The management of mental illness represents an important healthcare challenge. In the United States and Canada, around one fifth of adults experience mental illness, yet less than 50% have access to or are receiving treatment (1–6). Some barriers that have been reported include negative attitudes towards seeking help for mental illness, lack of availability, and long wait times due to mental health professional shortages, elevated costs, geographical and mobility factors. (7–12). These barriers were exacerbated by the COVID-19 pandemic, further highlighting the need for accessible mental healthcare solutions and more efficient systems (7–12). Thus, online mental healthcare solutions, such as digital mental healthcare interventions (DMHIs), have emerged as promising alternatives leveraging the benefits of online platforms to overcome the barriers inherent in in-person services (8, 13–16). Another recent solution has involved the use of artificial intelligence (AI), as a powerful tool capable of automating several aspects of the healthcare process, from healthcare monitoring and triage to diagnosis, risk assessment, and even treatment delivery (6, 17–23). The research and validation of online mental health alternatives and AI applications have experienced significant growth and development in recent years (6, 8, 13–23). However, the study of the applications of AI in mental healthcare remains in its infancy (6, 20).
Online mental healthcare presents several advantages compared to its in-person counterpart, notably additional privacy, and the ability to access healthcare anywhere with an internet connection (11, 24). Additionally, studies have shown that despite some concerns over the strength of the therapeutic relationship, online mental healthcare has similar effectiveness as in-person options for the management of mental health conditions (11, 24). For instance, a study by Alavi et al. (2023) showed that an online cognitive behavioral therapy program (eCBT) for depression had similar effectiveness and attrition rates as its in-person counterpart, with a medium to large effect size in the management of depression symptoms (25). Nevertheless, despite the promising results obtained with online mental healthcare, some gaps and barriers remain, limiting its use (5, 15, 24, 26). For instance, similarly to in-person options, diagnosis-specific online mental healthcare often requires a diagnosis and triage process by a healthcare professional. This can result in similar long wait times as that experienced by individuals seeking in-person care (5, 15, 24, 26). Monitoring of treatment progress, risk assessment, personalization of the therapy experience, and training of new therapists also pose a challenge to online mental healthcare (5, 15, 19, 20, 24, 26). Especially in the case of fully self-guided online psychotherapy, the lack of monitoring, risk assessment and personalization may place the patient at an increased risk of dropping out from the treatment or experiencing an exacerbation of psychiatric symptoms (20, 24). AI technology can help address these issues by supporting a versatile, scalable, and powerful approach that can analyze and respond to large amounts of data and adapt to individual healthcare needs (6, 17–23).
In recent years AI has garnered significant attention for a variety of applications, showcasing its significant versatility and ability to integrate and enhance a range of services and fields of study (6, 17–23). In healthcare, AI is now being used to aid in clinical decision-making, facilitating disease detection and monitoring, optimizing medical management, and even the discovery of novel therapies (20, 22, 23). This is possible due to AI’s ability to process and learn from large amounts of data either through a supervised or unsupervised approach (6, 19). In the supervised approach, the AI algorithm uses known outputs (training sets) to develop patterns to predict outcomes in other datasets, while in the unsupervised approach, AI learns from unknown outputs to find patterns in the data (6, 19). The use of ML algorithms, which can analyze large datasets, has garnered significant attention in online mental healthcare with several models now being proposed in this field including: probabilistic latent variable model, linear ML model, random forest model, Latent Dirichlet Allocation (LDA) topic models, elastic net model, inductive logic programming, decision tree model, support vector machine, deep learning (DL), and artificial neural networks (ANN) (27–33). However, several concerns have been raised in terms of the validity, generalizability, and reliability of the results obtained using ML, which can be impacted by insufficient or not representative training datasets, improper model fitting or hyperparameter fine-tuning, improper handling of training data sets resulting in data leakage, lack of validation and reproducibility assessments, among others (34–37). Therefore, to support the robustness of studies using ML algorithms, recent guidelines have proposed six essential elements: justification of the need to use ML, adequacy of the data, description of the algorithm used, results including model accuracy and calibration, availability of the programming code, and discussion of the model’s internal and external validation (34–37).
If carefully implemented, ML can produce results with a precision that can rival or surpass seasoned clinicians (20). For instance, recent advances in AI-powered tools have shown similar or better sensitivity for detecting minuscule deviations from normal anatomy, compared to a human assessor (20, 38–40). In contrast, the application of AI in mental healthcare remains in its infancy (6, 20). Nevertheless, recent studies have shown promising results of AI applications including suicide risk assessment and risk prediction, identification of mental illness predictors, mental health monitoring, psychoeducation and psychotherapy delivery, therapist training, mental healthcare personalization, mental health triage and decision-making, and promoting treatment engagement (6, 17–23). In terms of therapy content, this includes CBT, acceptance and commitment therapy (ACT), dialectic behavioral therapy, mindfulness, and supportive therapy (6, 41). However, because of the novelty of this technology, there are several unanswered questions and considerations such as limitations in the interpretation of language, biases in the interaction with patients from diverse backgrounds, and unanswered ethical, patient safety, and health policy considerations (6, 17–23).
