Fungal infections have become a significant global concern. Among the known fungal pathogens, Candida tropicalis, an important opportunistic species, is associated with both superficial and systemic infections, has a higher mortality rate (41%) than other Candida species (Andes et al., 2012; de Souza et al., 2023). In 2022, the World Health Organization released its first-ever list of fungal priority pathogens, classifying C. tropicalis as a “high priority” fungal pathogen of considerable importance (World Health Organization, 2022). In recent years, the prevalence of drug-resistant C. tropicalis has increased, making it a serious-public health threat and a challenge to the management of invasive fungal diseases (Lima et al., 2022).
The most common antifungal drugs used against Candida infections were polyenes, fluoropyrimidines, echinocandins, and azoles (de Oliveira Santos et al., 2018). The widespread overuse of fungistatic azoles has led to the emergence of azoles-resistant C. tropicalis through various mechanisms, including modification and/or overexpression of the drug target, upregulation of drug-efflux pumps, and compensatory alterations within the ergosterol biosynthesis pathway (Revie et al., 2018). Thus, the clinical usefulness of antifungals is hampered by the undesirable side effects and the emergence of resistance. Additionally, biofilm formation by C. tropicalis has enhanced its ability to adhere to epithelial and endothelial cells, and led to an increase in its antibiotic resistance (Queiroz et al., 2023). Therefore, the severity of invasive infections caused by azole-resistant C. tropicalis is of the utmost clinical concern, and there is an urgent need for novel strategies to combat these life-threatening infections. These developments have generated greater interest in research on new antifungal drugs with multiple synthetic and natural molecules.
Antimicrobial peptides have emerged as a promising alternative for combating a diverse range of microbial pathogens. Nisin, a 34-amino acid pentacyclic peptide, is one of the oldest known antimicrobial compounds that was first discovered as a possible food-preserving agent in the food industry in 1928 (Rogers and Whittier, 1928). This inhibitory peptide is naturally produced by Lactococcus lactis subsp. lactis and was approved by the US Food and Drug Agency in 1988, and it generally recognized as safe (de Arauz et al., 2009). Twelve natural variants of nisin have been reported so far, and several bioengineered variants have been developed for various biological applications (Chan et al., 2023). Currently, the focus of research on nisin is shifting from food preservation to its therapeutic use for the treatment of bacterial infections. Nisin exhibits potent activity against Gram-positive bacterial pathogens (Field et al., 2010; Reiners et al., 2020), by causing the formation of pores in the cytoplasmic membrane, disrupting the proton motive force and pH balance, and eventually causing cell death. Another proposed mode of action of nisin is the inhibition of cell wall biosynthesis by binding lipid II, which inhibits cell wall biosynthesis (Field et al., 2008). However, the activity of nisin is substantially weaker against Gram-negative bacteria than against Gram-positive bacteria (Field et al., 2012; Zhou et al., 2023a). While little is known about the antimicrobial activity of nisin against fungi and viruses, a few studies have shown that nisin Z can inhibit the adhesion, growth, and morphological transformation of C. albicans (Le Lay et al., 2008; Akerey et al., 2009). However, there are few reports on the antifungal activity of nisin against C. tropicalis.
In this study, we have explored the antifungal activity of nisin against clinically isolated azole-resistant C. tropicalis and investigated its impact on biofilm formation and the expression of related genes, in order to provide important information for the potential application of nisin as an alternative antifungal therapeutic strategy.
Materials and methods Fungal strainsFifty-six Candida strains including 35 C. tropicalis and 21 C. albicans strains, isolated from sterile body fluids and blood specimens from patients admitted to Nanjing Drum Tower Hospital between 2013 and 2022 were included. All the strains were identified using the VITEK 2 system (bioMérieux, France) and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) (bioMérieux, France). Prior to the experiments, Candida strains were inoculated on Sabouraud dextrose agar and incubated at 35°C for at least 24 h. Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as quality control strains for antifungal susceptibility testing.
Antifungal susceptibility testingSensititre YeastOne™ YO10 (ThermoFisher Scientific, Cleveland, OH, USA) was utilized for antifungal susceptibility analysis following the manufacturer’s instructions. Current clinical breakpoints or epidemiological cut-off values were employed for interpretation of the susceptibility results (Clinical and Laboratory Standards Institute, 2022). The MICs of nisin (MCE, USA) against 35 clinical isolates of C. tropicalis and 21 isolates of C. albicans were determined using the broth microdilution method in accordance with the CLSI guidelines (M27-Ed4) (Clinical and Laboratory Standards Institute, 2017). Briefly, nisin was dissolved in 0.02 mol/L HCl and prepared at a concentration of 2048 μg/mL for storage (Santos et al., 2019). RPMI-1640, buffered to pH 7 with 3-[N-morpholino] propanesulfonic acid, was used as the growth medium, and the fungal solutions were incubated in a U-shaped 96-well sterile microtiter plate for 24 h at 35°C. The MIC was determined as the lowest concentration resulting in 50% inhibition of growth compared to the growth of the control. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were included with each assessment as quality controls.
