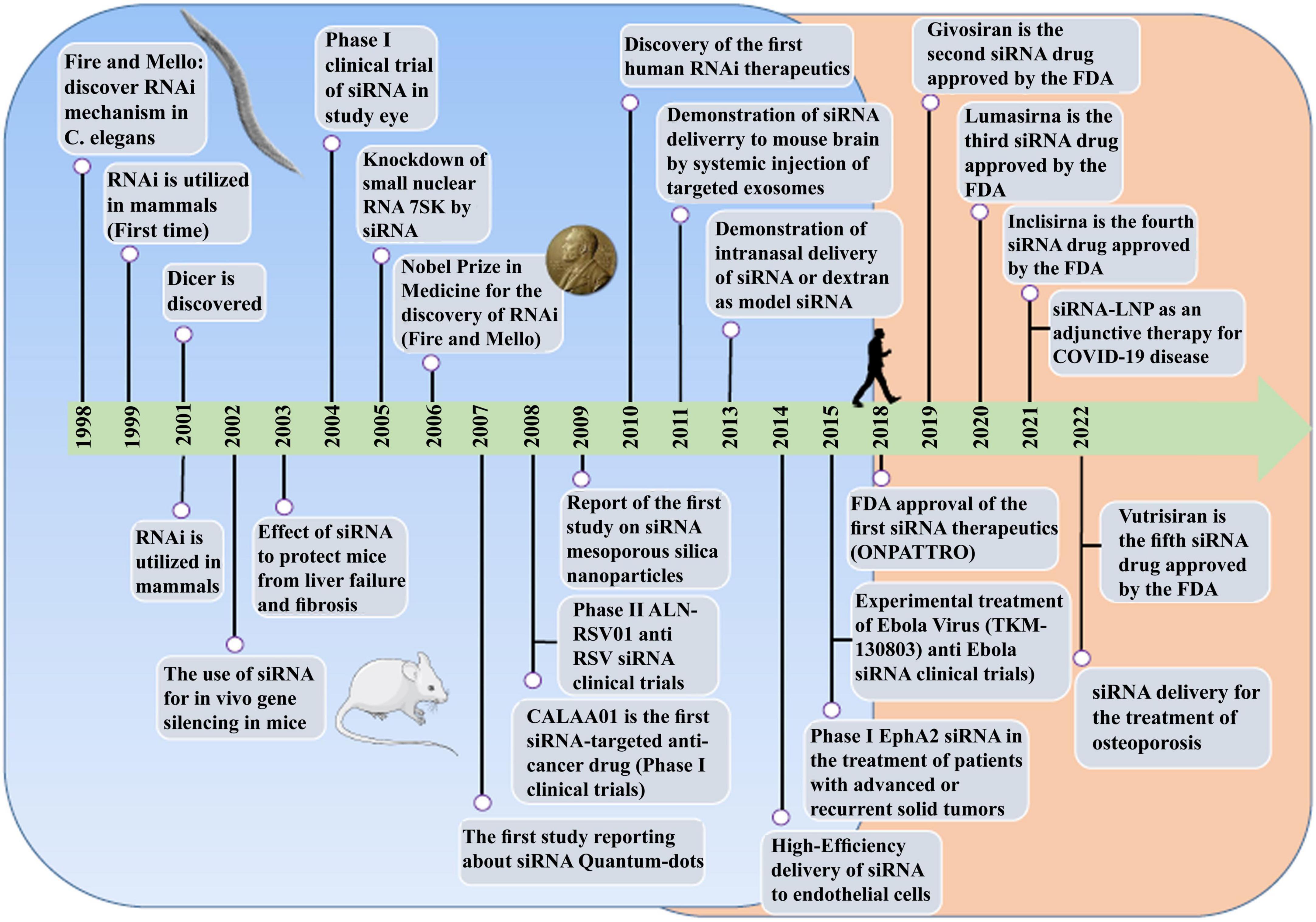
Figure 1. The comprehensive event of the discovery and elucidation of the RNAi pathway.
Small interfering RNA molecules are potential therapeutic agents for a wide range of diseases, including cancer, viral and bacterial infections (Yanagihara et al., 2006; Lares et al., 2010). SiRNAs can protect the cells by inhibiting the replication of viral agents like human immunodeficiency virus (HIV), influenza virus (INFV), hepatitis B virus (HBV), hepatitis C virus (HCV), SARS coronavirus (SARS−CoV), human papillomavirus (HPV), and West Nile virus (Tan and Yin, 2004; Qureshi et al., 2018). Accordingly, it seems that RNAi-based drugs are suitable options for the treatment of several severe viral infections (Qureshi et al., 2018),but more studies are needed for the use of these molecules as preventable vaccines for viral infections. On the other hand, siRNAs have inhibited the expression of genes in bacterial infections (Yanagihara et al., 2006; Zhang et al., 2007; Xu et al., 2009). Also, bacteria can be used as carriers to transfer silencing genes. For example, attenuated Salmonella enterica serovar typhimurium (Salmonella typhimurium) was used as a vectors to deliver hairpin silencing RNA (shRNA) expression plasmids to mammalian cells in 2007. This approach led to gene silencing in vitro (cancer cell lines) and in vivo (mice containing implanted tumors) (Zhang et al., 2007).
The potential application of siRNAs as a probable therapeutic is being investigated in several clinical trials (Setten et al., 2019). Since 2004 until now, when the first clinical trial targeting siRNA was introduced by intravitreal injection in patients with age-related macular degeneration (AMD), extensive research has been conducted on the application of siRNA in therapy. The clinical trials were performed to investigate the effects of siRNA to treat diseases like non-arterial anterior ischemic optic neuropathy (Solano et al., 2014), delayed graft function (Lam et al., 2012; Peddi et al., 2014), transthyretin-mediated amyloidosis (Coelho et al., 2013; Suanprasert et al., 2014), cancer (Davis et al., 2010; Tabernero et al., 2013), hepatitis B (Wooddell et al., 2013), Ebola (Davis et al., 2010), hypercholesterolemia (Fitzgerald et al., 2014), diabetic macular edema (Lee et al., 2012), etc. Interestingly five siRNA-based drugs (Patisiran, Givosiran, Inclisiran, Lumasiran, and Vutrisiran) have been approved by the FDA (Table 1) and several potent drugs are in the final stages of phase III clinical trials (Table 2; Friedrich and Aigner, 2022).
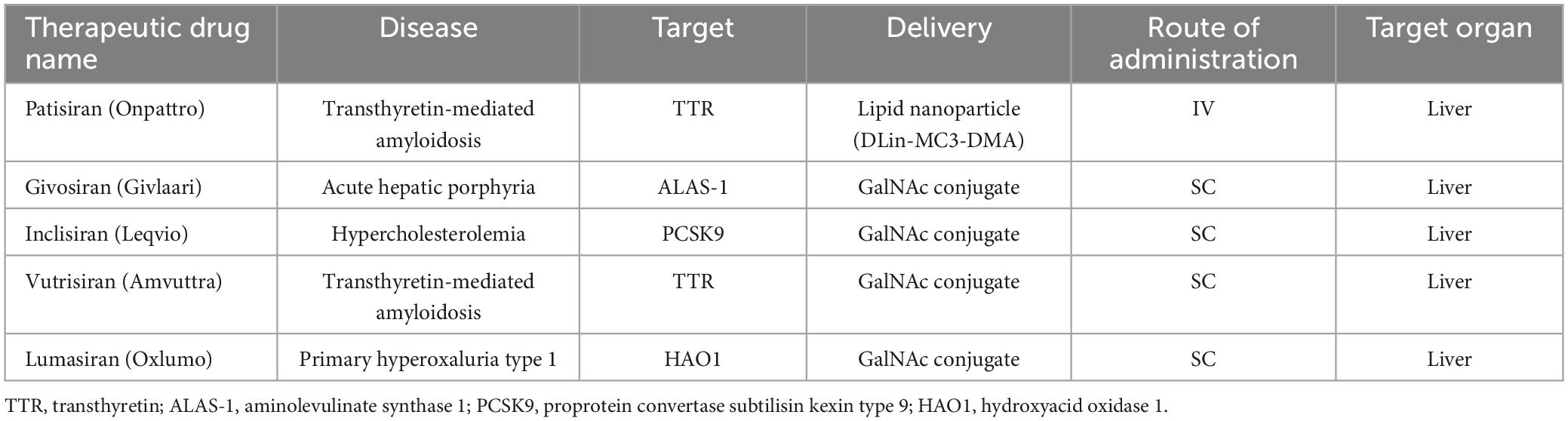
Table 1. Smallinterfering RNA drugs approved by the FDA.