The benefits provided by AI technologies can revolutionize online mental healthcare in many ways, supporting shorter wait times, enhanced accessibility, personalization, and engagement. Therefore, to supplement the available literature, this review presents the most comprehensive and first mixed-methods review, which implements both meta-analytic and network meta-analytic approaches to synthesize the available evidence on the applications of AI in online mental healthcare. This exhaustive review considered a broad range of AI applications from triage processes, and the delivery of psychoeducation and psychotherapy, to the monitoring of therapy progress and the ability to support therapy engagement. Based on the available evidence, we hypothesized that the results of this mixed methods review will support the implementation and applications of AI technology in online mental healthcare. Hence, applying a mixed methods review approach, we aimed to determine the impact that AI technologies are having on online mental healthcare, and the challenges and important considerations of this technology in clinical practice. Finally, future directions and recommendations will be discussed to help guide the development and implementation of AI technologies in online mental healthcare.
2 Methods2.1 Protocol and registrationWe registered this mixed methods systematic review with PROSPERO (CRD42023443575, https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=443575) and followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines, including the extension for NMAs (42–44).
2.2 Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
2.3 Search strategyWe developed a comprehensive search strategy following the approach taken by previous reviews on online mental healthcare and AI applications in the field (6, 17–23, 45–49). The review question and article eligibility and exclusion criteria was defined by using a populations-interventions-comparators-outcomes-study (PICOS) design framework (50). The Medical Subject Headings (MeSH) terminology and specific keywords included terms related to AI, applications of AI, and online mental healthcare including different modalities of psychoeducation and psychotherapy can be found in Supplementary Material 1. Then, we searched MEDLINE, CINAHL, PsycINFO, EMBASE, and Cochrane for relevant articles. The search was limited to articles written from inception of the searched terms until October 2023. We searched the bibliographies of previous reviews in the field and included articles to identify additional studies that may not have been identified by our systematic search strategy.
2.4 Study selection and eligibilityWe conducted the review on COVIDENCE, a web-based systematic review manager (51). Four co-authors (GG, CS, JE, and KA) independently screened the identified articles considering the eligibility and exclusion criteria. Two votes were required to approve any screening decisions, and conflicts or disagreements were resolved by consensus between the co-authors involved in the decision or a third co-author. The eligibility criteria included case studies, observational studies, open-label trials, and randomized controlled trials (RCTs) written in English on the applications of AI-augmented intervention and AI tools and algorithms in online mental healthcare. AI-augmented interventions correspond to any online mental healthcare intervention that integrates AI technology in some capacity (i.e., delivery, monitoring, assessment, etc.) and AI tools and algorithms correspond to any AI based analysis of online mental healthcare data. Exclusion criteria included review studies, editorial comments, grey literature, secondary analysis of data, and protocols.
2.5 Data extraction processFour reviewers (GG, CS, JE, and KA) independently extracted the selected articles on COVIDENCE. Two votes were required to approve any extraction decisions, and conflicts or disagreements were resolved by consensus between the co-authors involved in the decision or a third co-author. We extracted: name of the study, authors, sample size, demographic information, study location, intervention characteristics (modality, frequency, focus, application of AI, comparators), outcome measures, treatment duration and follow-up. For outcome measures, we followed two extraction approaches based on the type of the articles. For RCTs, observational cohort, and non-randomized experimental studies, we extracted pre- and post-intervention outcomes for all the study arms. In preparation for this step, through consensus amongst the co-authors we identified depression symptom reduction and anxiety symptom reduction as the most common outcome measures in the included studies. We focused on the extraction of these outcome measures to avoid underpowered meta-analytic comparisons. For studies reporting on the results of large dataset analysis using ML algorithms, data extraction followed the recent research guidelines and standards for ML studies, which recommend that these studies report on the adequacy of the data for the intended outcomes, model training and fine-tuning, features analyzed, validation, interpretability, and code and data availability (34–37). Additionally, we extracted the clinical and practical insights and implications that the studies obtained and discussed regarding the implementation of ML. These studies were not included in the meta-analysis or network meta-analysis calculations, and their results were only included and discussed in a narrative fashion.
2.6 Risk of bias within studiesFour reviewers (GG, CS, JE, and KA) independently appraised the quality of the selected articles using the Cochrane Risk of Bias Tool (RoB2) (52). Two votes were required to approve any appraisal decisions, and conflicts or disagreements were resolved by consensus between the co-authors involved in the decision or a third co-author. The RoB2 considers high, unclear, or low risk of bias for six domains: randomization, allocation concealment, blinding of participants and evaluators, incomplete outcome reporting, and selective reporting (52). The randomization, allocation concealment and blinding of participants and evaluators domains are more closely related to the assessment of RCTs. Thus, to account for the inclusion of other study types we also included selection, confounding, information, allegiance, and adherence bias assessments. Selection bias occurs when the researcher can influence who gets recruited to the study. Confounding bias occurs when extraneous or unaccounted elements could have impacted the outcomes assessed by the study. Information bias occurs when the outcomes are not adequately measured with validated tools. Allegiance bias corresponds to the relation between the developer of the studied treatment and the researchers, and adherence bias refers to any changes or deviations in the protocol. The risk of bias in each study was considered to be high if any of the assessed domains scored high for risk of bias, or if they scored unclear on two or more domains (52, 53).
2.7 Risk of bias across studiesWe assessed the risk of bias across studies or certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines (54). The quality of the evidence was downgraded if the risk of bias within studies, publication bias, imprecision in outcomes, indirectness or heterogeneity were considered to be high (54). We analyzed publication bias using funnel plots (55) and Rosenthal’s fail-safe N. These techniques show the number of hypothetical null results that would be required to make the results of the analysis insignificant (56). Imprecision referred to the significance of the reported results using a 95% confidence interval (95%CI) and the appropriateness of the sample size to achieve a power of 0.8, and 95%CI (54, 57, 58). Indirectness is assessed by considering the applicability of the outcomes, the use of surrogate outcomes, and the number of indirect comparisons (54). We calculated transitivity or heterogeneity using τ2 (the total variation) and I2 (the percentage of τ2 not related to random error) with higher values being associated with higher heterogeneity between studies (43, 59–62).