Growth inhibition curvesCandida strains were grown in yeast extract peptone dextrose broth (YPD, containing 1% yeast extract, 2% peptone, and 2% glucose) at 35°C for 18 h. The fungal inoculum standardized until a cell concentration of 105 CFU/mL was reached and then treated with nisin, which was diluted to concentration of 1,024 μg/mL, 512 μg/mL, and 256 μg/mL in the final incubation, with shaking at 180 rpm. HCl at concentrations of 0.01 mol/L, 0.005 mol/L and 0.0025 mol/L were set up as reagent controls. Cells cultivated only with YPD broth was set as the blank control. Subsequently, absorbance was measured at OD600 nm using a MB-580 microplate reader (HEALES, China) at 0, 2, 4, 8, 12, 16, and 24 h, respectively. All assays were performed in triplicate.
Cryo-scanning electron microscopeA cryo-scanning electron microscope (Hitachi Regulus 8,100, Japan) was utilized to observe the effect of nisin on C. tropicalis. The cells were cultured in both drug-free medium and a medium containing nisin (8 μg/mL) for 24 h. The process involved loading the fungal sample onto the sample holder, mounting the sample holder onto the sample transfer rod device, and then inserting the device into solid nitrogen at −210°C for approximately 30 s for pre-cooling and cryofixation. The sample was then transferred under vacuum to the cryo-preparation chamber at −90°C, where it was sublimated for 2 min at −95°C to remove water from the sample and coated with a conductor by spraying with platinum for 50 s. Once the cryogenic sample preparation was completed, the sample could be transferred to the cold stage of the scanning electron microscope, with the temperature set at −175°C, for observation and photography.
Biofilm inhibitionThe biofilm formation ability of C. tropicalis and C. albicans strains was evaluated using the microtiter plate method, as described previously (Zhou et al., 2023b). The strains were first grown overnight on a YPD agar plate, adjusted to a concentration of 0.5 McFarland, and then seeded at a concentration of 106 CFU/mL in YPD broth. Subsequently, diluted broth containing nisin at 1/2 MIC or a corresponding concentration of HCl was added, with a final volume of 200 μL per well, and the microtiter plate was incubated at 35°C for 24 h. After incubation, the solution was discarded, and the plate was washed with sterile phosphate-buffered saline three times and dried. The plate was then stained with 100 μL of 0.5% crystal violet (Beyotime Biotechnology, China) for 15 min and washed with sterile PBS three times. Following this, 200 μL of absolute ethanol was added, and the absorbance was measured at OD570 nm using a microplate reader (Zhou et al., 2022). Each assay was performed with three replicates for each strain. Sterile YPD broth served as a blank control, while S. epidermidis ATCC 12228 and ATCC 35984 were used as the negative and positive controls, respectively (Solis et al., 2016).
Quantitative reverse transcription polymerase chain reactionQuantitative reverse transcription polymerase chain reaction (qRT-PCR) was utilized to assess the expression of the ERG11, MDR1, CDR1, UPC2, and BCR1 genes. RNA extraction was carried out using the RNA Enhancement and FreeZol Reagent (Vazmye, China) according to the manufacturer’s instructions. C. tropicalis strains were initially cultured on YPD medium, and this was followed by incubation in YPD broth containing nisin (1/2 MIC) at 35°C with shaking at 180 rpm for 8 h. Subsequently, the culture was collected for RNA extraction. cDNA synthesis was immediately performed for each sample in two steps using a HiScript RT SuperMix qPCR kit (Vazmye, China) based on the manufacturer’s instructions. qPCR was conducted in a 20-μL reaction volume using ChamQ Universal SYBR qPCR Master Mix (Vazmye, China). The reactions commenced with primary denaturation at 95°C for 2 min, which was followed by 40 cycles each of 95°C for 10 s and 60°C for 30 s. ACT1 was employed as an internal reference. All treatments were carried out in triplicate, and the 2−ΔΔCt method was used to determine the fold changes of the candidate genes.
Statistical analysisThe data were analyzed using the SPSS statistical software (version 20.0) and GraphPad (Version 9, GraphPad Software, United States). Quantitative data were subjected to the t-test or the Mann–Whitney U-test. Statistical significance was set at 0.05.
