High blood glucose, indicative of diabetes, leads to a significant and often irreversible decline in health, contributing to cardiovascular diseases, kidney failure, and vision loss (1–4). It represents a major cause of disability worldwide, with a growing prevalence especially alarming among the older adult (5). These highlight the urgency in identifying effective primary prevention measures for managing high blood glucose (BG) levels (6, 7).
One of the myriad factors influencing BG levels is the concentration of heavy metals in serum. Beyond dietary influences, the prevalence of diabetes mellitus is notably exacerbated by exposure to various environmental contaminants, including heavy metals, whose presence in the atmosphere has surged alongside industrialization (8–10). Research indicates a substantial positive correlation between the concentration of heavy metals in the blood and their atmospheric levels, suggesting that the accumulation of specific heavy metals in the human body impacts BG concentration (11). For instance, cadmium (Cd) accumulation in insulin-producing β-cells can diminish insulin release and elevate BG levels, while lead (Pb) has been linked to increased insulin resistance and a higher risk of diabetes mellitus (12, 13).
Inflammation also plays a critical role in regulating BG levels. Insights from the Framingham Offspring Study reveal a direct correlation between insulin resistance and elevated markers of oxidative stress. This oxidative stress compromises the ability of muscle and adipose tissues to absorb glucose, as well as impairing pancreatic islet cells’ insulin secretion capacity, thereby contributing to higher BG concentrations (14). Reactive oxygen species generated within the body inflict damage on cellular DNA, membranes, lipids, and proteins, further inducing the expression of inflammatory genes. Such inflammation disrupts insulin-mediated metabolic pathways, culminating in insulin resistance (15).
Serum heavy metal concentrations are mainly related to the external environment, with heavy metals entering the body through inhalation, ingestion, and dermal contact, while inflammation and oxidative stress are usually caused by other in vivo abnormalities (16). Relevant studies have shown that heavy metal exposure induces systemic inflammatory responses, and disruption of metal ion homeostasis can also lead to oxidative stress, for example, Cu induces oxidative stress through two pathways, namely, catalyzing the formation of ROS via a related reaction as well as decreasing glutathione levels, and zinc deficiency increase oxidative damage levels to some degree, thus it is envisioned that serum heavy metals affect BG levels as being mediated by inflammation and oxidative stress in vivo (16–18).
However, although existing researches have highlighted the roles of diet and genetic factors in the onset of diabetes, in-depth studies into the impact of environmental factors, particularly heavy metals, on diabetes were scarce. As environmental exposure to heavy metals such as Cd has been confirmed to increase the risk of diabetes, there still remains a significant research gap regarding how copper, a common environmental metal, affects blood glucose levels through internal biological processes. Moreover, although inflammation was widely considered key pathways in the progression of diabetes, systematic studies on how these pathways mediate the interaction between copper and blood glucose levels are extremely limited. Current research tends to focus on individual biomarkers, overlooking the complexities of the combined effects of multiple markers.
Therefore, the present study was to analyze the correlation between Cu and the concentration of BG and the mediating role of inflammatory factors therein by means of a large-scale cross-sectional study based on the NHANES database.
2 Materials and methods 2.1 Study populationLed by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC), NHANES is a biannual program of studies designed to assess the health and nutritional status of adults and children in the United States. NHANES is designed as a multiyear, stratified, clustered four-stage sample of non-institutionalized civilians with fixed sample-size targets for sampling domains defined by age, sex, race and ethnicity, and socioeconomic status, with data released in 2-y cycles.
Participants gave informed consent of the survey process and their rights as a participant, and the survey was approved by the NCHS Review Board.27 Questionnaires were administered in-home followed by standardized health examinations in specially equipped mobile examination centers. Publicly available, de-identified, and detailed health data sets are available on the NHANES website. We acquired all data from the NHANES database that measured Cu levels in serum, including 3 2-y cycles (2011–2016) and all of the cycles with available data on blood and urinary metals and detailed drug use, to create a larger and more geographically diverse sample.
2.2 Exclusion criteriaData from 3 cycles of NHANES from 2011 to 2016 were used in this study. First, a total of 29,902 individuals participated in the cross-sectional study. After excluding participants without serum copper concentration, inflammatory markers, and BG concentration data types, 2,318 participants were included in this study. In addition to performing PCA by substituting missing values using mean or median for missing values, we excluded participants with missing data, and 2,162 participants were included in subsequent statistical analyses. Finally, for sensitivity analyses (when adjusted for demographic variables and metal concentrations), we excluded participants with missing data on demographic variables, and a total of 1873 participants were included (Figure 1).