2.8 Summary measuresWe used Cohen’s d standardized mean differences (SMD or d) to summarize continuous outcomes (e.g. symptom severity), and risk ratios (RRs) and odds ratios (ORs) for dichotomous data (e.g. dropouts and treatment response) (53, 63, 64). The outcome measures were reported using a 95% CI to determine statistical significance (65).
2.9 Planned methods of analysisThis mixed-methods review presented two types of analysis to maximize the comprehensive assessment of the identified articles: meta-analysis and network meta-analysis (66). This approach accounted for the scarcity of articles in this field and supported the generation of insights about the current state of AI technology and its applications to online mental healthcare. We analyzed four treatment outcomes: “Main effects” considered all the primary outcomes reported by the relevant included studies regardless of type of symptom management. We focused on main effects to support a more robust assessment of the impact of AI-augmented interventions on non-specific mental health symptoms, and to avoid underpowered estimates due to the scarcity of available studies. “Depression” considered only interventions specific to the management of depression symptoms and changes in depression symptoms severity scores. “Anxiety” considered only interventions specific to the management of anxiety symptoms and changes in anxiety symptoms severity scores. And “Treatment dropouts” considered all reported participant dropouts in the included studies. Following this outcome analysis approach, all the studies presenting pre- and post-intervention data resulting from the implementation of AI-augmented interventions, were analyzed using a meta-analytic approach. This analysis supported the understanding of the effect of AI-augmented interventions in the management of psychiatric symptoms. Then, only the RCTs presenting post-intervention psychiatric symptoms data using validated scales were analyzed using a network meta-analytic (NMA) approach. This analysis supported a deeper exploration of the effects of the available AI-augmented interventions in relation to other interventions in the field through direct and indirect comparisons (67). Articles reporting on the results of large data set analysis using ML algorithms were not included in the meta-analysis or network meta-analysis calculations.
Missing data was collected by contacting the authors when appropriate, otherwise, this data was excluded from the analysis. For both meta-analysis and NMA, we employed a random-effects model to calculate the SMD or RR for relevant outcomes in the included studies, and heterogeneity using τ2 and I2 with the meta package in R version 3.5.3, computed using RStudio and the NMA tool, Meta Insight (59, 61, 68–70). We employed a random-effects model to account for study heterogeneity (I2>50%, and to accommodate different types of measurement for the same outcome (e.g., different assessment scales for depression) (59, 61). We also plotted forest plots for each outcome measure. For the NMA, we employed the assumption that the participants would have a similar probability of being allocated to any available treatment, meaning that the network was jointly randomizable (59, 71). The NMA league plots were used to present a more comprehensive assessment of all available direct and indirect head-to-head comparisons and to rank the interventions based on the desirability of their effect compared to other interventions. Additionally, we analyzed inconsistency to determine the goodness of fit of the NMA model (p>0.05 indicates network consistency) (68, 71, 72). Finally, treatment dropouts and any identified side effects were presented as a proportion for all studies. For RCTs reporting treatment dropouts, we employed an NMA approach as detailed above to obtain a deeper understanding of the attrition rates among the identified AI-augmented interventions.
3 Results3.1 Study selectionOur systematic search identified a total of 2276 citations from inception to October 2023 (Figure 1). From these, 38 reports were assessed for full-text assessment, and 29 studies met the eligibility criteria for inclusion in this review. 9 records were excluded due to wrong intervention, and wrong study design. Kappa Interobserver agreement was determined to be good (k = 0.78), and disagreements were solved by consensus.

Figure 1 PRISMA guidelines – Study selection process.
3.2 Study characteristicsThe identified studies (n=29) were mostly conducted in the USA (n=10), Sweden (n=6), and the UK (n=6). The studies included a diverse sample of adults (n=22), college students and young adults (n=4), anonymous users (n=2), and adolescents (n=1) (28–33, 41, 73–94). Eight studies reported on the ethnicity of the participants (30, 32, 41, 74, 76, 77, 80, 83), and in most of the studies between 31% and 91% of the participants identified as White. The studies focused on a population with moderate psychiatric symptoms including depression, anxiety, obsessive-compulsive disorder (OCD), insomnia, stress, body dysmorphic disorder (BDD), opioid misuse, social anxiety disorder (SAD), and bipolar personality disorder. Most studies (n=20) included >50% of female participants, with n=4 studies not reporting the percentage of males or females in their study (31, 33, 84, 90). The selected studies reported on several AI-augmented interventions modalities and AI tools and algorithms including: AI-self-guided eCBT: AI agent or chatbot delivering eCBT with no therapist guidance (n=7), AI-guided eCBT: AI agent or chatbot delivering eCBT with asynchronous therapist guidance (n=1), AI-modified eCBT: AI based modifications to eCBT program (n=3), AI-self-guided ACT: AI agent or chatbot delivered acceptance and commitment therapy (ACT) with no therapist guidance (n=1), Combined: Remote CBT and AI-self-guided eCBT combined (n=1); AI prediction of treatment dropout (n=2), AI identification of useful therapy aspects (n=1), AI identification of themes in patients’ utterances and written assignments (n=2), AI matching patients to appropriate treatments (n=1), AI prediction of short and long-term response (n=9), and AI prediction of treatment engagement and adherence (n=2). The included RCTs, observational cohort and non-randomized experimental studies included the following comparators: no intervention (n=2), remote CBT: standard CBT program delivered over the phone (n=1), psychoeducation (n=4), AI chatbot: regular interactions with an AI chatbot not trained or designed to deliver therapeutic content (n=1) (Table 1). These studies assessed the following outcomes: main effects (n=10), depression symptoms (n=7), anxiety symptoms (n=4) and treatment dropouts (n=13) (Table 2). On average the interventions were 7.33 (SD=4.16) weeks long, and 10 studies reported follow-up measurements. The length of the follow-up was on average 30.44 (SD=29.21) weeks long. Figure 2 presents all the head-to-head comparisons between the AI-augmented interventions and comparators as a network developed using NMA for the four main studied outcomes (main effects, depression, anxiety, and treatment dropouts).