Results Susceptibility of Candida tropicalis to antifungal drugsAntifungal susceptibility testing was conducted on 35 strains of C. tropicalis, which were divided into azole-resistant (21 strains) and azole-sensitive (14 strains) groups based on their MIC values for fluconazole, itraconazole, voriconazole, and posaconazole. The MICs were higher in the azole-resistant groups (fluconazole: 128–256 μg/mL, itraconazole: 0.5–16 μg/mL; voriconazole: 4–8 μg/mL; and posaconazole: 0.25–2 μg/mL) than in the azole-sensitive group (fluconazole: ≤ 2 μg/mL; itraconazole: ≤ 0.25 μg/mL; voriconazole: ≤ 0.12 μg/mL; and posaconazole: ≤ 0.12 μg/mL). All the 35 strains were sensitive to amphotericin B, anidulafungin, caspofungin, and micafungin.
Expression of genes related to azole resistance in Candida tropicalisIn order to investigate the resistance mechanisms of the azole-resistant C. tropicalis strains included in our study, the relative expression levels of the ERG11, UPC2, MDR1, and CDR1 genes in azole-resistant and azole-sensitive strains were evaluated using qRT-PCR. The results indicated that the relative expression levels of the ERG11 and UPC2 genes were significantly higher in the azole-resistant group than in the azole-sensitive group (Figures 1A,B, p< 0.0001), while no differences were observed for the MDR1 and CDR1 genes (Figures 1C,D, p > 0.05). These results suggest that overexpression of the ERG11 and UPC2 genes may be the main mechanism in the isolated azole-resistant C. tropicalis strains investigated here.

Figure 1. Azole-resistant related genes expression of C. tropicalis. Expression levels of resistant-related genes EGR11 (A), UPC2 (B), MDR1 (C) and CDR1 (D) in azole-sensitive and azole-resistant groups by qRT-PCR, respectively. S: azoles sensitive group, n = 14. R: azoles resistant group, n = 21. Mann–Whitney U test was used to compared the genes expression between two groups.
Antifungal activity of nisin against Candida tropicalisThe MIC values of nisin were determined for various strains and were found to range between 2 to 8 μg/mL for clinical C. tropicalis isolates (MIC90 and MIC50 were both 4 μg/mL) and 1 to 2 μg/mL for C. albicans strains (MIC90 and MIC50 were both 2 μg/mL). With regard to the controls, the MIC values of nisin against C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were 2 μg/mL and 4 μg/mL, respectively, as shown in Table 1.
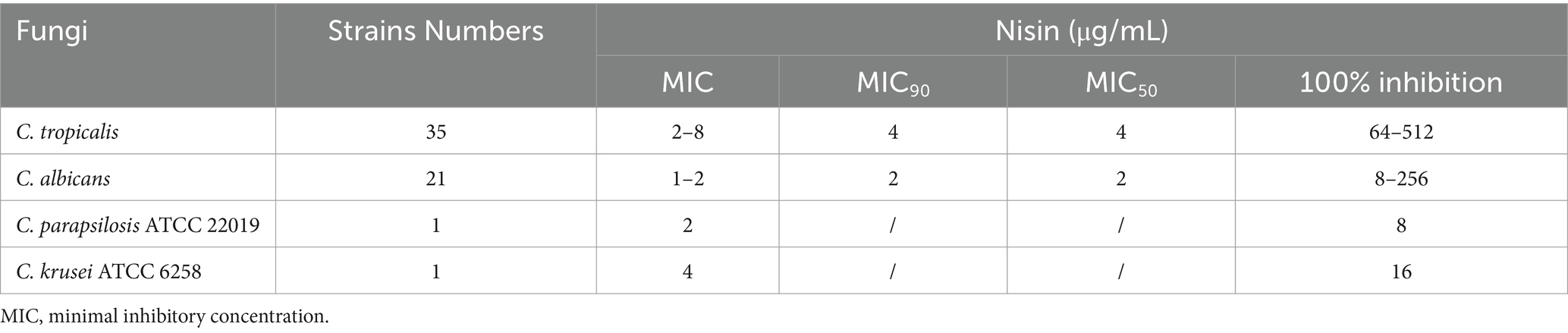
Table 1. MIC values of nisin against fungi strains.