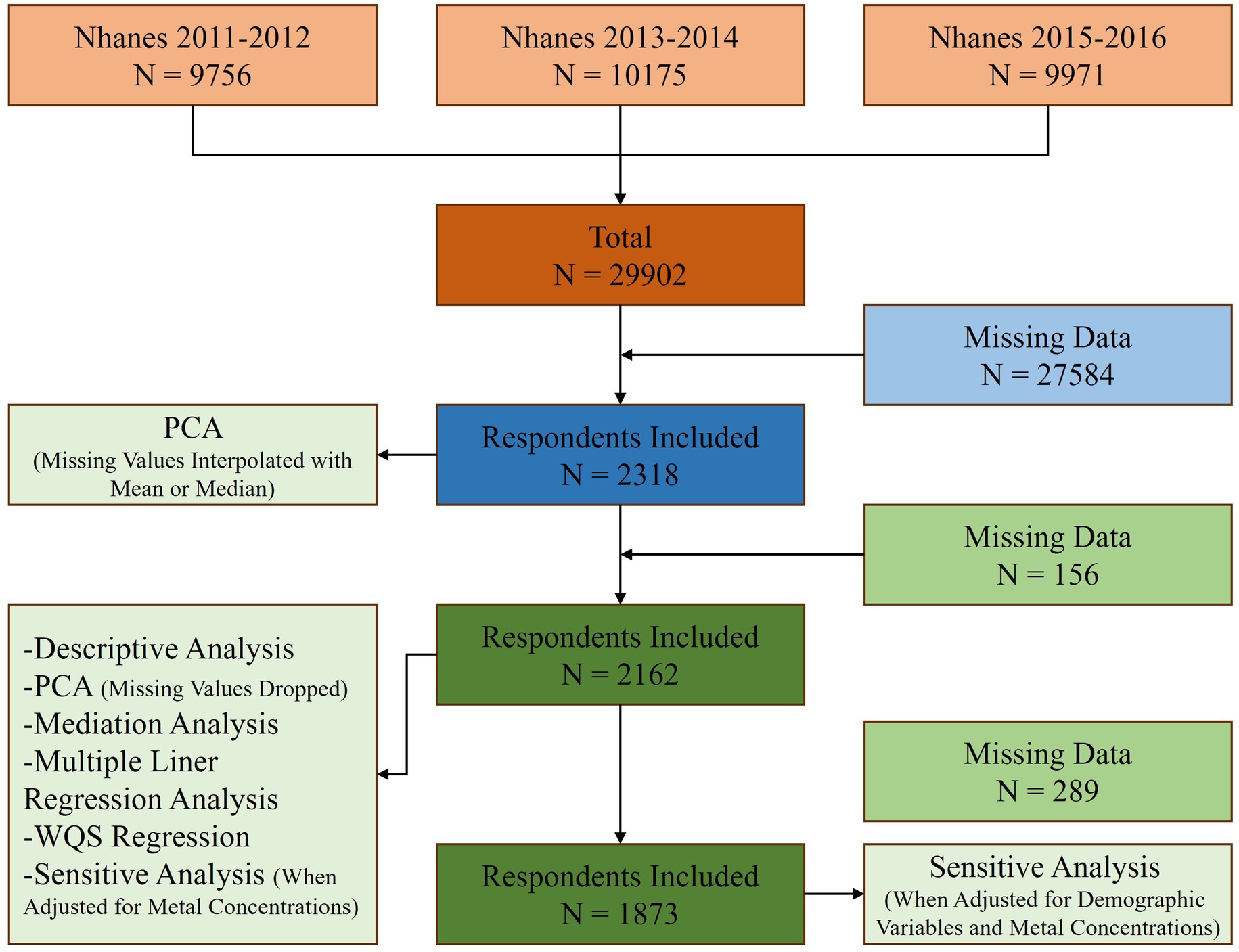
Figure 1. Study flowchart.
The NHANES database was approved by the National Center for Health Statistics and was directly accessible to researchers who met eligibility requirements. All participants in the database signed an informed consent form.
2.3 Measurement of serum CuSerum specimens were processed and stored under appropriate frozen (−70°C) conditions until they were shipped to the National Center for Environmental Health for analysis. Serum Cu concentrations were measured by inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS)—a multi-element analytical technique capable of trace-level elemental analysis. Liquid samples were introduced into the ICP through a nebulizer and spray chamber carried by a flowing argon stream. Radio-frequency power was coupled into flowing argon to form a plasma. The sample passed through a region of the plasma, and the thermal energy atomized the sample and then ionized the atoms. The ions, along with the argon, entered the mass spectrometer through an interface that separated the ICP from the mass spectrometer. The ions passed through a focusing region, dynamic reaction cell, and the quadrupole mass filter, and finally, were counted in rapid sequence at the detector allowing individual isotopes of an element to be determined. The isotopes measured by this method included Zn (m/z 64), Cu (m/z 65), and Se (m/z 78), as well as the internal standard, gallium (m/z 71). Serum samples were diluted 1 + 1 + 28 with water and diluent containing gallium (Ga) for multi-internal standardization.
2.4 Measurement of inflammation biomarkersFasting blood sample of the participants for the laboratory tests was collected by the mobile examination center phlebotomist. The NHANES laboratory manual provides the reference ranges on laboratory parameters in the form of lower and upper limits. Analysis for the complete blood count was done in the mobile examination center, and refrigerated or frozen blood samples were transported and analyzed in the central laboratories for the other parameters.
The DxC800 with lactate dehydrogenase (LDH) reagent (using lactate as substrate) utilizes an enzymatic rate method to measure LDH activity in biological fluids. The DxC600i system or DxC800 system uses a kinetic rate method using a 2-Amino-2-Methyl-1-Propanol (AMP) buffer to measure alkaline phosphatase (ALP) activity in serum or plasma. For the processing of CRP, latex-enhanced nephelometry with particle-enhanced assays was used for quantitation. These assays were performed on a Behring Nephelometer for quantitative CRP determination. The methods used to derive complete blood count (CBC) parameters [white blood cell (WBC), segmented neutrophils (Nsg), lymphocyte (Lym), monocyte count (Mono), eosinophils (Eos) and basophils (Baso)] are based on the Beckman Coulter method of counting and sizing, in combination with an automatic diluting and mixing device for sample processing. The Beckman Coulter MAXM instrument in the Mobile Examination Centers (MECs) produces a CBC on blood specimens.
2.5 Measurement of BGThe method for measuring BG levels involves using the Roche Cobas C311 system, which first requires the patient to be fasting or to undergo an oral glucose tolerance test. Blood is collected via venipuncture, and plasma samples are collected in fluoride-containing gray top tubes, with a minimum volume of 200 microliters. To reduce sugar decomposition, the collected samples must be immediately placed in an ice water bath, and plasma and cells must be separated within 30 min. If testing is not conducted immediately, the samples are then frozen and stored at −70°C. The measurement principle is based on the reaction catalyzed by hexokinase between glucose and ATP to produce glucose-6-phosphate (G-6-P) and ADP, then G-6-P is further oxidized by glucose-6-phosphate dehydrogenase, simultaneously generating NADH directly proportional to the glucose concentration, which is measured by spectrophotometry at 340 nanometers. System calibration and quality control are performed before testing to ensure the accuracy and repeatability of the results. The Roche Cobas C311 automatically calculates the glucose concentration, and the results are reported in mg/dL or mmol/L.