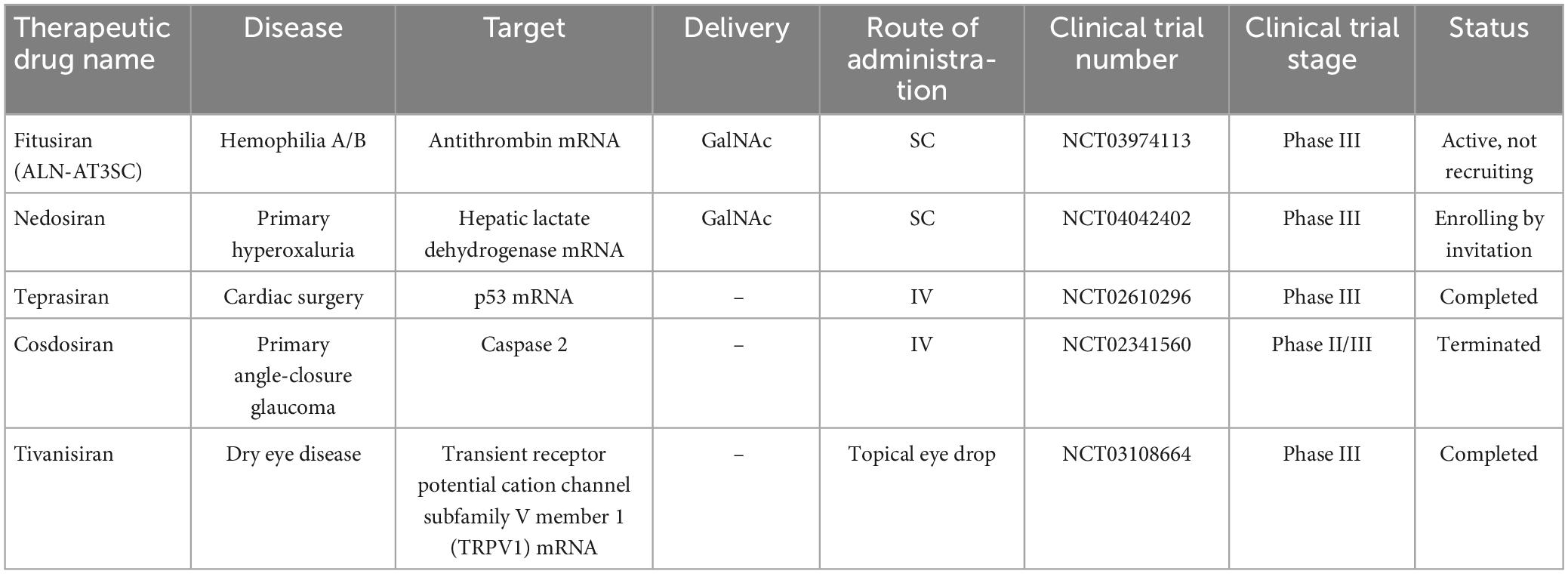
Table 2. Small interfering RNA-based drugs in the final stages of phase III clinical trials.
In this review, we have discussed the key and promising advances in the development of siRNA-based drugs in preclinical and clinical stages, the impact of these molecules in bacterial and viral infection diseases, delivery system issues, the impact of administration methods, limitations of siRNA application and how to overcome them and a glimpse into future developments.
2 Non-coding RNABased on the coding potential, RNA molecules are categorized into coding RNAs and ncRNAs (Figure 2). NcRNAs were initially identified in 1973 as functional non-coding transcripts that do not encode any proteins (Li and Liu, 2019). The regulatory functions of the ncRNAs in eukaryotic and prokaryotic cells were published in the 1980s (Arraiano, 2021). The mechanisms of gene regulation of ncRNAs were recognized in 2002. Considering the nucleotide size, ncRNAs are categorized to small ncRNAs (less than 200 nt) and long ncRNAs (lncRNAs, more than 200 nt). Small ncRNAs classified into the housekeeping ncRNAs like transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear RNA (snRNA), and regulatory ncRNAs like miRNAs, siRNA, and circular RNAs (circRNAs). LncRNAs are categorized based on their structure (linear and circular), area of activity (sense, anti-sense, intronic, and intergenic), and mechanism of action (transRNA, cisRNA and CeRNA) (Delihas, 2015; Bhatti et al., 2021; Winkle et al., 2021). lncRNAs consists of stand-alone lncRNAs, natural antisense transcripts, pseudogenes, and abundant short transcripts (Kung et al., 2013). circRNAs are another type of ncRNAs that have a circular structure that makes them resistant to ribonucleases (Arraiano, 2021).
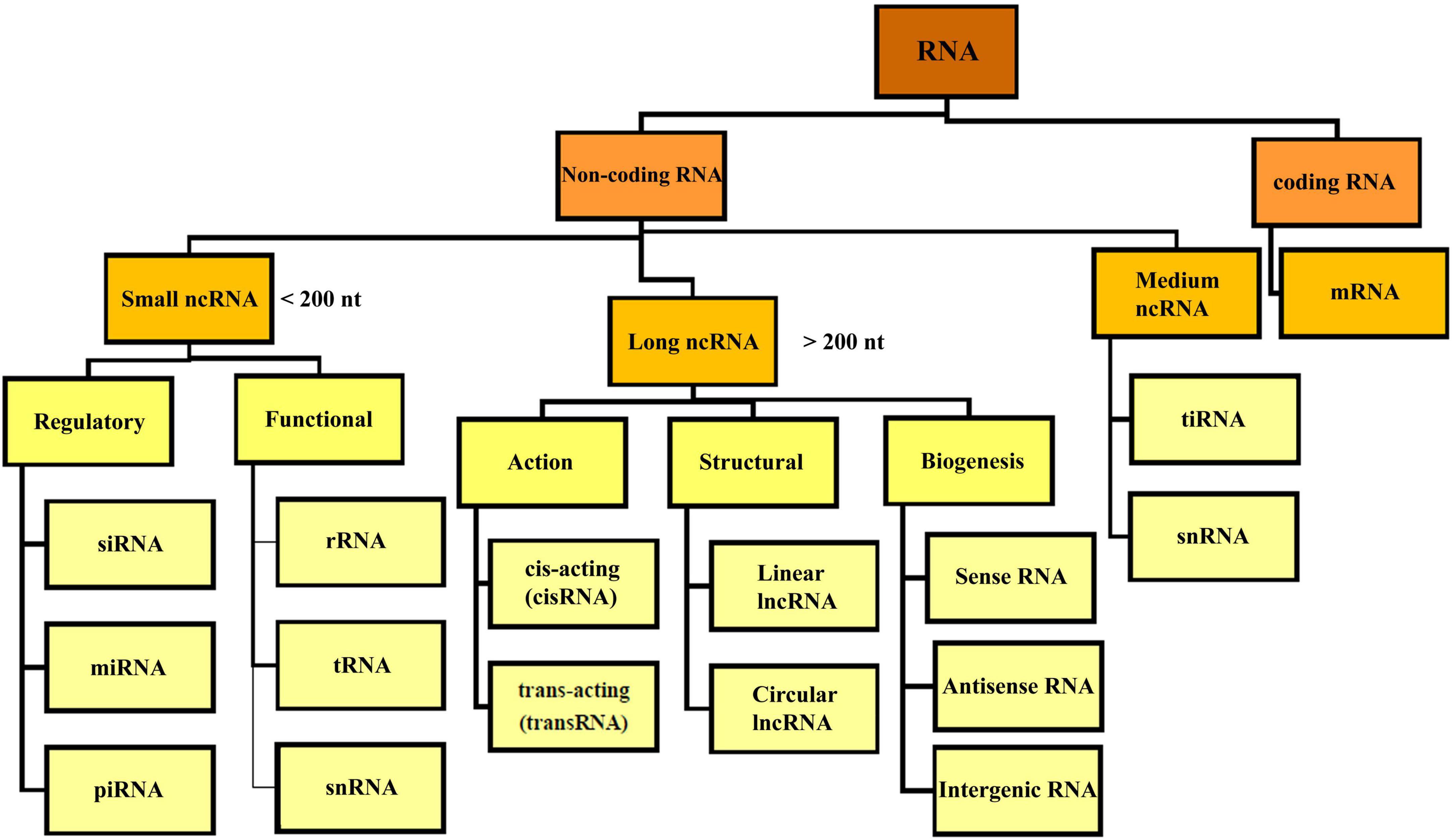
Figure 2. Overview classification of RNA. There are different types of RNA in the cell which are divided into coding and non-coding RNA. NcRNA, non-coding RNA; siRNA, small interfering RNA; miRNA, micro-RNA; piRNA, piwi interacting RNA; rRNA, ribosomal RNA; mRNA, messenger RNA; tRNA, transfer RNA; ciRNA, circular intronic RNA; snRNA, small nuclear RNA; tiRNA, tRNA-derived stress-induced RNA.