Candida tropicalis clinical isolates numbered 197 and 420 were randomly selected for nisin inhibition fungal growth curve analysis. The concentration at which nisin completely inhibited fungal growth was 512 μg/mL. Consequently, we selected concentrations of 1,024 μg/mL, 512 μg/mL and 256 μg/mL of nisin for the analysis of growth inhibition curves. After 24 h of growth observation, we assessed the inhibitory effect of the above concentrations of nisin on C. tropicalis by measuring the reduction in absorbance at OD600 nm. Our results demonstrated that nisin at concentrations of 1,024 μg/mL, 512 μg/mL and 256 μg/mL resulted in a significant decrease in the growth of C. tropicalis over 8–24 h (Figures 2A,B). Nisin exhibited dose- and time-dependent inhibition of C. tropicalis growth. Specifically, treatment of strain 197 with 512 μg/mL of nisin resulted in a more than four-fold reduction in absorbance at the 8-h time point (0.8611 ± 0.0191 vs 0.1813 ± 0.0301, p < 0.0001). Moreover, the concentrations of HCl with 0.005 mol/L did not have interference with the viability of fungal growth.

Figure 2. Impact of nisin on the growth of C. tropicalis isolates. The growth inhibition curves of C. tropicalis no.197 (A) and 420 (B) were performed. The fungal inoculum was standardized until reaching a cell concentration of 105 CFU/mL, then treated with nisin which was diluted to the concentration of 1,024 μg/mL, 512 μg/mL and 256 μg/mL in the final incubation with shaking at 180 rpm in tubes. Subsequently, growth was measured using absorbance at OD600 nm in YPD medium at times 0, 2, 4, 8, 12, 16, and 24 h in a MB-580 microplate reader (HEALES, China). YPD broth and YPD containing 0.01 mol/L, 0.005 mol/L, 0.0025 mol/L HCl in final concentrations were used as reagent controls. Cells cultivated only with YPD broth was set as the blank control. All assays were performed in triplicate.
Effects of nisin on fungal micromorphologyTo investigate the potential impact of nisin on the morphology of azole-resistant C. tropicalis, we employed cryo-scanning electron microscopy and Gram staining. C. tropicalis was cultured in both a drug-free medium and a medium containing nisin (8 μg/mL) for 24 h. Our observations using cryo-scanning electron microscopy and Gram staining revealed significant alterations in the morphology of the nisin-treated cells compared to the control cells. Under normal culture conditions, C. tropicalis exhibited a typical cellular morphology characterized by a rounded or oval shape with a smooth surface (Figures 3A–C). However, after 24 h of incubation with nisin, we observed apparent transition from the yeast to the hyphal form, as well as the presence of ultrastructural collapse with loss of shape (Figures 3D–F). In addition, vesicle-like structures were found when C. tropicalis was cultured in liquid media (Figures 3B,E).

Figure 3. Nisin promoted C. tropicalis yeast-hypha transition. The C. tropicalis cells were cultured in drug-free medium and medium with nisin (8 μg/mL) for 24 h. Control, cryo-scanning electron microscope 1.0KV × 600 (A). Control, cryo-scanning electron microscope 1.0KV × 4000 (B). Control, Gram staining (C). Nisin, cryo-scanning electron microscope 1.0KV × 600 (D). Nisin, 1.0KV × 4000 (E). Nisin, Gram staining (F). Scale: 10 μm.
Inhibitory effect of nisin on biofilm formation and expression of related genesCandida tropicalis exhibited a stronger ability to form biofilms than C. albicans (Figure 4A). However, there was no significant difference in biofilm formation ability observed between the azole-resistant and azole-sensitive groups of C. tropicalis (Figure 4B). However, treatment with nisin at 1/2 MIC for 24 h, resulted in a significant decrease in the OD570 nm value of the 21 azole-resistant C. tropicalis strains compared to the controls (Figure 4C, U = 40, p < 0.0001). Among the 21 azole-resistant C. tropicalis strains, the biofilm formation was inhibited in 17 strains (17/21, 81%). The biofilm biomass of C. tropicalis was significantly decreased compared to the control (Figures 4D,E), and the rate of inhibition of biofilm formation was more than 85% in strain 420 (Figure 4F). Consequently, our findings indicate that nisin effectively reduces the biofilm production by azole-resistant C. tropicalis strains.

Figure 4. Inhibitory effect of nisin on the in vitro biofilm formation of C. tropicalis. Absorbance at OD570 nm of C. albicans (n = 21) and C. tropicalis (n = 35) strains (A), also azole-sensitive and azole-resistant groups of C. tropicalis (B). S: azoles sensitive group, n = 14. R: azoles resistant group, n = 21. Absorbance at OD570 nm of control and nisin treatment group (C). The crystal violet stain of control (D) and nisin treatment group (E) under microscope. The inhibition for representative strains with their specific controls (F). The concentration of nisin is 1/2 MIC. Mann–Whitney U test was used to compared the inhibitory effect between two groups.