2.6 CovariatesAge, sex, race and ethnicity, education, marriage, and PIR were acquired from self-reported questionnaires on demographic information. Race and ethnicity are classified as Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Race (including Multiple Races) based on self-identification originally designated by NHANES. “Other Race” encompasses all other races, including Non-Hispanic Asians and individuals reporting multiple races. Common exposures to other metals (e.g., dietary) may vary by race and ethnicity, hence are adjusted for in our models. Education is reclassified into Less than 9th grade, 9–11th grade (Includes 12th grade with no diploma), High school graduate/GED or equivalent, Some college or AA degree, and College graduate or above. Marital status is divided into married, widowed, divorced, separated, never married, living with partner, refused, and missing.
2.7 Statistical analysisFirst, we utilized PCA to determine the combination of inflammatory markers adopted in this study. Second, we conducted a descriptive analysis of the serum copper (Cu), inflammatory markers, and BG concentration in the included population, reporting mean, standard deviation, median, and quartiles.
Third, we performed mediation analysis to explore the direct and indirect relationships and the extent of mediation effect using non-parametric bootstrapping (n = 1,000).
Fourth, we conducted linear regression analysis to investigate the mixed effects of different inflammatory markers on glucose concentration. Fifth, we performed WQS regression analysis to assess the comprehensive and individual effects of inflammatory markers on BG concentration by calculating weighted linear indices and assigning corresponding weights. In this study, 10,000 bootstrap iterations were used to construct positive and negative WQS indices. When the WQS index was significant, the corresponding weights were examined to determine the relative contribution of each heavy metal in the index to glucose concentration. The dataset was randomly split, with 40% allocated to the training set and the remaining 60% as the validation set.
Finally, we conducted the sensitivity analysis. We adjusted for demographic variables in mediation analyses and for serum copper concentrations as well as demographic variables in MLRA, WQS regression analyses. We also performed sensitivity analyses of PCA using different missing value treatments to determine the optimal combination of inflammatory indicators for inclusion in the study.
Adjusted for demographic factors, weighted data was not used. All analyses, including WQS, MLRA (Multiple Linear Regression Analysis), and mediation analysis, were conducted using R software. A p-value of <0.05 was considered statistically significant.
3 Results 3.1 General informationThis study involved 2,318 participants (Table 1), evenly split between males (50.17%, n = 1,163) and females (49.83%, n = 1,155), with an average age of 42.70 years (SD = 15.34). The population was diverse, including Mexican American (14.41%), other Hispanic (12.21%), non-Hispanic White (33.69%), non-Hispanic Black (22.30%), and other races (17.39%). Educational levels ranged from less than 9th grade (8.02%) to college graduate or above (26.92%). Marital status varied, with 47.63% married and 21.05% never married. The median poverty income ratio (PIR) was 1.94 (IQR = 0.97–3.81).
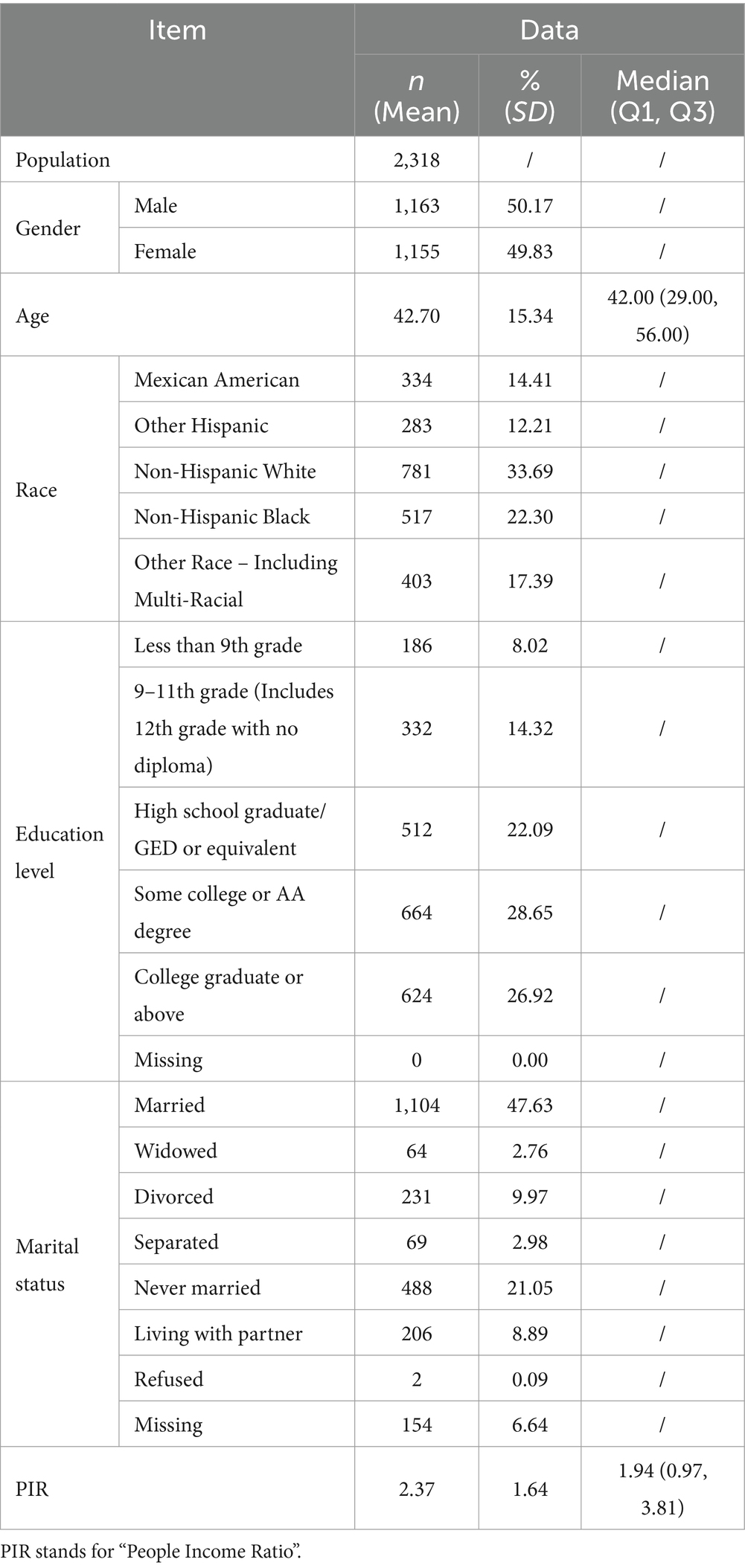
Table 1. Baseline characteristics of participants included in the study.