MicroRNA was discovered by Victor Ambros in 1993 during his research on lin-4 gene of Caenorhabditis elegans (Lee et al., 1993). MiRNAs are endogenous 17–25 nucleotides single-stranded molecules which are applied for diagnostic purposes and treatment of diseases like lung adenocarcinoma, prostate, gastric, breast, liver, and colorectal cancers (Moran et al., 2014; Arraiano, 2021; Ho et al., 2022). Following the synthesis of long miRNAs by RNA polymerases, pri-miRNAs are produced by ribonucleases in the nucleus and converted into pre-miRNAs with a loop structure. Pre-miRNAs are transferred from the nucleus to the cytosol by RanGTP-dependent double-stranded RNA (dsRNA)-binding proteins like Exportin 5. The pre-miRNAs turn to mature double-stranded miRNA by the RNase III Dicer. MiRNAs accompanied by RNA helicase and the RISC protein complex can perform the task as a gene regulator complex (Winkle et al., 2021). There are potential drugs like RG-012 and MGN-2677 targeting miR-122 and miR-143/145 for the treatment of nephropathy and vascular disease which are under investigation in different clinical trial stages. MRX34 and Remlarsen targeting miR-34a and miR-29 are in phase I clinical trial to study cervical, ovarian and colon cancer, and cutaneous and pulmonary fibrosis, respectively. Currently, Miravirsen targeting miR-122 is being investigated in phase II clinical trial for the treatment of HCV (Chakraborty et al., 2020).
Long noncoding RNAs were first discovered in the 1990s. The biogenesis of lncRNAs are similar to miRNAs, but they are longer about 200 nucleotides (Cabili et al., 2011; Kung et al., 2013). LncRNAs consist of two main parts; the interactor which is important in the interaction with lipid, protein and nucleic acids, and the structural elements that create the secondary and tertiary structures. Such interactions in secondary structures make the lncRNAs more functional compared to miRNAs. lncRNAs have a role in the gene expression regulating, transcriptional and post-transcriptional regulation (Zhu et al., 2013). In addition to regulating gene expression, they contribute in biological pathways such as the immune (Amit et al., 2020) and the nervous system (Andersen and Lim, 2018). Different lncRNAs can have vital roles in regulation of transcription (e.g., TARID, APOLO, and ANRIL), post-transcriptional regulation (PNCTR, PNUTS, TINCR, and TINCR), cellular organelles (RMRP and SAMMSON), structural functions (NEAT1 and MALAT1), and genome integrity (GUARDIN, lincRNA-p21, and DINO) (Statello et al., 2021). LncRNAs based therapy is a promising method to treat various diseases and cancers and several cardiovascular diseases, e.g., heart failure and hypertension, neurological diseases such as Alzheimer, Parkinson, and metabolic disorders like diabetes (Chen Y. et al., 2021). LncRNAs also regulate viral life cycle, viral gene expression and their pathogenesis (Li et al., 2022). Triggering of the immune system leads to the activation of host lncRNAs which are involved in the antiviral response by stimulating the secretion of interferons (IFN-1), so it can be proposed for treatment of coronavirus disease 2019 (COVID-19) (Guttman et al., 2009; Peng et al., 2010). AC009088, LINC02384, AL392172, and HOTAIRM1 are examples of lncRNAs involved in the regulation of COVID-19 by downregulation Pycard, regulating IFN-γ, and IL-17 signaling pathway, respectively (Moazzam-Jazi et al., 2021; Yang et al., 2021). CRISPR–Cas9, siRNAs, antisense oligonucleotides (ASOs), ASO anti-microRNAs (antimiRs), shRNAs, and circRNAs are a number of RNA based therapies (Ling et al., 2013; Rupaimoole and Slack, 2017) which among the aforementioned molecules, siRNAs or ASOs based therapies are approved and licensed by FDA (Winkle et al., 2021). LncRNAs therapeutic application for preeclampsia (PE) and lung cancer are being investigated in different clinical trials.
Small interfering RNA showed successful inhibitory effects on tumor growth, high specificity, low adverse effects, cost-effectiveness, safety, and high efficiency at very low doses. Influencing factor on the efficiency outcomes of siRNA are including as the availability of target sequence on mRNA, stability and structural characteristics of siRNA. In contrast to antisense agents, siRNAs are resistant against nuclease enzymes degradation (Li et al., 2006). In comparison to monoclonal antibody-based drugs, siRNA recognizes its target through pairing the Watson–Crick pattern with mRNA (Hu et al., 2020). As specified by Gupta et al. (2019), the safety of siRNA refers to the fact that there are no chemicals and dangerous materials used in siRNA synthesis processes. Moreover, in contrast to other antisense agents, mode of action of siRNA and their inhibitory effects on gene expressions is carried out in post-translation stages without interfering with DNA or induction of any mutation in its structure (Xu and Wang, 2015; Subhan and Torchilin, 2020). Also, siRNA synthesis is cost-effective because it does not require expensive complex tools. They can undergo changes by some modifications which can confer beneficial effects on their stability in serum and inhibitory efficacy (Braasch et al., 2003; Chiu and Rana, 2003).
3 Mechanism of action of RNAiRNA interference has evolved to control gene expression in various organisms (Robb et al., 2005). The mechanism of miRNA and siRNA gene silencing at the post-transcriptional level is depicted in Figure 3. Although some structural similarities are observed in the molecules, the mechanism of action and clinical applications are different (Lam et al., 2015). MiRNAs are small non-coding RNAs (18–24 nucleotides) that regulate gene expression at the translational level (Kobayashi and Singer, 2022). MiRNA biogenesis initially begins by processing from precursor molecules (primiRNA) in the nucleus. The primiRNA are either transcribed from independent miRNA genes or are part of introns of protein-coding RNA polymerase II transcripts. Pri-miRNAs are folded into hairpin structures and further processed by RNA binding protein DiGeorge syndrome critical region gene 8 (DGCR8) and RNase III type endonucleases Drosha (Filipowicz et al., 2008; O’Brien et al., 2018). DGCR8/Pasha is an essential cofactor for Drosha which has been identified in Drosophila melanogaster (Wu et al., 2012). Unlike Drosha, DGCR8 directly and stably interacts with pri-miRNAs. Microprocessor complex (Drosha-DGCR8) processes pri-miRNAs to about 70-nucleotide hairpins known as pre-miRNAs. Then, the pre-miRNA located in the nucleus is transported to the cytoplasm by Exportin 5 and further cleaved by Dicer (RNase III endonuclease) to generate the 18–25 nucleotide miRNA duplex. The Dicer enzyme cleaves the terminal loop and finally an RNA-induced silencing complex (RISC)-loading is formed with Argonaute protein (Ago1–Ago4). MiRNA duplex associates with RISC and forms a complex called miRISC. The miRNA duplex is denatured; the passenger strand is discarded. In siRNA processing, AGO2 cleaves the siRNA passenger strand (Liu et al., 2004). In association with the miRNA pathway, miRISC is directed by the guide strand to the target mRNA by partially complementary binding. In the siRNA pathway, the guide strand (antisense) directs the active RISC to the target mRNA, and full complementary binding between the siRNA guide strand and the target mRNA leads to mRNA cleavage.