To further investigate these effects of nisin at the gene level, we quantified the difference in the expression of the BCR1 and UPC2 genes in three representative C. tropicalis strains incubated with or without nisin for 8 h using qRT-PCR. The results demonstrated that the expression level of the BCR1 gene, a regulator of biofilm generation, was downregulated by nisin (1/2 MIC) in strains 197, 420, and 10,476 (Figures 5A–C, p < 0.05). Additionally, the resistance-related gene UPC2 also exhibited decreased expression after treatment with nisin (1/2 MIC) (Figures 5D–F, p < 0.05).

Figure 5. Nisin inhibited the expression of BCR1 and UPC2 genes in C. tropicalis. Relative mRNA expression levels of BCR1 (A–C) and UPC2 (D–F) in strains of C. tropicalis 197, 420 and 10,476 were detected with qRT-PCR. Unpaired t-test.
DiscussionNisin, a well-studied and widely used lantibiotic, has been shown to have extremely potent against a variety of Gram-positive bacteria, while little is known about the antimicrobial activity of nisin against fungi (Khan et al., 2023). A few studies suggested that nisin may possess antifungal activity on C. albicans. Le Lay et al. first investigated the effect of nisin Z on C. albicans and demonstrated its ability to inhibit the growth of C. albicans strains at concentrations of 500 μg/mL, in a dose- and time-dependent manner (Le Lay et al., 2008). Other studies have suggested that nisin Z may inhibit C. albicans adhesion and transition (Akerey et al., 2009). However, there were few reports on the effectiveness of nisin against C. tropicalis, which is one of the most important Candida species with high rate of mortality and an increasing azole-resistant rates (Wang et al., 2021b). Our study demonstrated, for the first time, that nisin has antifungal activity and significant anti-biofilm activity against clinical isolates of azole-resistant C. tropicalis strains.
Some factors that contribute to the azole resistance of Candida spp. include mutations and/or overexpression of the following genes: ERG11 (encodes cytochrome P450 lanosterol 14a-demethy-lase), UPC2 (encodes the transcription factor of ERG11 gene), CDR1 (encodes efflux protein of ATP binding-cassette (ABC) family) and MDR1 (encodes efflux protein of major facilitator superfamily (MFS) family) (Sasani et al., 2021). In the present study, we observed that clinically isolated azole-resistant C. tropicalis exhibited high expression of ERG11 and UPC2 genes (as shown in Figures 1A,B). Further, the azole-resistant strains exhibited higher MIC values for fluconazole and voriconazole. These findings are similar to those reported in Wang’s study (Wang et al., 2021a). However, our study did not find any significant difference in the expression levels of CDR1 and MDR1 genes between azole-resistant and azole-susceptible C. tropicalis isolates. These results imply that the ERG11 and UPC2 genes play a role in the molecular mechanisms of azole resistance in C. tropicalis isolates.
The discovery of new antifungal drugs for clinical treatment of the resistant strains is urgently needed, and nisin has piqued our interest as a potential safe and effective choice against C. tropicalis, especially for drug-resistant strains. In this study, we investigated the efficacy of nisin against C. tropicalis, with a primary focus on its ability to control fungal growth. Our findings revealed that concentrations of 2–8 μg/mL of nisin can inhibit the growth of clinical strains of C. tropicalis, including azole-resistant strains with MIC50 and MIC90 both being 4 μg/mL. Compared to clinical C. tropicalis strains, the MIC of nisin against C. albicans was lower than that against C. tropicalis. Furthermore, considering that the concentration at which nisin completely inhibited C. tropicalis growth was 512 μg/mL, we used nisin at concentrations of 1,024 μg/mL, 512 μg/mL and 256 μg/mL to perform the fungal growth inhibition experiments. The results showed that nisin at concentrations of 1,024 μg/mL, 512 μg/mL, and 256 μg/mL significantly inhibited fungal growth in dose and time-dependent manner. These results indicate that nisin has potential as an alternative antifungal strategy to target azole-resistant C. tropicalis. Recently, nisin-loaded PCL nanoparticles have been suggested as a potential strategy for preventing vaginal candidiasis by inhibiting C. albicans growth (de Abreu et al., 2016). Further, the use of nisin as an additive may help reduce the use of antibiotics, minimize side effects, and prevent resistance in clinical scenarios (Chan et al., 2023). Therefore, nanoformulations or combination treatment therapies that include nisin may hold promise for combating fungal infections and entail exciting prospects for future applications.