3.2 Principal component analysisFirst, we conducted a principal component analysis (PCA) to explore the combination of inflammatory markers in the study. We calculated the standard deviation (S.D.) and explained variance (VER) for each marker, resulting in the cumulative explained variance (CVER) across the components.
After imputing missing values with the mean, the analysis indicated that WBC count, Lym percentage, Mono percentage, Nsg percentage, Eos percentage and Baso percentage exhibited higher variances, with their cumulative variance accounting for a substantial majority of data variability (Table 2). Similarly, imputing missing values with the median or removing missing values altogether yielded comparable results.
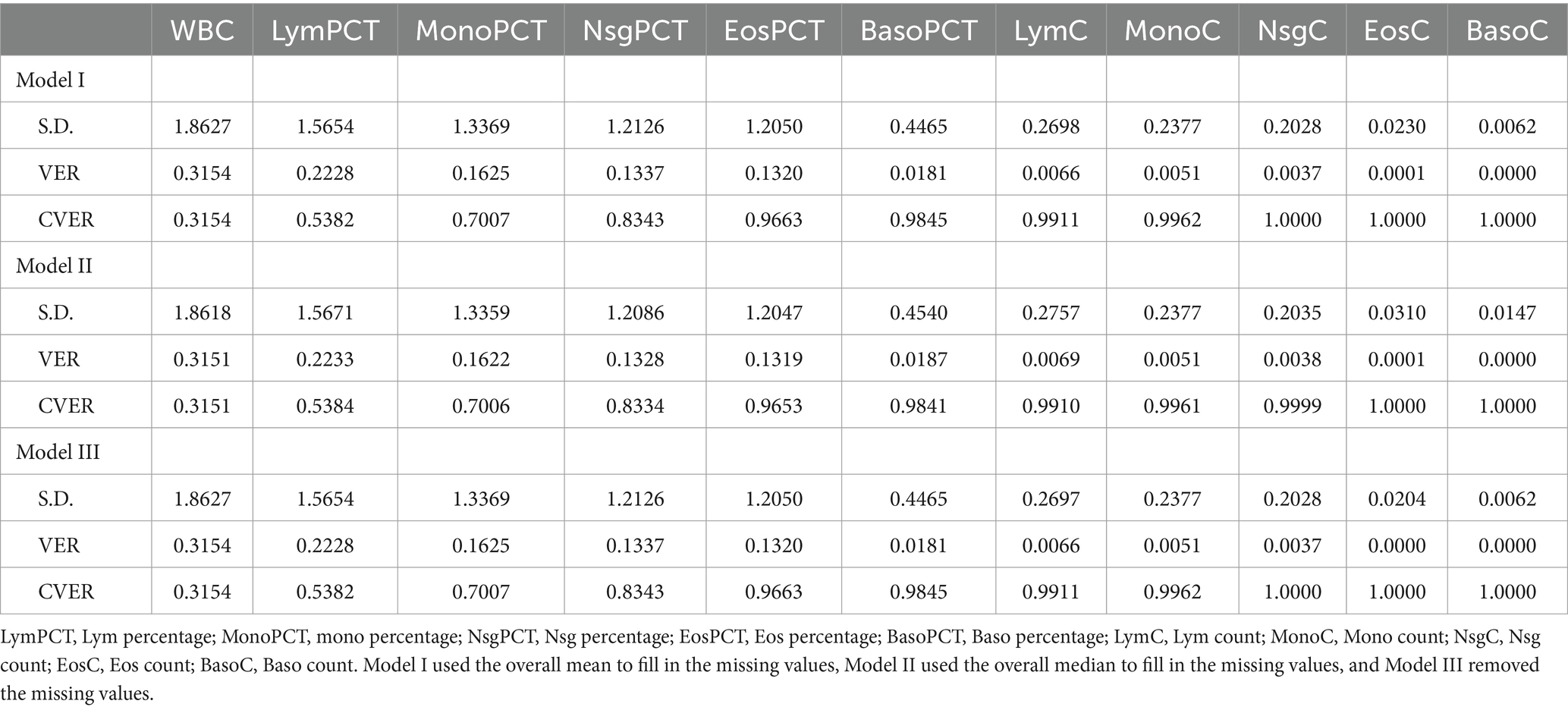
Table 2. Factor loadings of 11 inflammation indicators based on principal component analysis.
3.3 Level of serum Cu, inflammation markers and BGThen, we analyzed the baseline levels for serum Cu, WBC count, Lym percentage, Mono percentage, Nsg percentage, Eos percentage, Baso percentage, and BG (Table 3). The mean serum Cu level was found to be 119.50 μg/dL, with a standard deviation (SD) of 32.04, highlighting a moderate variability among individuals. WBC count averaged at 6.82 × 109/L. Lym percentage, Mono percentage, Nsg percentage, Eos percentage, and Baso percentage demonstrated diverse immune cell distribution, with mean values of 31.67, 7.89, 56.77, 2.98, and 0.76%, respectively. The mean BG level was noted at 107.10 mg/dL (SD = 36.62).