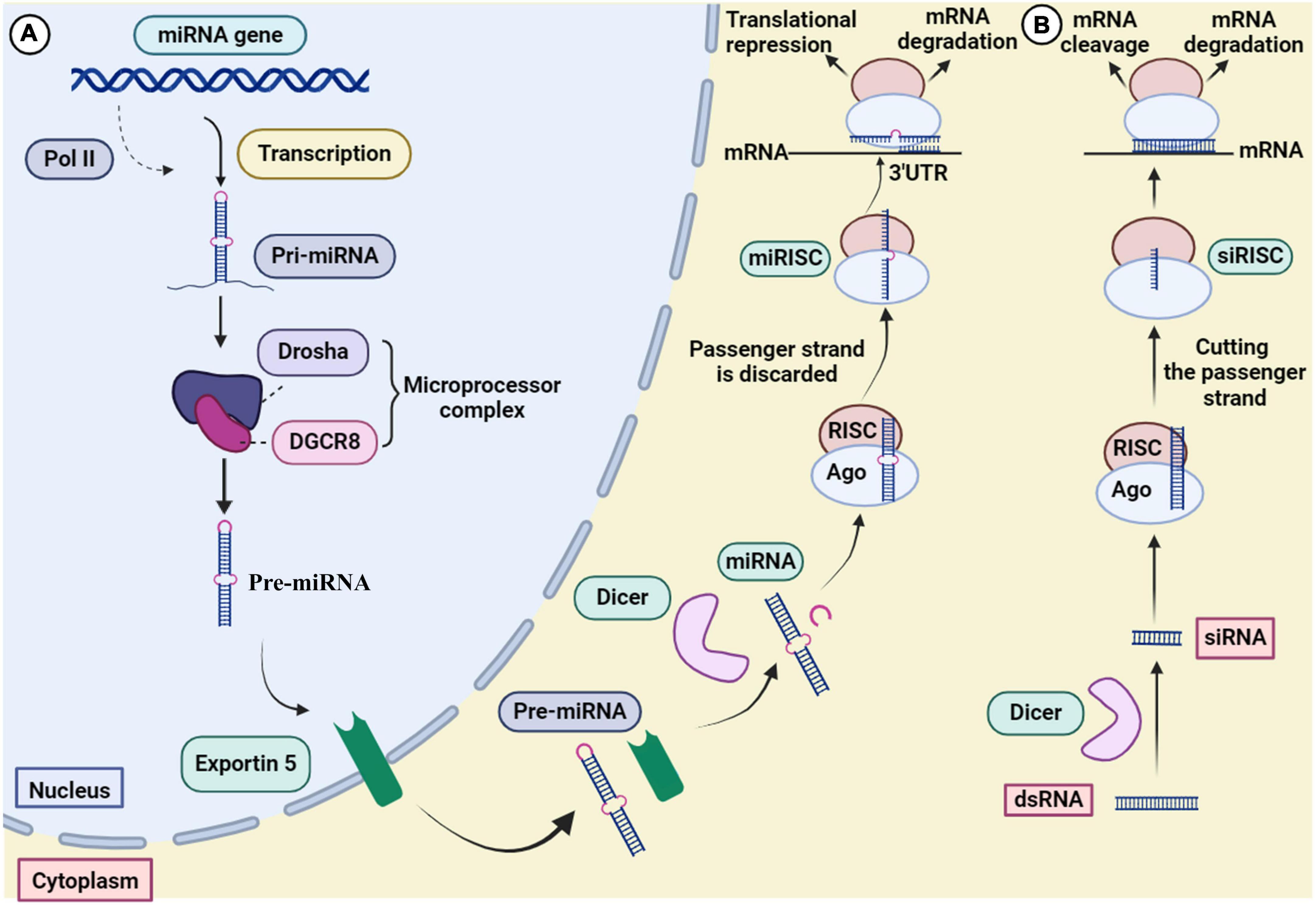
Figure 3. Mechanism of gene silencing through (A) miRNA and (B) siRNA.
4 Developments in siRNA therapeutics for cancerRNA interference manage to switch off the main genes involved in cancer and viral infections (Saraswathy and Gong, 2014; Siegel et al., 2014). Same as viral diseases, siRNAs are effective in the treatment of cancers by blocking malignancy-related genes (Table 3; Elbashir et al., 2001; Debela et al., 2021). SiRNA affect the tumor in three ways: inhibition of angiogenesis, inhibition of tumor survival and induction of apoptosis. The process of angiogenesis and vascular endothelial growth factor receptor (VEGFR-1 and VEGFR-2) as the main glycoprotein involved molecule play role in regulation of the of tumor cells growth, metastasis and preventing the death of vasculature by activating signaling pathways like Ras-MAPK (mitogen-activated protein kinase) pathways (Chen and Huang, 2008). The aforementioned pathways are induced by phosphorylation of VEGFR which then followed by cell migration as the initial step of metastasis. VEGF gene and VEGFR gene targeting siRNAs reduce VEGF expression and receptor blocking, and finally inhibition of angiogenesis (Kou et al., 2005). Proto-oncogenes and oncogenes as the main causes of cell growth induction and/or anti-apoptosis effectors can potentially be inactivated by siRNAs. Also, the inhibitory function of siRNAs in silencing of the Wnt pathway was shown in breast and lung cancers (Rosell et al., 2006; Wieczorek et al., 2008).
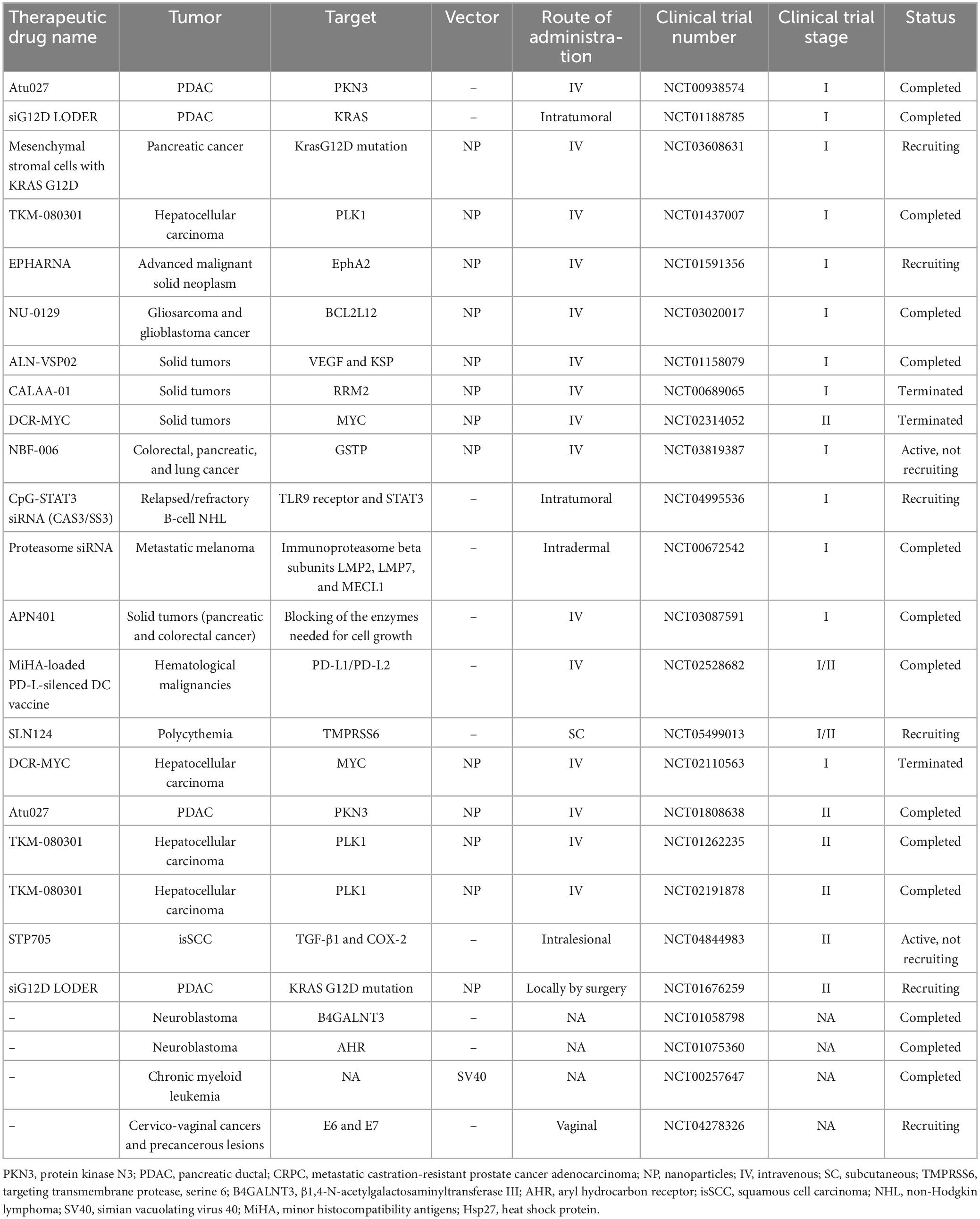
Table 3. Anticancer siRNA in pre-clinical and clinical trials.