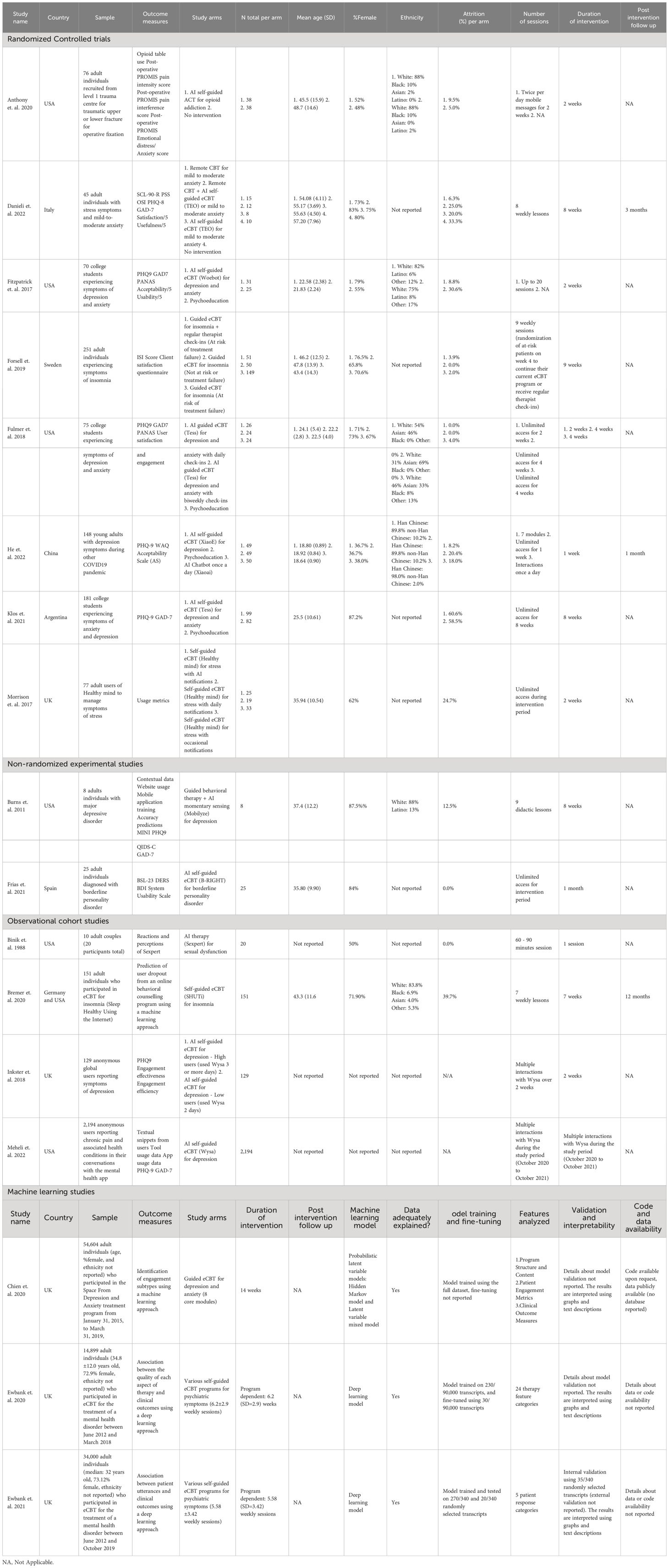
Table 1 Characteristics of the included studies.