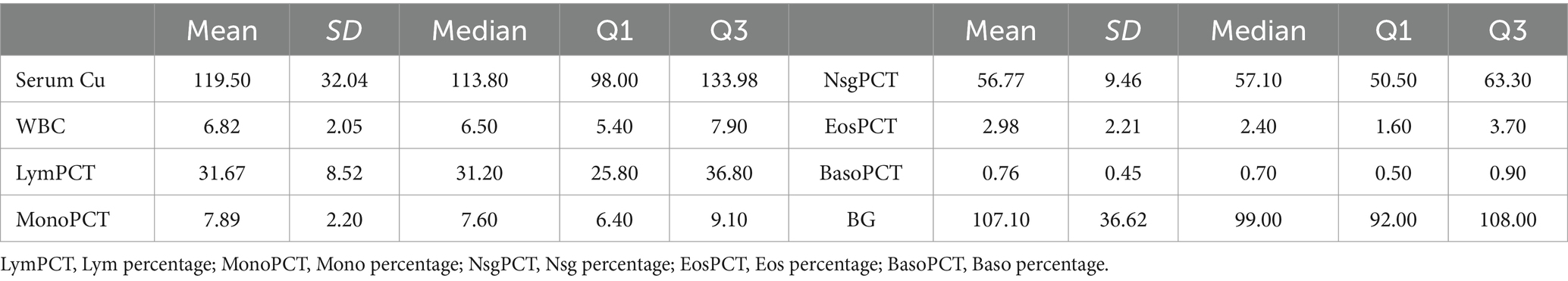
Table 3. Level of serum Cu, inflammation markers and blood glucose among the participants included in the study.
3.4 Mediation analysisThen, we conducted the mediation analysis investigating the role of inflammatory factors in the relationship between serum Cu levels and BG, we focused on four intermediary variables: WBC count, Lym percentage, Mono percentage, Nsg percentage (Table 4). The analysis revealed significant mediation effects, with WBC count showing a substantial mediation proportion of 39.78% (p = 0.0004), indicating a strong mediator role. Lym percentage displayed a moderate mediation effect with a proportion of 10.16% (p = 0.0421), while Mono percentage also demonstrated significant mediation, with a proportion of 33.37% (p = 0.0006). Nsg percentage contributed notably as well, with a mediation proportion of 20.82% (p = 0.0076).
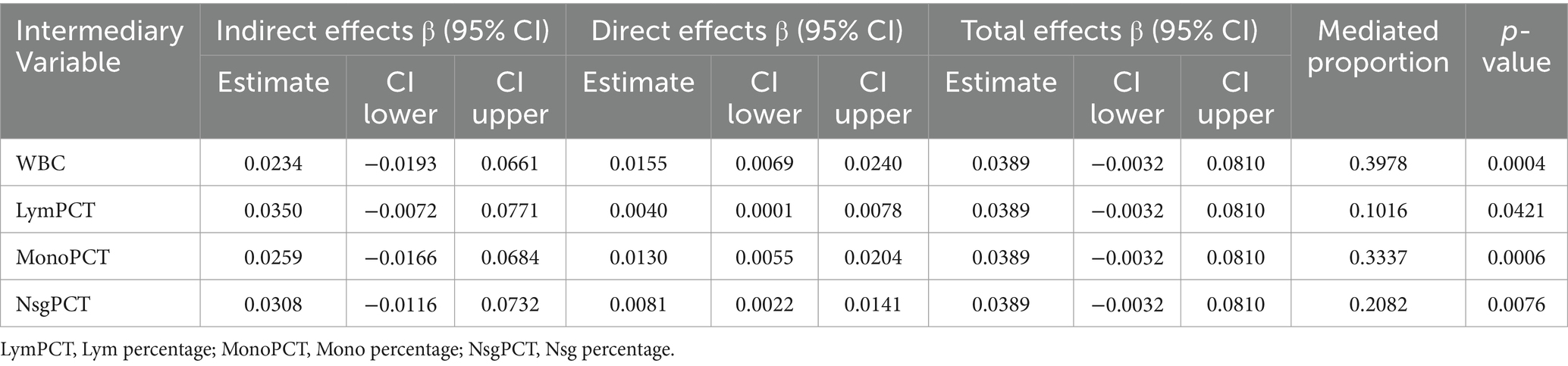
Table 4. The mediating effects of the relationship between serum Cu and BG.
3.5 Multiple linger regression analysisBuilding on the results from the mediation analysis, we further investigated the influence of significant mediatory variables on the relationship between serum Cu levels and BG through multiple linear regression analysis.
The WBC presented a positive and significant association with serum Cu (estimate: 1.077, 95% CI: 0.432 to 2.490, p = 0.013), suggesting higher WBC counts correspond with increased serum Cu levels. Mono showed a notable negative relationship (estimate: −1.573, 95% CI: 0.520 to −3.025, p = 0.003), indicating that higher Mono is associated with decreased serum Cu levels. Lym and Nsg did not demonstrate statistically significant associations (p-values of 0.126 and 0.210, respectively) (Table 5).