Heparin-binding EGF-like growth factor (HB-EGF) is involved in biological processes like skin wound healing, heart and eyelid development, and the formation of malignant tumors through the interaction with the signaling molecules downstream of ErbB receptors. As HB-EGF levels are increasing in cancers, it seems that HB-EGF expression is essential in tumorigenicity. Introducing specific drug to target HB-EGF can inhibit tumor growth. For example, Okamoto et al. (2018) showed a reduction in HB-EGF expression in Breast Cancer cell line using lipid nanoparticles encapsulating siRNA with a Fab′ antibody against heparin-binding EGF-like growth factor (αHB-EGF LNP-siRNA). Recently, it has been found that NF-κB activation is related to malignant cell survival by inhibition of apoptosis-related genes (Guo et al., 2005; Yu et al., 2020). Direct or indirect suppression of NF-κB pathway, increase the sensitivity of cancerous cells to apoptosis. For example, considering the fact that HIF-1α is important in NF-κB activation, Chen et al. investigated the adeno-associated virus carrying siRNA targeting HIF 1α (rAAV-siHIF) to induce apoptosis in pancreatic cancer cells. rAAV-siHIF results in reduction of HIF-1α expression and activation of apoptosis in MiaPaCa2 cells (Miyamoto et al., 2006).
Human telomerase is composed of two components, human telomerase reverse transcriptase (hTERT) and RNA (hTR). Telomerase manage immortality and malignancy development through hTERT. hTERT exist in low levels in healthy cells compared to the same cancer cells. SiRNA targeting hTERT-sensitized cervical cancer cells to radiation therapy by decreasing hTERT mRNA (Wang et al., 2007). HER-2/neu and Bcl-2 as an anti-apoptotic proteins are highly expressed in several cancers like human breast cancer and gastric cancer, respectively. HER-2/neu, as an epidermal growth factor receptor (EGFR) family make cancers resistant to apoptosis and it activates protein kinase B (PKB, or Akt) to phosphorylation and ubiquitination of the mouse double minute 2 (MDM2), which this protein degrades the tumor suppressor p53 protein. MDM2 is mostly found in osteosarcoma, breast and ovarian cancer. Obstructing Bcl-2 and Akt pathway or HER-2/neu by blocking agents like siRNA, decrease Bcl-2 expression in gastric cancer cell, decrease telomerase activity, and increases p53 level which regulate apoptosis and suppress cancer cell growth (Wang et al., 2001; Zhou et al., 2001; Hao et al., 2007; Chen and Huang, 2008).
5 Therapeutics siRNA for microbial infections 5.1 Bacterial infectionUsually, bacteria are not affected by the silencing action of siRNAs because they do not use host cell replication tools (Lieberman et al., 2003; Leung and Whittaker, 2005; López-Fraga et al., 2009). Some bacterial pathogens like Mycobacterium tuberculosis (MTB), Listeria monocytogenes, Mycobacterium fortuitum, S. typhimurium, and Yersiniaceae need host cell facilities for entry and invasion (Agaisse et al., 2005). Inactivating the invasion related genes like SEC22A, Rab1B, and VPS33B by siRNA can prevent bacterial cell entrance to the host cells (Menanteau-Ledouble et al., 2020). In our recent in vitro study, we designed specific siRNA against urease B subunit (ureB) and cytotoxin-associated gene A (CagA) genes from Helicobacter pylori. Both virulence factors play an important role in gastric cancer caused by H. pylori. The findings of our study showed that targeting ureB and cagA genes with siRNA is a new strategy to inhibit urease enzyme activity, reduce inflammation and colonization rate (Motamedi et al., 2023). Menanteau-Ledouble et al., reported that siRNA targets and silence zipper and trigger processes related to genes in Yersinia ruckeri, the causative agent of enteric red mouth in fish. They reported that Rab1A, myotubularin, Lama2, and Rac1 were more strongly silenced by siRNA (Criss and Casanova, 2003; Menanteau-Ledouble et al., 2020). Silencing of Caveolin-2 as a host gene involved in the invasion of Pseudomonas aeruginosa by siRNAs leads to the reduction of bacterial pathogenesis (Zaas et al., 2005). SiRNA accelerates the clearance of microorganisms by regulating inflammation (Gong et al., 2014). For example, siRNA can reduce the excessive amount of tumor necrosis factor-α (TNF-α) in inflammatory conditions like sepsis (Sørensen et al., 2003). The genes of glutamine synthetase and β-hexosaminidase, the enzymes involved in Mycobacterium cell wall biosynthesis and peptidoglycan hydrolase activity, respectively, can be considered as potential targets for silencing by siRNA (Harth et al., 2000; Koo et al., 2008). Also, siRNA is a promising tool for combating drug-resistant bacterial infections. Yanagihara et al. (2006) showed that silencing coagulase gene reduce staphylocoagulase gene expression and MRSA count in pulmonary infections. In another study, application of siRNA targeting MexB efflux pump gene in P. aeruginosa significantly decreased the bacterial load in lung infections (Gong et al., 2014). Bacterial strains like attenuated Salmonella are used as vehicles for siRNA delivery to melanoma, cervical, and colorectal cancer cells (Zhao et al., 2019; Chen J. et al., 2021). Combination of chloroquine as an anti-malarial agent and novel anti-cancer drug with an anti-Programmed Death-1 (PD-1) siRNA which carried by recombinant attenuated Salmonella to Colon Cancer, showed a significant reduction in cancer cell survival by induction of apoptosis (Lu et al., 2021). Co-administration of PD-L1 targeting siRNA and lenvatinib can improve the treatment of Hepatocellular (HCC) carcinoma (Chen et al., 2022).
As electrostatic interaction and hydrophobic-hydrophilic balance consider for are considered an antibacterial polycation carrier and optimized gene delivery, Zhang et al. (2022) showed siRNA delivery with (triblock amphiphilic polycation micelles) TDDE-3 micelles confer strong antibacterial activity with low MIC need for Escherichia coli and Staphylococcus aureus.
5.2 Viral infectionIn recent years, scientists have widely used RNAi to target a number of viral genes to inhibit their expression as well as therapeutic applications (Leonard and Schaffer, 2006). SiRNAs offer a promising therapeutic strategy to combat viral pathogenesis as these molecules target various genes of lethal viruses such as HBV, HCV, HIV, influenza virus, SARS−CoV, HPV, and WNV in infected cells displayed encouraging results in inhibiting viral replication. We have referred to the clinical trials that have been conducted so far for various human viruses based on the direct effect of siRNA to inhibit viral infections.
5.2.1 Respiratory syncytial virusRespiratory syncytial virus (RSV) belongs to the Paramyxoviridae family and is a nonsegmented negative-strand enveloped RNA virus that is recognized as a major viral respiratory pathogen (Zhang et al., 2005). RSV is the most common cause of hospitalization in infants and also the main cause of bronchiolitis, otitis media and pneumonia in children (less than 1-year-old) (Zhang et al., 2005; Zhang and Tripp, 2008). Currently, there is no effective vaccine for this virus. The RSV genome contains about 15,200 nucleotides, which encode 11 different proteins, including two non-structural (NS1 and NS2) proteins and nine structural proteins (Zhang et al., 2005). For this reason, the use of siRNA to knock down these proteins is an important point that can play a potential role in the treatment of RSV.