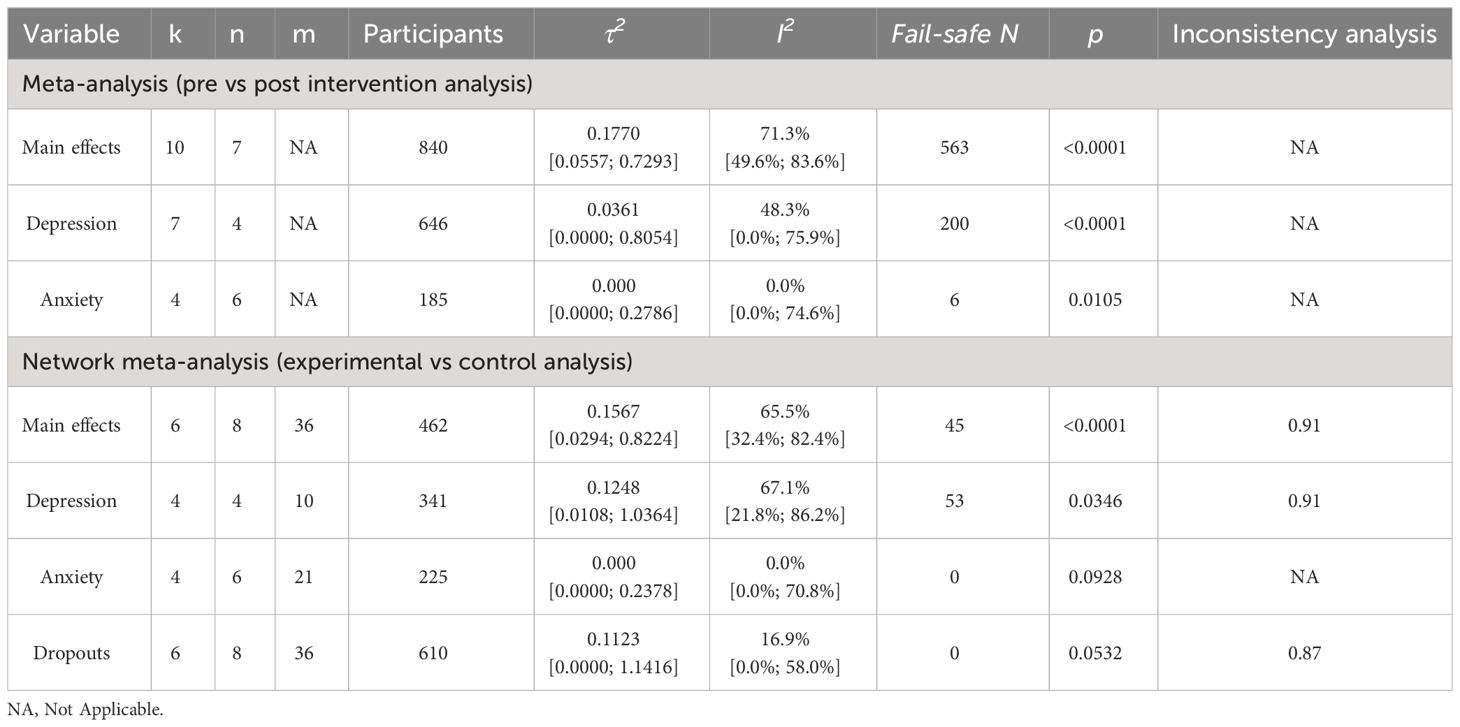
Table 2 Synthesis of data indices.
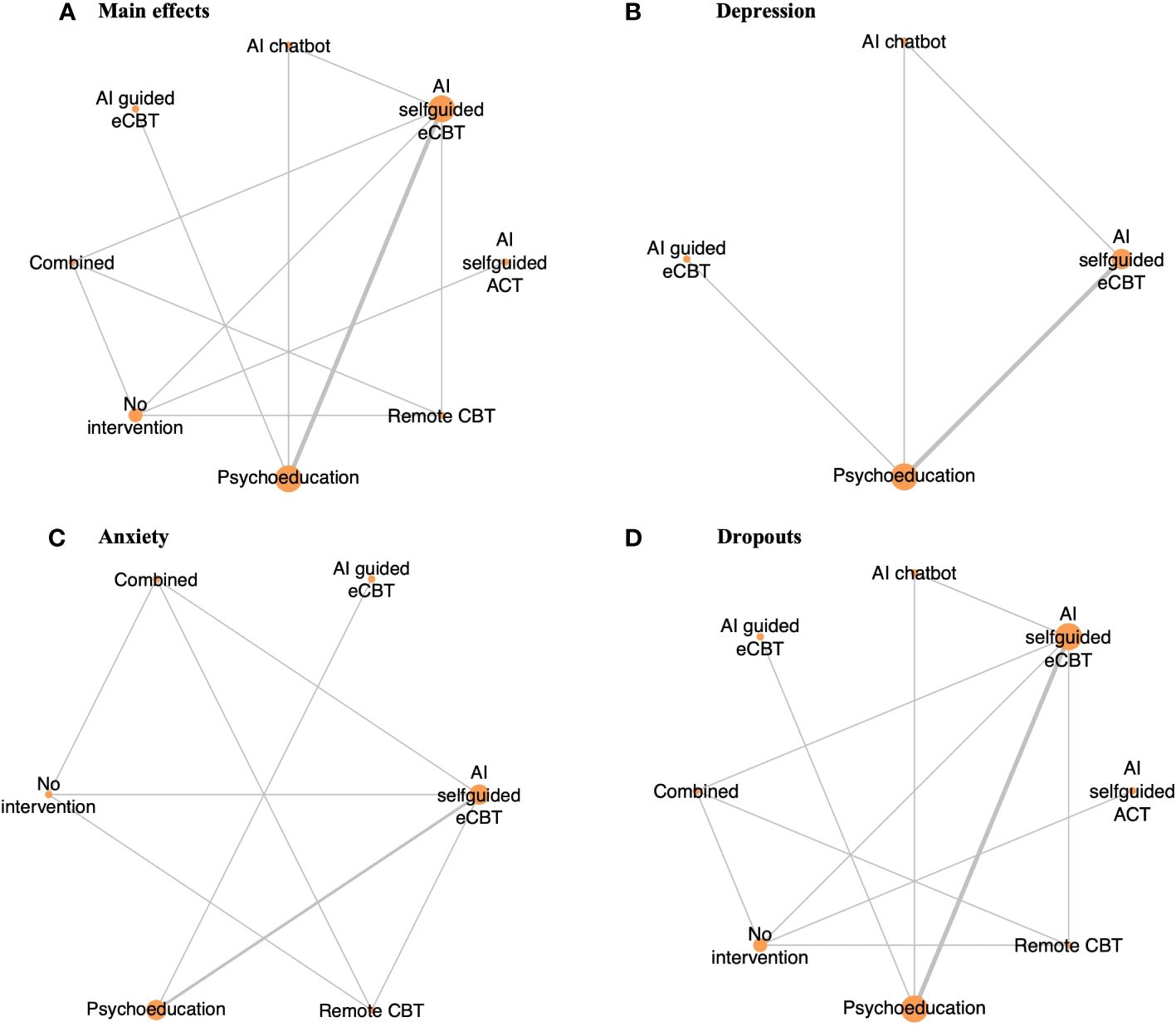
Figure 2 Network plot of RCTs included in this review, comparing the identified interventions (“AI self-guided eCBT”: AI agent delivering eCBT with no therapist guidance; “AI guided eCBT”: AI agent delivering eCBT with asynchronous therapist guidance; “Remote CBT”: Standard CBT delivered over the phone; “AI self-guided ACT”: AI agent delivered ACT with no therapist guidance; “Combined”: Remote CBT and AI self-guided eCBT; “AI chatbot”: AI agent providing general conversational responses not associated with CBT or therapy; “Psychoeducation”; and “No intervention”). Circle sizes represent the aggregated sample size relative to each intervention, and the thickness of the lines represents the number of studies comparing the respective interventions. (A) Main effects of all the included RCTs (n=6), (B) Depression scores in RCTs specific to the study of interventions for depression symptoms (n=4), (C) Anxiety scores in RCTs specific to the study of interventions for anxiety symptoms (n=4), (D) RCTs reporting participant dropouts (n=6).
3.3 Risk of bias within studiesUsing the Cochrane Risk of Bias Tool and the additional risk of bias domains (selection, confounding, information, adherence, and allegiance bias), most studies were generally of moderate to low quality, presenting a high or unclear level of bias in multiple domains. Among the included studies, the domains that commonly presented a high risk of bias were allegiance bias (n=14) and selective reporting (n=8). In terms of allegiance bias, because of the scarcity and novelty of AI-augmented interventions and other AI algorithms, most of the included articles developed and studied their own AI tools, instead of AI tools developed by other researchers (29, 33, 73, 74, 