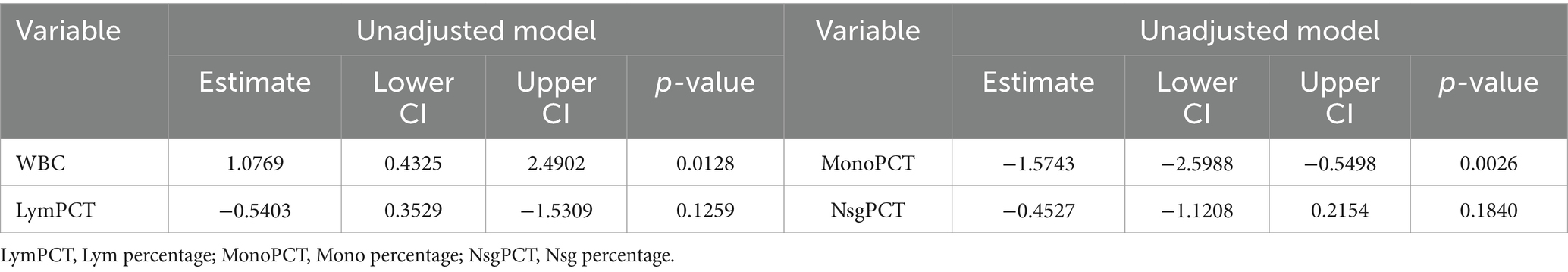
Table 5. Association between potential mediators and BG determined by MLRA.
3.6 WQS regression modelsSubsequently, we employed WQS regression analysis to further explore the weighted impact of inflammatory factors on BG. The WQS regression results showed that the overall model estimate was 4.7167, with statistical significance (p-value = 0.0216), indicating a significant effect of the combination of inflammatory factors on BG. Among the inflammatory factors, Nsg had the highest weight coefficient (0.4472). WBC and Lym had weight coefficients of 0.3558 and 0.1954, respectively. Meanwhile, the weight coefficient for Mono was 0.0015, indicating its relatively minor impact on BG.
3.7 Sensitive analysisWe conducted several sensitivity analyses to verify the stability of our research results. Initially, after adjusting for demographic variables in the mediation analysis, the results showed a significant increase in the mediation effects of WBC and Mono (p < 0.005), while the mediation effect of Lym became non-significant (p = 0.7203) (Supplementary Table S1). Secondly, upon adjusting for serum Cu levels and then for serum Cu levels plus demographic variables in the MLRA, the negative correlation between Mono and serum Cu levels remained significant across all models (P < =0.003), whereas the correlation of WBC became non-significant after including demographic variables (p = 0.072) (Supplementary Table S2). Additionally, in the WQS regression model, after adjusting for serum Cu levels and for serum Cu levels plus demographic variables, the overall estimate of the model significantly increased, with the p-value decreasing from 0.0216 to 0.0002, indicating an enhanced significance of the model under different adjustments (Supplementary Table S3).
4 DiscussionLong-term hyperglycemia can lead to serious complications, including cardiovascular diseases, diabetic neuropathy, nephropathy, and retinopathy (19–23). These conditions significantly increase morbidity and mortality among diabetic patients. Recent studies have also indicated that heavy metals are key factors in the pathogenesis of several diseases, including obesity, metabolic syndrome, and hypertension (24–26). Environmental pollution caused by heavy metals has become an ongoing concern worldwide (27, 28). In recent years, the impact of exposure to individual heavy metals on blood glucose levels has garnered widespread attention. These metals, by interfering with the body’s normal metabolic functions, may increase the risk of diabetes. Research covering various heavy metals, including manganese (29), nickel (30), mercury (31), cadmium (32), and lead (33), has indicated that they disrupt glucose metabolism and insulin sensitivity through different mechanisms, thereby affecting blood glucose levels. However, all of the above studies did not include copper exposure, so it remains uncertain whether copper exposure is associated with abnormal changes in blood glucose in heavy metal mixtures.
In this study, we investigated the serum copper levels, inflammatory markers, and fasting blood glucose levels among 2,318 participants, uncovering a range of meaningful results. The average serum Cu level was found to be 119.50 μg/dL, indicating moderate variability among individuals. Through PCA, we identified that WBC, Lym, Mono, and Nsg as inflammatory markers occupied significant positions in the variability of the data. Mediation analysis further revealed the important mediating roles of WBC, Lym, Mono, and Nsg in the relationship between serum Cu levels and BG, with WBC showing the highest mediation proportion at 39.78%, and Nsg showing the lowest at 20.82%. During MLRA, we observed a positive correlation between WBC and serum Cu levels, while Mono showed a negative correlation with serum Cu levels. Additionally, using the WQS regression model, we explored the cumulative impact of inflammatory factors on BG and found a significant effect of the combination of inflammatory markers on BG levels. Sensitivity analysis confirmed the robustness of these findings, with the estimates of mediation effects and the WQS regression model being strengthened after adjusting for demographic variables.
Accumulating evidence supports the role of inflammation in the abnormal changes in blood glucose (34). Lin et al. (35) revealed the relationship between excessive intake of sugary drinks and abdominal obesity with diabetes and the elevation of C-reactive protein (CRP) levels, emphasizing the positive correlation between sugar intake and CRP levels in adults with prediabetes. Moreover, Mi et al. (36) found that the Dietary Inflammatory Index (DII) is positively associated with insulin resistance in adults of normal and healthy weight, highlighting the potential value of anti-inflammatory diets in the prevention or management of insulin resistance. Furthermore, Yuan et al. (37) explored the relationship between the DII and long-term all-cause and cardiovascular mortality, pointing out that high DII scores are associated with an increased risk of long-term all-cause and cardiovascular mortality in patients with diabetes.
Given this common pathogenesis, it is reasonable to investigate whether Cu exposure leads to abnormal changes in BG through inflammation. In this study, we found that WBC, Lym, Mono, and Nsg were involved in the positive correlation between serum Cu concentration and changes in BG concentration, accounting for 39.78, 10.16, 33.37, and 20.82%, respectively, in the mediation analysis. Therefore, we hypothesized that excessive Cu exposure promotes inflammation and thus increases blood glucose concentration.
The results of our sensitivity analysis underscore the stability of our findings when considering the effects of serum copper levels and demographic variables. Notably, the negative correlation between Mono and serum Cu levels remained consistent across various model adjustments, highlighting the potential of Mono as a robust indicator of serum Cu levels. However, the association between WBC and serum Cu levels became non-significant after adjusting for demographic variables, suggesting the need to consider a broader range of demographic and biological factors when evaluating the relationship between biomarkers and environmental exposure (38, 39). Finally, the results of the WQS model further support the importance of the relationship between serum Cu levels and health outcomes under different adjustments.