The beginning of research related to the role of siRNA in targeting RSV proteins met with good progress. Meanwhile, in 2001 an almost 90% reduction of phosphoprotein (P) from RSV virus was demonstrated using 10 nM dsRNA (Bitko and Barik, 2001). In 2004, researchers were able to specifically prevent and inhibit RSV and parainfluenza by siRNA injected intranasally in mice, with or without transfection reagents (Bitko et al., 2005). In 2005 researchers targeted the RSV NS1 protein and showed that human dendritic cells transfected with siNS1 increased type-I interferons and induced the differentiation of naive CD4+ T cells into T helper type 1 (TH1) cells after RSV infection. They also showed that siNS1 nanoparticles may provide an effective inhibition of RSV infection in humans (Zhang et al., 2005). Three years later, the first clinical trial was conducted in lung transplant patients infected with RSV using siRNA. The siRNA designed for ALN-RSV01 is a 19-bp RNA duplex plus two (2′-deoxy) thymidine at both 3′ ends to prevent its nuclease degradation. The siRNA, ALN-RSV01, targets a highly conserved region in the RSV nucleocapsid (N) protein mRNA. ALN-RSV01 (NCT00658086) is a randomized, double-blind, placebo-controlled and multi-center trial which evaluated the safety and antiviral activity of vaccine in phase II of clinical trial. According to the latest update of NCT00658086, a phase II immunogenicity and safety of the candidate vaccine was performed in 24 participants who received RSV inoculation. ALN-RSV01 was administered daily by nasal spray, 2 days before and 3 days after RSV inoculation. Intranasal injection of ALN-RSV01 was safe and well tolerated and also showed a similar safety profile to saline placebo. Overall, ALN-RSV01 resulted in a 38% reduction in the number of infected individuals and a 95% increase in the number of uninfected individuals (DeVincenzo et al., 2010).
On the other hand, Khaitov et al. (2014) used siRNA to suppress allergen-induced responses for interleukin (IL)-4 and RSV P protein coding gene in BALB/c mouse model. Combined intranasal administration of anti-IL-4 and anti-RSV siRNAs resulted in a significant reduction of IL-4 mRNA and RSV viral RNA, and finally reduced eosinophils in bronchoalveolar lavage fluid and airway inflammation (Khaitov et al., 2014).
The authors investigated the M2-2 protein of RSV, which is important in the regulation of viral RNA transcription and replication. They designed siRNAs that specifically targeted the RSV M2-2 gene and determined their effectiveness at the protein (98%) and mRNA (83.1%) levels (Chin et al., 2016). According to these studies, it seems that important steps have been taken in the treatment of RSV based on siRNA, but more research is needed.
5.2.2 Hepatitis C virusHepatitis C virus is a positive-sense, single-stranded, enveloped RNA virus belonging to the Hepacivirus genus of the Flaviviridae family. HCV is responsible for chronic liver diseases such as hepatocellular carcinoma and cirrhosis (Blanchard et al., 2002). This pathogen contains four structural proteins (Core, E1, E2, and P7) and six non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B). Core, E1 and E2 are known as the main viral components of HCV particles. Meanwhile, P7 and NS2 are essential “cofactors” for virus assembly and NS3 to NS5B form a membrane-bound replicase complex (Jirasko et al., 2010). Currently, due to the high diversity of HCV strains, an effective treatment strategy for this virus infection has not been introduced. Several reports have shown the potential activity of siRNA against HCV, but it has not yet reached the clinical trial stage.
In 2003, Yokota et al. designed several siRNAs to target different parts of the HCV genome. They designed siRNAs to target the 5′ untranslated region (5′ UTR) of HCV genome, which resulted in 80% suppression of HCV replication with low concentrations (2.5 nM) of siRNA (Yokota et al., 2003). Subsequently, another study showed that the designed siRNA could inhibit HCV replication and expression of proteins (NS3-1948 and NS5B-6133) in Huh-7 cells stably replicating the HCV genome (Kapadia et al., 2003). These results indicate that RNAi can be a potential tool to help treat HCV in future studies.
Because HCV replicates in the cytoplasm of hepatocytes, RNA-based antiviral strategies are likely to successfully block the HCV replication cycle. Also, this molecule may inhibit cellular cofactors, such as proteasome a-subunit 7 (PSMA7) or Hu R antigen (HuR). Therefore, siRNAs have been shown to significantly reduce the levels of NS5B protein and HCV replicon RNA by silencing PSMA7 and HuR (Korf et al., 2005). A study transfecting the transcriptional plasmid DNA encoding the HCV 1a genome shown that three siRNAs targeting the E2, NS3, and NS5B regions effectively inhibited the expression of the core protein and the NS5A protein (Prabhu et al., 2005). On the other hand, it has been shown that siRNA targeted against NS5A of HCV genotype 1a inhibits the expression of NS5A and main protein in human hepatoma cells (HepG2) (Sen et al., 2003). In another method, the combination of RNAi mediated by lentiviral vector and IFN-alpha was used to evaluate HCV treatment and the results showed that IFN-α increases gene silencing and inhibits HCV proliferation (Pan et al., 2009). Several other studies have been conducted on the successful effect of siRNAs on non-structural proteins of HCV (Randall et al., 2003; Wilson et al., 2003; Kim et al., 2006; Liu et al., 2006).
Few studies have been conducted to evaluate the antiviral effect of anti-HCV siRNAs. In 2005, RNAi-mediated gene inhibition was first reported in an animal model after direct delivery of shRNAs. In this study, shRNAs against the conserved region of HCV internal ribosome entry site (IRES) were designed to measure their ability to inhibit HCV IRES-mediated reporter gene plasmid expression in human tissue culture cells and a mouse model. Finally, it was shown that specific shRNAs were effective in reducing the expression of luciferase-based on HCV IRES (Wang et al., 2005). Also, a designed siRNA targeting the NS5B region in a mice model reduced luciferase expression from a protein-luciferase fusion by 75% (McCaffrey et al., 2002). Kim et al., demonstrated the use of DTC-Apo consisting of cationic liposomes (DTC) and apolipoprotein A-I (apo A-I) with liver-specific siRNA delivery technology. They considered the potency and durability of gene silencing in mice after a single intravenous injection with DTC-Apo and showed that DTC Apo/HCV-specific siRNA administration inhibited viral gene expression by 65%–75% on the second day in the liver (Kim et al., 2009). Also, other studies showed the effect of siRNA against HCV in a mouse model for liver diseases (Pan et al., 2012).
One of the limitations of siRNA for target gene silencing is the lack of an efficient in vivo siRNA delivery system. The usage of the cationic lipid DOTAP was expanded in studies because it increases the complex formation with polyanionic nucleic acids such as siRNA and facilitates the interaction with the cell membrane. A cationic lipid-based anti-HCV (DOTAP) approach called nanosome was investigated along with several siRNAs targeting different sites of the HCV 5′-UTR. Meanwhile, systemic administration of combined siRNA-nanosomes in BALB/c mice is well tolerated without liver damage or tissue toxicity, and this indicates a significant reduction of HCV proliferation in a liver tumor-xenotransplant mouse model (Chandra et al., 2012). In addition, the evaluation of the effect of siRNA on protein kinase C-related kinase 2 (PRK2) was shown in vivo. PRK2 specifically phosphorylates NS5B by interacting with the N-terminal finger domain of NS5B and promotes HCV replication. Administration of PRK2 siRNA formulated with lipidoid nanoparticles (ND98 lipidoid, cholesterol, and PEG-ceramide C16) resulted in a decrease in serum HCV RNA titer, in the subcutaneous and orthotopic xenograft of mouse (Moon et al., 2016). Also, systemic administration of siPRK2 using galactosylated lipidoids caused more silencing of host PRK2 in mouse liver (≈80%) and faster suppression of HCV replication in HCV-xenograft mice (Park et al., 2016). The aim of all these studies is to show a promising path for siRNA-based HCV therapy in the future.
5.2.3 Evaluation of siRNA in HBV preclinical and clinical studiesHepatitis B virus is a hepatotropic virus with a partially double-stranded DNA genome of 3.2 kilobases (kb) (Washizaki et al., 2022). Proteins encoded by HBV include Core, pre-Core, Small (S), Middle S, Large S, Polymerase, and hepatitis B virus protein X (HBx) (Song et al., 2021). HBx, which is essential for the initiation and maintenance of replication, is considered the main cancer-associated protein in HBV infection (Zhao et al., 2021). This virus leads to chronic liver diseases such as chronic hepatitis, cirrhosis and hepatocellular carcinoma (McMahon, 2009). One of the promising therapeutic approaches that support the potential functional treatment of hepatitis B is siRNA (Wooddell et al., 2013; Huang et al., 2022). There have been several studies investigating the performance of RNAi in both preclinical and clinical studies (Table 4). The most important molecules in preclinical studies are ARC-520, ARB-1467, ARB-1740, and ALN-HBV.
Table 4. Clinical studies on siRNA in chronic hepatitis B.