76, 79, 80, 82–87, 90). The studies that ranked high for selective reporting bias tended to report only user satisfaction data and treatment adherence, despite their methods considering the collection and assessment of psychiatric scale data and other effectiveness outcomes (29, 76, 79, 85–88, 92). Other sources of bias, such as adherence, selection, confounding or information bias commonly presented a low risk of bias (Supplementary Material 2).
3.4 Effectiveness and tolerability of AI-augmented interventions for the management of mental health symptoms3.4.1 Main effectsThe meta-analysis assessing the impact of AI-augmented interventions on mental health symptoms considered n=10 studies (41, 73–78, 80, 84, 90) presenting pre- and post-intervention outcomes. The results of this meta-analysis showed that these interventions had a significantly large effect size in the reduction of mental health symptoms. The sub-group analysis showed that AI-self-guided eCBT had a significant medium effect size, AI-guided eCBT effect had a non-significant effect, and AI-modified eCBT, AI-self-guided ACT and Combined had a significant large effect size in the reduction of mental health symptoms (Figure 3A). The NMA considered n=6 RCTs (41, 73, 74, 76–78). The head-to-head comparisons against AI-self-guided eCBT determined that this intervention was significantly more effective than AI chatbot with a large effect and psychoeducation with a medium effect size for the reduction of mental health symptoms. AI-self-guided ACT was significantly more effective than AI-self-guided eCBT with a large effect size for this outcome (Figure 4A). The head-to-head NMA ranked AI-self-guided ACT as the best treatment option for the reduction of mental health symptoms, with psychoeducation and AI chatbot being the lowest-ranked interventions (Supplementary Material 7A).