Nisin exhibits significant antimicrobial activity primarily through electrostatic interactions with bacterial cell wall constituents, such as lipid II, teichoic acid, and polysaccharides. This interaction leads to the formation of stable toroidal pores, which inhibit cell wall biosynthesis and ultimately cause cell death (Bauer and Dicks, 2005). Based on these observations, it is possible that the antifungal mechanism of nisin also relies on disrupting the integrity of fungal cell walls and affecting the transition between yeast and hyphal forms. Accordingly, previous studies have reported that nisin Z-treated C. albicans had distorted cell wall surfaces and highly vacuolated cells (Le Lay et al., 2008). Further, it has also been reported that the transition between the yeast and hyphal forms is closely linked to the adaptability and pathogenicity of Candida in the host environment (Jain et al., 2008). In the case of C. albicans, it has been demonstrated that nisin Z effectively inhibits the transformation of C. albicans from blastospores to hyphal forms, thereby disrupting its transition (Le Lay et al., 2008). This differs from our observation on C. tropicalis treated with nisin, as shown in Figure 3: C. tropicalis treated by nisin showed yeast-hypha transition and exhibited ultrastructural collapse with loss of shape. However, the impact on internal cell damage is unclear and needs to be further investigated. The regulatory mechanisms of filamentation in C. tropicalis exhibit both conserved and divergent features (Zhang et al., 2016), so, further research is needed to determine if the regulation of filamentation by nisin is specific to C. tropicalis. Additionally, recent research indicated that fungal extracellular vesicles can regulate yeast-to-hypha differentiation in C. albicans (Honorato et al., 2022). In our study, vesicle-like structures were also found through observation using cryo-electron microscopy after culturing of C. tropicalis in liquid media (Figures 3B,E).
Biofilm formation is one of the main virulence factors in C. tropicalis. A recent study has demonstrated that biofilm production in C. tropicalis was associated with high mortality rates in patients with candidemia (Vitális et al., 2020). Furthermore, biofilm growth is accompanied by resistance to antifungal drugs, especially azoles, in C. tropicalis (Borghi et al., 2016; Cavalheiro and Teixeira, 2018). According to our results, C. tropicalis has stronger ability to form biofilms than C. albicans, but no differences were found between azole-resistant and azole-sensitive strains. In the clinical environment, the colonization adherence of a pathogen to abiotic surfaces is the first step in biofilms formation on medical devices. Catheter-related blood C. tropicalis isolates exhibit a stronger adhesion ability to polystyrene microspheres than that of other sources of Candida species (Zuo et al., 2021), and C. tropicalis strains isolated from the urinary tract can form biofilms on silicone and latex urinary catheters (Negri et al., 2011). Several studies have shown that nisin has anti-biofilm activity against clinical isolates of Staphylococcus and Pseudomonas aeruginosa (Field et al., 2016; Chen et al., 2023). Furthermore, nisin Z, in combination with gingival cells, downregulated C. albicans adhesion (Akerey et al., 2009). Further, a new study has shown that the curcumin-nisin-poly (L-lactic acid) nanoparticle could serve as an excellent orthodontic acrylic resin additive against S. mutans and C. albicans biofilms (Pourhajibagher et al., 2022). In our results, as shown in Figures 4C–F, we found for the first time that nisin can significantly inhibit biofilm formation of azole-resistant C. tropicalis strains, 81% inhibition of biofilm was observed (17/21), while more than 85% inhibition of biofilm formation was observed in the representative strains. Thus, due to its effectiveness and low toxicity, nisin has potential value in inhibiting the colonization of C. tropicalis in the clinical environment.
The formation of biofilms in Candida species is regulated by a heterogeneous gene network involved in adhesion, extracellular matrix, and filamentation (de Souza et al., 2023). Nisin used independently or in combination with other antimicrobials has been found to reduce adhesion to polystyrene and the expression of genes related to biofilm formation (Mathur et al., 2018). In C. tropicalis, the transcription factor Bcr1 regulates the expression of adhesin-associated genes such as CtrALS1, CtrALS3, and HWP1 (Zhang et al., 2016; Staniszewska, 2020). Accordingly, our results demonstrate that nisin can decrease the expression of the BCR1 gene, which may contribute to the regulation of biofilms and cell walls. Sasani et al. (2021) showed an increase in the expression of both the ERG11 and UPC2 genes in fluconazole-treated biofilms of C. tropicalis. Here, we found that nisin can reduce the expression of the UPC2 gene in azole-resistant C. tropicalis strains (Figure 5). As a transcription factor of ERG11 genes, the UPC2 gene plays an important role in the mechanism of azole resistance in C. tropicalis. However, whether the downregulation of UPC2 by nisin implies that combining nisin with azole drugs could have a synergistic clinical effect needs further investigation. By elucidating the inhibitory effects of nisin on both biofilm formation and gene expression in C. tropicalis, this study provides valuable insights into the potential application of nisin as a therapeutic agent against biofilm-related infections caused by azole-resistant strains. Further research is warranted to explore the underlying mechanisms and optimize the utilization of nisin in clinical settings.