A variety of viral and non-viral systems are being developed to deliver siRNA to the liver, tumors, and other tissues in vivo. In 2007, researchers were able to deliver siRNA to liver cells (both in vitro and in vivo), and the result was siRNA Dynamic PolyConjugates (DPC) (Rozema et al., 2007). Six years later, Wooddell et al. (2013) coinjection cholesterol-siRNA with N-acetylgalactosamine-conjugated melittin-like peptide (NAG-MLP) targeting liver cells, which was proposed as a promising treatment method for patients with HBV. The result of this coinjection was the suppression of multilogs of viral RNA and DNA, and proteins with a long duration of action (Wooddell et al., 2013).
Huang et al. (2022) showed significant results of ionizable liposomal siRNA in strong and continuous treatment of hepatitis B. In this study, a potent siRNA targeting HBV was selected and encapsulated with RBP131 with an approximate pKa value of 6.21 to construct a therapeutic formulation named RB-HBV008. They used mouse models (transient and transgenic) to investigate the effectiveness, and the results showed that the expression of viral RNAs and antigens (HBsAg and HBeAg), as well as viral DNA, was dose-dependent and time-dependent in the range of multilog reduction, both in the circulation and in liver tissue is suppressed (Huang et al., 2022).
The chronic agent of HBV, specifically the covalently closed circular DNA (cccDNA), is a highly stable and active nuclear episomal form of the viral genome that plays a key role in the viral life cycle. ARC-520 was the first RNAi therapy that included two siRNAs, cholesterol-siHBV74 and cholesterol-siHBV77, located at positions 118 and 71 bp upstream, respectively, and increased delivery of siRNAs to hepatocytes.
A randomized (phase I, NCT01872065), double-blind, placebo-controlled, single-center study was conducted in Melbourne (Australia) in 54 healthy volunteers (half male and half female) who received an intravenous dose of ARC-520 or placebo. Since the injection of ARC-520 is associated with the release of histamine, oral antihistamine treatment was recommended before starting the injection. The aim of this study was to evaluate parameters such as safety, tolerability, pharmacokinetics, and pharmacodynamics (Schluep et al., 2017). Further studies evaluated the effect of ARC-520 in combination with Entecavir for participants with hepatitis B surface antigen (HBsAg) in a clinical study (phase II, NCT02065336) in chronic HBV patients. The results showed that HBsAg decreased significantly in patients who were negative for HBV e antigen (HBeAg), while it decreased significantly in patients who were not HBeAg positive. On the other hand, ARC-520 reduced serum levels of HBsAg, HBeAg, and HBV DNA in chimpanzees. Arrowhead Pharmaceuticals recently conducted two multicenter, randomized, double-blind, placebo-controlled, multiple-dose phase II studies to evaluate levels of HBsAg reduction after intravenous administration of investigational product ARC-520 in a population of adults with CHB infection (Yuen et al., 2020). These two studies were stopped by the company decision.
Arrowhead Research Corporation was able to introduce another siRNA, ARC-521, into a phase I (NCT02797522) study in 47 participants. This intravenous combination was performed to evaluate the safety, tolerability, pharmacokinetics and antiviral activity in normal adult volunteers and patients with CHB, but a serious side effect was an increase in ALT up to 678 IU/ml as possibly related to the studied drug, it was recorded in the condition of non-adherence of nucleosid(t)e analogs (NA) (van den Berg et al., 2020). Overall, the ARC-520 and ARC-52 studies were discontinued due to lethal toxicity of the delivery formulation EX1 (a version of NAG-MLP).
Recently, a phase IIa clinical trial (NCT03365947) evaluated the efficacy of siRNA JNJ-73763989 (JNJ-3989) plus NA, with/without assembly encapsulant JNJ-56136379 (JNJ-6379, NCT03361956) in patients with to CHB. JNJ-3989 (formerly ARO-HBV), which is being developed in collaboration with Janssen Pharmaceuticals, is administered subcutaneously and is capable of targeting all HBV transcripts. JNJ-3989 was well tolerated in patients with CHB, reducing their HBsAg levels (<100 IU/ml in patients) and maintaining them for 336 days in 38% of patients after the last dose (Yuen et al., 2022).
Another siRNA, ARB1467, was evaluated in a phase IIa (NCT02631096) clinical trial by Arbutus Biopharma Corporation. ARB-1467, administered intravenously, targets viral RNA transcripts packaged within specific lipid nanoparticles (LNPs). Overall, treatment with this combination was well tolerated and no significant elevation of ALT was observed (except for one patient). In addition, the reduction of HBsAg after several doses was shown in HBeAg-negative and HBeAg-positive patients.
ALN-HBV is another combination of RNAi that is provided by Alnylam Pharmaceuticals in phase I/II. The compound, which has a subcutaneous injection, is planned to be studied in several directions: in healthy volunteers, a single ascending dose study and a multiple ascending dose study in CHB patients. The company discontinued development of ALN-HBV01 to advance a new development candidate, ALN-HBV02, which Enhanced Stabilization Chemistry-Plus (ESC+) GalNAc conjugate technology.
5.2.4 Ebola virusEbola virus, a fatal hemorrhagic viral disease, is a negative-sense RNA virus belonging to the Filoviridae family (Yuan et al., 2022). In a preclinical study, LNP-encapsulated siRNAs designed to target Ebola virus were able to protect rhesus monkeys against this challenge (Thi et al., 2015). On the other hand, Tekmira (TKM-100802) has been evaluated in guinea pig, non-human primate (NHP), and human phase I clinical trials (Geisbert et al., 2010). TKM-Ebola consists of two siRNAs and targets three of the seven Ebola proteins (L, VP24, and VP35). Administration of TKM-100802 to patients infected with this virus (five patients) and one person as prevention after exposure showed that the efficacy or safety of this compound in the treatment of Ebola virus is not possible (Kraft et al., 2015; Liddell et al., 2015). Then a new formula of TKM-100802, TKM-130803 was presented. This new formulation included two nucleotide substitutions in siRNA VP35 and one nucleotide substitution in siLpol-2. Administration of TKM-130803 by intravenous infusion had no survival benefit (Kraft et al., 2015). However, according to the mentioned studies, siRNAs showed poor clinical results against the Ebola virus.
5.2.5 Human immunodeficiency virusHuman immunodeficiency virus belongs to the genus Lentivirus and is grouped in the family Retroviridae, which causes acquired immunodeficiency syndrome (AIDS) (Baba et al., 2000). Two types of HIV have been identified: HIV type 1 (HIV-1) and HIV type 2 (HIV-2). HIV-1 is more dangerous and infectious than HIV-2. In 2002, for the first time, a study was conducted to treat HIV-1 infection using siRNAs. In this study, with designed siRNAs, they were able to 4 logs of inhibition of expression from the HIV-1 DNA (Lee et al., 2002). Two strategies can be defined to inhibit the replication of HIV through RNAi. The first is to target the structural genes gag, pol, and env, the regulatory genes rev and tat, and the accessory genes vpu, nef, vpr, and vif, and the second is to target the cellular genes required by HIV for replication (Bennasser et al., 2007; Bobbin et al., 2015). Several studies have been conducted in relation to the inhibition of HIV replication by siRNAs using cell culture methods, but only a few of them have reached the clinical trial level.
6 Criteria for designing any anti-cancer and anti-infection siRNA drugsThere are several criteria proposed for designing siRNA and siRNA delivery systems, especially in cancer therapy. SiRNA delivery is limited by their large size, which is sometimes up to 13 kDa and negatively charged because of phosphorylation at both 3′ ends which led to low bioavailability and weak penetration across the cancerous cell membranes (Whitehead et al., 2009). Formulation of siRNA with nanoparticles, polymers and protein or lipid-based systems, co-administration of siRNA with anticancer drugs as well as applying chemical modifications to the structure of siRNA are good solutions for the aforementioned limitations. Suitable chemical alterations leads to increase the siRNA stability and persistence in serum, a reduction in its side effects and better penetration into vascular barriers and tissues (Singh et al., 2018). Therefore, ideal siRNA delivery systems results in reduced interaction with normal body cells and serum proteins, specific delivery of siRNA to the target area compared to normal tissues, resistance to fast clearance and resistance to degradation by serum nucleases, lack of immunogenicity, while it must be degradable and compatible with the environment (Alexis et al., 2008; Whitehead et al., 2009; Singh et al., 2018). The criteria for the design of siRNAs are having 30%–52% G/C content, 3 “A/U” bases at positions 15–19, an “A” base at position 19, an “A” base at position 3, “U” base at position 10 in sense strand, absence of internal repeats and stability of sense and antisense strands (Reynolds et al., 2004). Thermodynamic properties of siRNA and accessibility of the target mRNA, as well as the availability of free ends of antisense siRNA are other factors that affect on the efficiency of siRNA (Kurreck, 2006). Off-targeting and immune stimulation also must be considered in drug specificity (Bumcrot et al., 2006; Zhang et al., 2021).