Our study possesses several strengths. First, this research constitutes the inaugural investigation into the impact of Cu exposure on BG concentration via inflammatory factors. Second, we utilized a variety of statistical methodologies and adjusted for potential confounding variables to enhance the robustness and reliability of our findings. Third, all data were sourced from a large population database and adhered to stringent quality control measures. However, this study also has certain limitations. First, due to its cross-sectional design, we cannot ascertain the causality between copper exposure and alterations in blood glucose levels. Second, the NHANES database lacks data on uncontrollable factors, such as exposure to wastewater and cosmetics, which might affect the accuracy of our results. Third, not considering the cumulative amount of copper exposure could impact the outcomes.
As for the future direction and suggestion, it is necessary to conduct long-term longitudinal studies to determine the causal relationship between Cu exposure and BG levels, and explore its molecular mechanisms, particularly how Cu affects BG regulation through inflammatory pathways. Moreover, we should further investigate how environmental Cu exposure interacted with lifestyle factors such as diet and physical activity to collectively influenced the development of abnormal BG concentration. Most importantly, for populations living in areas of high heavy metal exposure, healthcare providers should consider regular testing for serum Cu and other relevant biomarkers as part of routine health screenings. In addition, specific nutritional and medical advice should be provided to these populations to reduce the potential health risks of heavy metal exposure.
5 ConclusionThis research with 2,318 NHANES participants conclusively demonstrated that serum Cu levels significantly influenced BG concentrations through specific inflammatory markers, suggesting reducing heavy metal exposure in the environment could lower the risk of developing diabetes. Mediation analyses revealed the impact of inflammatory markers on BG, emphasizing the role of public health measures to reduce heavy metal exposure and consider environmental factors in reducing diabetes.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statementThe studies involving humans were approved by NCHS Ethics Review Board Protocol #2011–17. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsZC: Visualization, Writing – original draft. YK: Formal analysis, Project administration, Writing – original draft, Writing – review & editing. WY: Methodology, Project administration, Software, Writing – review & editing. HX: Investigation, Resources, Writing – review & editing. DT: Investigation, Methodology, Supervision, Writing – review & editing. YZ: Conceptualization, Investigation, Methodology, Writing – review & editing.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe acknowledge the members of the National Center for Health Statistics of the Centers for Disease Control and Prevention and all participants of the National Health and Nutrition Examination Survey. We would also like to thank Shuping Yang from the School of Mathematics and Statistics, Central South University, for providing initial guidance on statistical analysis.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1401347/full#supplementary-material
Footnotes References2. Kumar, S, Mittal, A, Babu, D, and Mittal, A. Herbal medicines for diabetes management and its secondary complications. Curr Diabetes Rev. (2021) 17:437–56. doi: 10.2174/1573399816666201103143225
Crossref Full Text | Google Scholar
3. Mello, GPC, Simões, EFC, Crista, DMA, Leitão, JMM, Pinto da Silva, L, and Esteves da Silva, JCG. Glucose sensing by fluorescent nanomaterials. Crit Rev Anal Chem. (2019) 49:542–52. doi: 10.1080/10408347.2019.1565984
Crossref Full Text | Google Scholar
5. Walker, AF, Graham, S, Maple-Brown, L, Egede, LE, Campbell, JA, Walker, RJ, et al. Interventions to address global inequity in diabetes: international progress. Lancet. (2023) 402:250–64. doi: 10.1016/s0140-6736(23)00914-5
PubMed Abstract | Crossref Full Text | Google Scholar
6. Agiostratidou, G, Anhalt, H, Ball, D, Blonde, L, Gourgari, E, Harriman, KN, et al. Standardizing clinically meaningful outcome measures beyond HbA(1c) for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF international, the Leona M. and Harry B. Helmsley Charitable Trust, the pediatric Endocrine Society, and the T1D exchange. Diabetes Care. (2017) 40:1622–30. doi: 10.2337/dc17-1624
Crossref Full Text | Google Scholar
7. Saeedi, P, Salpea, P, Karuranga, S, Petersohn, I, Malanda, B, Gregg, EW, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract. (2020) 162:108086. doi: 10.1016/j.diabres.2020.108086
PubMed Abstract | Crossref Full Text | Google Scholar
8. Fatma, UK, Nizami, G, Ahamad, S, Saquib, M, and Hussain, MK. Activated green tamarind pulp (AGTP) as an efficient adsorbent for removal of Pb2+, Cu2+, Zn2+, and Ni2+ from contaminated water. J. Water Proc. Engineering. (2024) 59:105048. doi: 10.1016/j.jwpe.2024.105048
Crossref Full Text | Google Scholar
9. Fatma, UK, Nizami, G, Ahamad, S, and Hussain, MK. Efficient removal of Pb2+, Cu2+, and Zn2+ by waste tea‐derived cost‐effective bioadsorbent. Chemistryselect. (2023) 8:e202300944. doi: 10.1002/slct.202300944
Crossref Full Text | Google Scholar