ConclusionIn conclusion, our study explored, for the first time, the antifungal activity of nisin against clinical isolates of azole-resistant C. tropicalis. We found that nisin was able to inhibit the growth of azole-resistant C. tropicalis and prevent biofilm formation; further, the mechanistic experiments showed that these effects involve the downregulation of the BCR1 gene. We also observed that nisin treatment can downregulate UPC2 gene expression, and this suggests its potential application in combating resistant strains or in combination with other antifungal drugs. One limitation of our study was the insufficient number of C. tropicalis strains, which will be increased in subsequent research. In addition, there were technical limitations on morphological research. Therefore, further in-depth observation and study are needed to explore the finer structural changes in C. tropicalis after the action of nisin. Although nisin did not inhibit filamentation in our study, further investigation to understand the possible molecular mechanisms behind this yeast-to-hyphae transition is necessary. Our findings contribute new evidence to the field of antifungal research on nisin. Still, the antifungal activity of nisin remains understudied and warrants further study, as it may emerge as a promising candidate for future strategies in treating clinical azole-resistant C. tropicalis infections.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributionsSG: Data curation, Writing – original draft, Conceptualization. YJ: Data curation, Software, Writing – review & editing. SX: Data curation, Methodology, Writing – review & editing. JJ: Formal analysis, Investigation, Writing – review & editing. BF: Data curation, Investigation, Writing – review & editing. YZ: Formal analysis, Methodology, Writing – review & editing. HS: Resources, Writing – review & editing. WZ: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Project of Chinese Hospital Reform and Development Institute, Nanjing University, Aid project of Nanjing Drum Tower Hospital Health, Education & Research Foundation (NDYG2022048).
AcknowledgmentsThe authors thank the staff of the Laboratory of Clinical Microbiology of Nanjing Drum Tower Hospital (Nanjing, China) for identification of the clinical isolates.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAkerey, B., Le-Lay, C., Fliss, I., Subirade, M., and Rouabhia, M. (2009). In vitro efficacy of nisin Z against Candida albicans adhesion and transition following contact with normal human gingival cells. J. Appl. Microbiol. 107, 1298–1307. doi: 10.1111/j.1365-2672.2009.04312.x
PubMed Abstract | Crossref Full Text | Google Scholar
Andes, D. R., Safdar, N., Baddley, J. W., Playford, G., Reboli, A. C., Rex, J. H., et al. (2012). Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis. 54, 1110–1122. doi: 10.1093/cid/cis021
PubMed Abstract | Crossref Full Text | Google Scholar
Borghi, E., Borgo, F., and Morace, G. (2016). Fungal biofilms: update on resistance. Adv. Exp. Med. Biol. 931, 37–47. doi: 10.1007/5584_2016_7
Crossref Full Text | Google Scholar
Chan, K. T., Song, X., Shen, L., Liu, N., Zhou, X., and Cheng, L. (2023). Nisin and its application in oral diseases. J. Funct. Foods 105:105559. doi: 10.1016/j.jff.2023.105559
Crossref Full Text | Google Scholar
Chen, H., Ji, P. C., Qi, Y. H., Chen, S. J., Wang, C. Y., Yang, Y. J., et al. (2023). Inactivation of Pseudomonas aeruginosa biofilms by thymoquinone in combination with nisin. Front. Microbiol. 13:1029412. doi: 10.3389/fmicb.2022.1029412
PubMed Abstract | Crossref Full Text | Google Scholar
Clinical and Laboratory Standards Institute (2017). Reference method for broth dilution antifungal susceptibility testing of yeasts. Wayne, PA: Clinical and Laboratory Standards Institute.
Clinical and Laboratory Standards Institute (2022). Performance standards for antifungal susceptibility testing of yeasts. Wayne, PA: Clinical and Laboratory Standards Institute.