7 SiRNA administration strategiesThere are two strategies for siRNA delivery based on route of administration; local and systemic methods. Local administration is used to treat diseases related to a specific organ of the body, such as the eye, lungs, skin, and oral cavity. Systemic administration is mostly used for the treatment of systemic diseases and metastatic cancer through nervous, gastrointestinal, and respiratory tracts (Figure 4; Chandela and Ueno, 2019). Reduced side effects and requiring a lower dose are the main advantages of local administration. Systemic administration is much more advanced than local administration, but it also has disadvantages, such as the degradation of siRNA in vessels by enzymes, endocytosis by other cells and their escaping, side effects, less specificity and faster rapid clearance in the body. Using nanocarriers improve systemic administration, which is more clinically used in blood-related diseases and cancers (Wang et al., 2010).
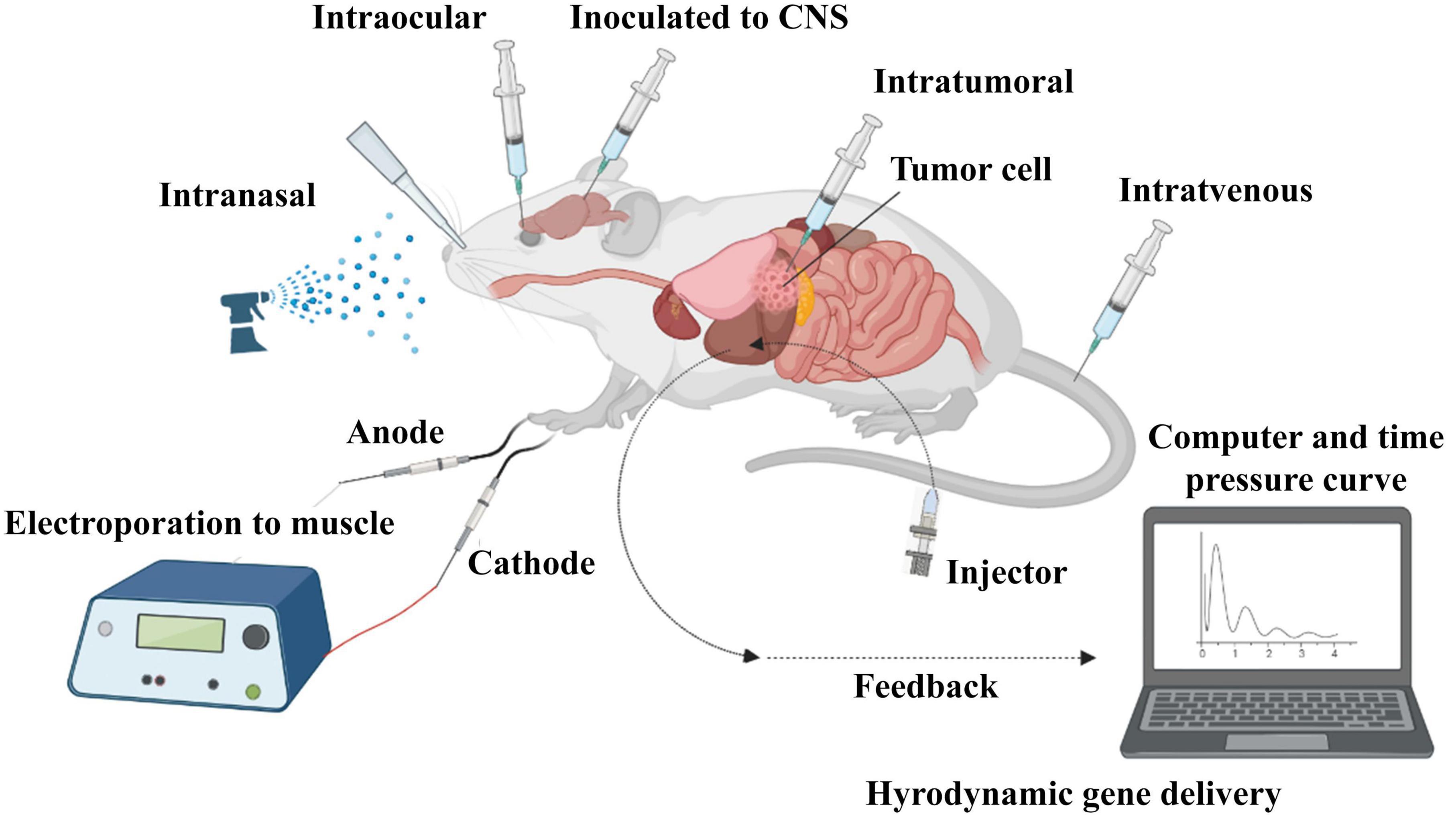
Figure 4. In vivo administration of siRNA. SiRNA was further evaluated in mouse models. Delivery routes are divided into local and systemic ways. Intravenous, intraperitoneal, intratumoral, intraocular, intranasal, intracerebral, and intramuscular are widely used for in vivo delivery of siRNA in studies.
7.1 Local administration of siRNA 7.1.1 Intraocular routeOcular administration is one of the first ways of siRNA delivery due to the ease of access to the eye space and the safety profile (Gupta et al., 2019) which its effectiveness was reported in the treatment of blindness (Conley and Naash, 2010). For example, retinal destruction ganglion cell (RGC), which is followed by two important eye complications, is caused by the action of caspase-2 nuclease. The injection of IVT siRNA selectively prevents the expression of caspase-2 nuclease gene and prevents the occurrence of disorders in anterior ischemic optic neuropathy and glaucoma-related blindness in people. This type of siRNA transfer is not only safe and prevent eye inflammation, but also siRNA is able to stay in the eye for a longer time and exert its effects (Ahmed et al., 2011). Physiological barrier due to constant washing of the eye by the tear film and the impermeability of the epithelial cells of the cornea, conjunctiva, and existence of blood-retinal barrier are the main drawbacks of ocular administration that lead to a decrease in bioavailability and absorption of drug (Bodor and Buchwald, 2005; de la Fuente et al., 2010).
7.1.2 Pulmonary routeTransfer of siRNA to the lungs through the pulmonary route is carried out in three ways: inhalation, intranasal route, and intra-tracheal administration. Pulmonary administration can protect the drugs against nucleases (Bodor and Buchwald, 2005). Local pulmonary administration is applied for treatment of bacterial and viral infections (mycobacterial infections and influenza), lung cancer, hypersensitivities, and respiratory fibrosis (pulmonary fibrosis) (Gupta et al., 2019). As an example, siRNA targeting RSV nucleocapsid synthesis and Na+ channel (ENaC) gene are applied for treatment of RSV upper respiratory tract infection and Cystic fibrosis (Khatri et al., 2012). The disadvantage of this administration is sensitivity to physiological barrier like mucus flow and respiratory cilia movement (Griesenbach et al., 2006; Gutbier et al., 2010).
7.1.3 Administration to CNSGenerally, there are three ways to access the brain space: Intravenous, Intracerebroventricular, and Intranasal administration. Intravenous and intranasal delivery of siRNA to the CSF are non-invasive ways by which siRNA can reach the brain by passing through the blood–brain barrier (BBB). However, intranasal administration is not commonly used due to its limitations in the absorption of siRNA by the nasal epithelium. Intracerebroventricular also has access to the BBB (Nishina et al., 2013). Brown et al. showed that the combination of 2′-O-hexadecyl (C16) with fully modified siRNAs enables safe, potent and durable silencing in the CNS, eye and lung in rodents and non-human primates. C16-siRNAs delivered intrathecally or intracerebroventricularly were active across CNS regions and cell types with sustained RNAi activity for at least 3 months (Brown et al., 2022). IV administration of siRNA conjugated to nine-arginine-conjugated rabies virus glycoprotein peptide (RVG-9R) reaches the neuronal cells and significant GFP silencing in them (Kumar et al., 2007; Shyam et al., 2014).
For instance, increased beta-secretase (BACE1) activity is directly related to amyloid precursor protein (APP) and Alzheimer’s disease (AD). B
留言 (0)