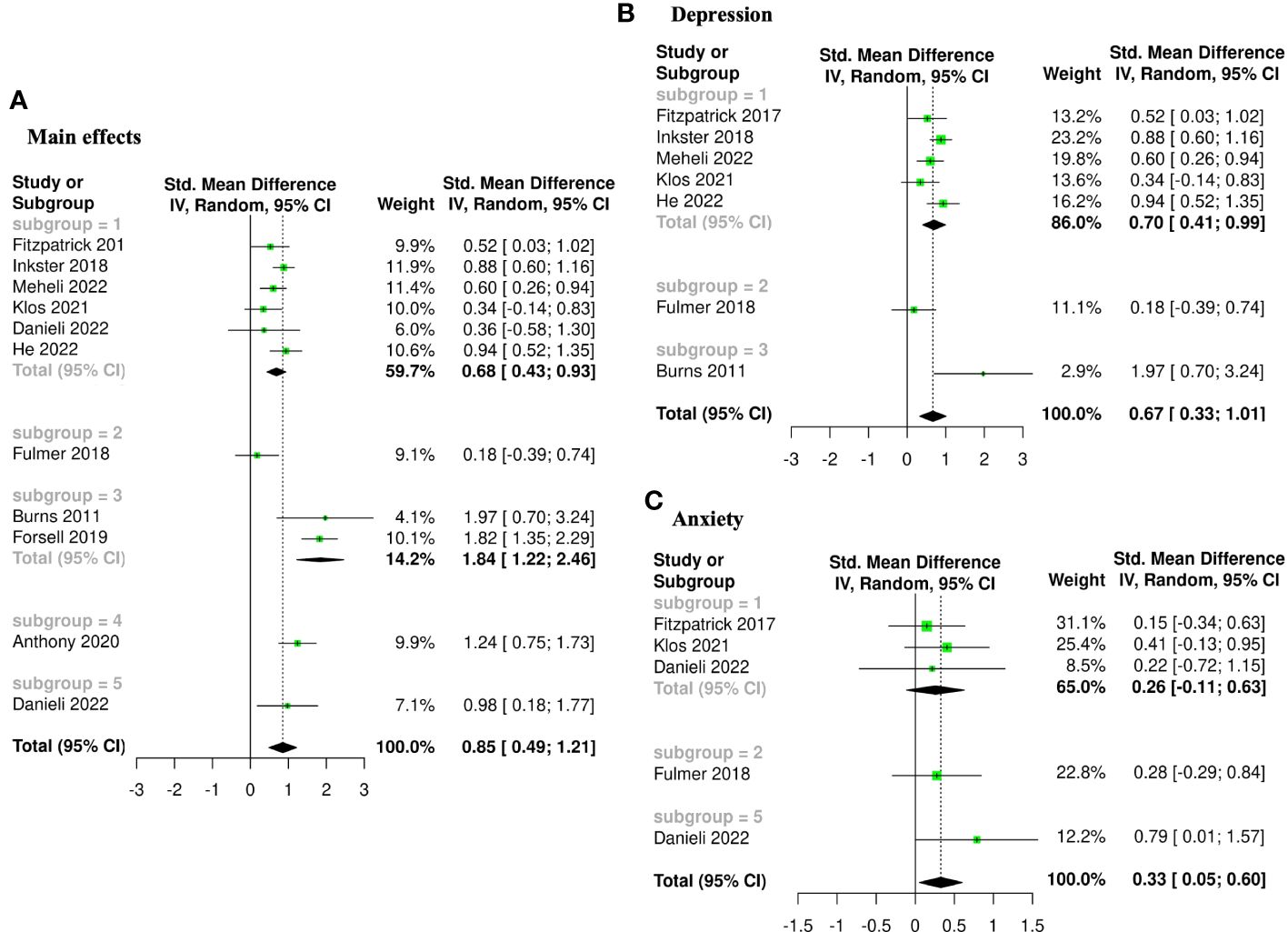
Figure 3 Forest plots of meta-analysis comparing pre- and post-intervention effects of five AI augmented interventions (Subgroup 1: AI self-guided eCBT, Subgroup 2: AI guided eCBT, Subgroup 3: AI modified eCBT, Subgroup 4: AI self-guided ACT, and Subgroup 5: Combined). (A) Main effects of all the included studies (n=10), (B) Interventions specific for the management of depression symptoms (n=7), (C) Interventions specific for the management of anxiety symptoms (n=4).
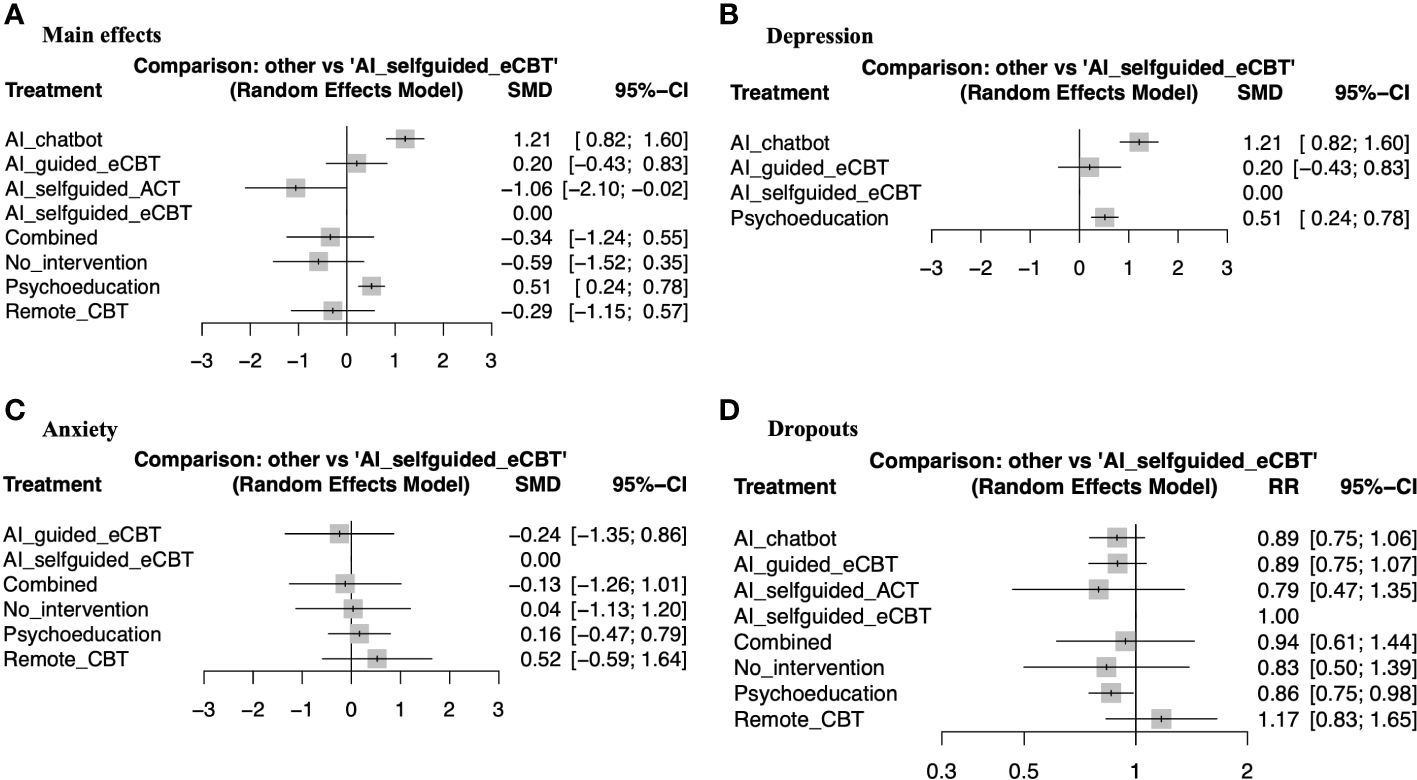
Figure 4 Forest plots of network meta-analysis of RCTs. (A) Main effects of all the included RCTs (n=6), (B) Interventions specific for the management of depression symptoms (n=4), (C) Interventions specific for the management of anxiety symptoms (n=4), (D) RCTs reporting participant dropouts (n=6).