de Abreu, L. C. L., Todaro, V., Sathler, P. C., da Silva, L. C. R. P., do Carmo, F. A., Costa, C. M., et al. (2016). Development and characterization of nisin nanoparticles as potential alternative for the recurrent vaginal candidiasis treatment. AAPS PharmSciTech 17, 1421–1427. doi: 10.1208/s12249-016-0477-3
PubMed Abstract | Crossref Full Text | Google Scholar
de Arauz, L. J., Jozala, A. F., Mazzola, P. G., and Vessoni Penna, T. C. (2009). Nisin biotechnological production and application: a review. Trends Food Sci. Technol. 20, 146–154. doi: 10.1016/j.tifs.2009.01.056
Crossref Full Text | Google Scholar
de Oliveira Santos, G. C., Vasconcelos, C. C., Lopes, A. J. O., de Sousa Cartágenes, M. D. S., Filho, A. K. D. B., do Nascimento, F. R. F., et al. (2018). Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front. Microbiol. 9:1351. doi: 10.3389/fmicb.2018.01351
PubMed Abstract | Crossref Full Text | Google Scholar
de Souza, C. M., Dos Santos, M. M., Furlaneto-Maia, L., and Furlaneto, M. C. (2023). Adhesion and biofilm formation by the opportunistic pathogen Candida tropicalis: what do we know? Can. J. Microbiol. 69, 207–218. doi: 10.1139/cjm-2022-0195
PubMed Abstract | Crossref Full Text | Google Scholar
Field, D., Begley, M., O'Connor, P. M., Daly, K. M., Hugenholtz, F., Cotter, P. D., et al. (2012). Bioengineered nisin a derivatives with enhanced activity against both gram positive and gram negative pathogens. PLoS One 7:e46884. doi: 10.1371/journal.pone.0046884
PubMed Abstract | Crossref Full Text | Google Scholar
Field, D., Connor, P. M., Cotter, P. D., Hill, C., and Ross, R. P. (2008). The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol. Microbiol. 69, 218–230. doi: 10.1111/j.1365-2958.2008.06279.x
PubMed Abstract | Crossref Full Text | Google Scholar
Field, D., O’ Connor, R., Cotter, P. D., Ross, R. P., and Hill, C. (2016). In vitro activities of Nisin and Nisin derivatives alone and in combination with antibiotics against Staphylococcus biofilms. Front. Microbiol. 7:508. doi: 10.3389/fmicb.2016.00508
PubMed Abstract | Crossref Full Text | Google Scholar
Field, D., Quigley, L., O’Connor, P. M., Rea, M. C., Daly, K., Cotter, P. D., et al. (2010). Studies with bioengineered Nisin peptides highlight the broad-spectrum potency of Nisin V. Microb. Biotechnol. 3, 473–486. doi: 10.1111/j.1751-7915.2010.00184.x
PubMed Abstract | Crossref Full Text | Google Scholar
Honorato, L., de Araujo, J. F. D., Ellis, C. C., Piffer, A. C., Pereira, Y., Frases, S., et al. (2022). Extracellular vesicles regulate biofilm formation and yeast-to-hypha differentiation in Candida albicans. MBio 13:e0030122. doi: 10.1128/mbio.00301-22
PubMed Abstract | Crossref Full Text | Google Scholar
Khan, F., Singh, P., Joshi, A. S., Tabassum, N., Jeong, G. J., Bamunuarachchi, N. I., et al. (2023). Multiple potential strategies for the application of nisin and derivatives. Crit. Rev. Microbiol. 49, 628–657. doi: 10.1080/1040841X.2022.2112650
PubMed Abstract | Crossref Full Text | Google Scholar
Le Lay, C., Akerey, B., Fliss, I., Subirade, M., and Rouabhia, M. (2008). Nisin Z inhibits the growth of Candida albicans and its transition from blastospore to hyphal form. J. Appl. Microbiol. 105, 1630–1639. doi: 10.1111/j.1365-2672.2008.03908.x
PubMed Abstract | Crossref Full Text | Google Scholar
Lima, R., Ribeiro, F. C., Colombo, A. L., and de Almeida, J. N. (2022). The emerging threat antifungal-resistant Candida tropicalis in humans, animals, and environment. Front. Fungal Biol. 3:957021. doi: 10.3389/ffunb.2022.957021
PubMed Abstract | Crossref Full Text | Google Scholar
Mathur, H., Field, D., Rea, M. C., Cotter, P. D., Hill, C., and Ross, R. P. (2018). Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes 4:9. doi: 10.1038/s41522-018-0053-6
PubMed Abstract | Crossref Full Text | Google Scholar
Negri, M., Silva, S., Henriques, M., Azeredo, J., Svidzinski, T., and Oliveira, R. (2011). Candida tropicalis biofilms: artificial urine, urinary catheters and flow model. Med. Mycol. 49, 1–9. doi: 10.3109/13693786.2011.560619
Crossref Full Text | Google Scholar
Pourhajibagher, M., Noroozian, M., Ahmad Akhoundi, M. S., and Bahador, A. (2022). Antimicrobial effects and mechanical properties of poly (methyl methacrylate) as an orthodontic acrylic resin containing curcumin-Nisin-poly (L-lactic acid) nanoparticle: an in vitro study. BMC Oral Health 22:158. doi: 10.1186/s12903-022-02197-z
留言 (0)