10. Kumar, A, Parveen, M, Ahamad, S, Khan, A, Ahmad, F, Kataria, R, et al. Synthesis and spectral studies of organotin (IV) dithiocarbamates derived from 2-aminoethyl piperazine: anticancer and anti-nematode activity. J Mol Struct. (2023) 1294:136462. doi: 10.1016/j.molstruc.2023.136462
Crossref Full Text | Google Scholar
11. Dawud, F, Takyi, SA, Arko-Mensah, J, Basu, N, Egbi, G, Ofori-Attah, E, et al. Relationship between metal exposures, dietary macronutrient intake, and blood glucose levels of informal electronic waste recyclers in Ghana. Int J Environ Res Public Health. (2022) 19:12768. doi: 10.3390/ijerph191912768
PubMed Abstract | Crossref Full Text | Google Scholar
12. Buha, A, Đukić-Ćosić, D, Ćurčić, M, Bulat, Z, Antonijević, B, Moulis, JM, et al. Emerging links between cadmium exposure and insulin resistance: human, animal, and cell study data. Toxics. (2020) 8:63. doi: 10.3390/toxics8030063
PubMed Abstract | Crossref Full Text | Google Scholar
13. Gangwar, C, Choudhari, R, Chauhan, A, Kumar, A, Singh, A, and Tripathi, A. Assessment of air pollution caused by illegal e-waste burning to evaluate the human health risk. Environ Int. (2019) 125:191–9. doi: 10.1016/j.envint.2018.11.051
PubMed Abstract | Crossref Full Text | Google Scholar
14. Luc, K, Schramm-Luc, A, Guzik, TJ, and Mikolajczyk, TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. (2019) 70, 809–824. doi: 10.26402/jpp.2019.6.01
PubMed Abstract | Crossref Full Text | Google Scholar
15. Lamb, RE, and Goldstein, BJ. Modulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int J Clin Pract. (2008) 62:1087–95. doi: 10.1111/j.1742-1241.2008.01789.x
PubMed Abstract | Crossref Full Text | Google Scholar
16. Wang, X, Bin, W, Zhou, M, Xiao, L, Xu, T, Yang, S, et al. Systemic inflammation mediates the association of heavy metal exposures with liver injury: a study in general Chinese urban adults. J Hazard Mater. (2021) 419:126497. doi: 10.1016/j.jhazmat.2021.126497
PubMed Abstract | Crossref Full Text | Google Scholar
17. Sun, X, Deng, Y, Fang, L, Ni, M, Wang, X, Zhang, T, et al. Association of exposure to heavy metal mixtures with systemic immune-inflammation index among US adults in NHANES 2011-2016. Biol Trace Elem Res. (2023) 202:3005–17. doi: 10.1007/s12011-023-03901-y
PubMed Abstract | Crossref Full Text | Google Scholar
19. Liu, Y, Liu, Y, He, W, Mu, X, Wu, X, Deng, J, et al. Fibroblasts: immunomodulatory factors in refractory diabetic wound healing. Front Immunol. (2022) 13:918223. doi: 10.3389/fimmu.2022.918223
PubMed Abstract | Crossref Full Text | Google Scholar
20. Maida, CD, Daidone, M, Pacinella, G, Norrito, RL, Pinto, A, and Tuttolomondo, A. Diabetes and ischemic stroke: an old and new relationship an overview of the close interaction between these diseases. Int J Mol Sci. (2022) 23:2397. doi: 10.3390/ijms23042397
PubMed Abstract | Crossref Full Text | Google Scholar
21. Ahmed, TS, Shah, J, Zhen, YNB, Chua, J, Wong, DWK, Nusinovici, S, et al. Ocular microvascular complications in diabetic retinopathy: insights from machine learning. BMJ Open Diabetes Res Care. (2024) 12:e003758. doi: 10.1136/bmjdrc-2023-003758
PubMed Abstract | Crossref Full Text | Google Scholar
22. Karthikeyan, A, Manjunath, PR, Latha, AT, and Sultana, S. Prevalence of cardiac autonomic neuropathy in patients with type 2 diabetes mellitus. J Family Med Prim Care. (2023) 12:2070–4. doi: 10.4103/jfmpc.jfmpc_609_23
PubMed Abstract | Crossref Full Text | Google Scholar
23. Yachmaneni, A, Jajoo, S, Mahakalkar, C, Kshirsagar, S, and Dhole, S. A comprehensive review of the vascular consequences of diabetes in the lower extremities: current approaches to management and evaluation of clinical outcomes. Cureus. (2023) 15:e47525. doi: 10.7759/cureus.47525
PubMed Abstract | Crossref Full Text | Google Scholar
24. Noor, N, Zong, G, Seely, EW, Weisskopf, M, and James-Todd, T. Urinary cadmium concentrations and metabolic syndrome in U.S. adults: the National Health and nutrition examination survey 2001-2014. Environ Int. (2018) 121:349–56. doi: 10.1016/j.envint.2018.08.029
PubMed Abstract | Crossref Full Text | Google Scholar
25. Peters, JL, Fabian, MP, and Levy, JI. Combined impact of lead, cadmium, polychlorinated biphenyls and non-chemical risk factors on blood pressure in NHANES. Environ Res. (2014) 132:93–9. doi: 10.1016/j.envres.2014.03.038
PubMed Abstract | Crossref Full Text | Google Scholar
26. Wang, X, Mukherjee, B, and Park, SK. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003-2014. Environ Int. (2018) 121:683–94. doi: 10.1016/j.envint.2018.09.035
PubMed Abstract | Crossref Full Text | Google Scholar
27. Wu, KG, Chang, CY, Yen, CY, and Lai, CC. Associations between environmental heavy metal exposure and childhood asthma: a population-based study. J Microbiol Immunol Infect. (2019) 52:352–62. doi: 10.1016/j.jmii.2018.08.001
PubMed Abstract | Crossref Full Text | Google Scholar
28. Chen, Y, Zhao, A, Li, R, Kang, W, Wu, J, Yin, Y, et al. Independent and combined associations of multiple-heavy-metal exposure with lung function: a population-based study in US children. Environ Geochem Health. (2023) 45:5213–30. doi: 10.1007/s10653-023-01565-0
PubMed Abstract | Crossref Full Text | Google Scholar
29. Yang, J, Yang, A, Cheng, N, Huang, W, Huang, P, Liu, N, et al. Sex-specific associations of blood and urinary manganese levels with glucose levels, insulin resistance and kidney function in US adults: national health and nutrition examination survey 2011-2016. Chemosphere. (2020) 258:126940. doi: 10.1016/j.chemosphere.2020.126940
留言 (0)