3.4.2 DepressionThe meta-analysis assessing the impact of AI-augmented interventions for the management of depression symptoms considered n=7 studies (74, 76–78, 80, 84, 90) presenting pre- and post-intervention depression score outcomes. The results of this meta-analysis showed that these interventions had a significant medium effect size in the reduction of depression symptoms. The sub-group analysis showed that AI-self-guided eCBT had a significant medium to large effect size, AI-guided eCBT effect had a non-significant effect, and AI-modified eCBT had a significant large effect size in the reduction of depression symptoms (Figure 3B). The NMA considered n=4 RCTs (74, 76–78). The head-to-head comparisons against AI-self-guided eCBT determined that this intervention was significantly more effective than AI chatbot with a large effect and psychoeducation with a medium effect size for the reduction of depression symptoms (Figure 4B). The head-to-head NMA ranked AI-self-guided eCBT as the best treatment option for the reduction of depression symptoms, with psychoeducation and AI chatbot being the lowest-ranked interventions (Supplementary Material 7B).
3.4.3 AnxietyThe meta-analysis assessing the impact of AI-augmented interventions for the management of anxiety symptoms considered n=4 studies (73, 74, 76, 78)presenting pre- and post-intervention anxiety score outcomes. The results of this meta-analysis showed that these interventions had a significant small effect size on the reduction of anxiety symptoms. The sub-group analysis showed that AI-self-guided eCBT had a significant small effect size, AI-guided eCBT effect had a non-significant effect, and AI-modified eCBT had a significant medium to large effect size in the reduction of anxiety symptoms (Figure 3C). The NMA considered n=4 RCTs (73, 74, 76, 78). The head-to-head comparisons against AI-self-guided eCBT determined that the effects of this intervention were not significantly different than AI-guided eCBT, combined, no intervention, psychoeducation, and remote CBT (Figure 4C). The head-to-head NMA ranked AI-guided eCBT as the best treatment option for the reduction of anxiety symptoms, with psychoeducation and remote CBT being the lowest-ranked interventions, though none of these comparisons were statistically significant (Supplementary Material 7C).
3.4.4 Adverse events and attrition rateNone of the included studies reported adverse events associated with the interventions. 12 of the included studies (41, 73–83) presented data on treatment dropout, online or remote psychotherapy interventions reported a dropout rate between 0% and 61%, psychoeducation a dropout rate between 4% and 60% and no intervention a dropout rate between 5% and 34%. No specific trends were identified when comparing the attrition rates with intervention length or whether the online intervention was therapist guided or not (Table 1). The NMA considered n=6 RCTs (41, 73, 74, 76–78). The head-to-head comparisons against AI-self-guided eCBT determined that this intervention had a 14% lesser risk of dropping out compared to psychoeducation, this comparison was statistically significant. The other head-to-head comparisons against AI chatbot, AI-guided eCBT, AI-self-guided ACT, Combined and Remote CBT were not statistically significant (Figure 4D). The head-to-head NMA ranked remote CBT as the best treatment option in terms of lower attrition rates, with psychoeducation and AI-self-guided ACT being the lowest-ranked interventions (Supplementary Material 7D).
3.4.5 Limitations in the assessment of effectiveness and tolerabilityAn important limitation of this meta and network meta-analysis is the scarcity of studies in this field. Aside from AI-self-guided eCBT [n=6 (73, 74, 77, 78, 84, 90)] and psychoeducation [n=4 (74, 76–78)], other interventions and comparators were reported in three or fewer studies – AI-modified eCBT [n=3 (75, 79, 80)], no intervention [n=2 (41, 73)], AI-guided eCBT [n=1 (76)], AI-self-guided ACT [n=1 (41)], AI chatbot [n=1 (77)], combined [n=1 (73)], remote CBT [n=1 (73)]. This can increase the imprecision, the indirectness, and the impact of bias in the results obtained with these analyses (54). Supplementary Materials 3-6 shows the full forest plots of the NMA analysis, for main effects, depression, anxiety, and dropout-related data respectively.
3.4.6 AI treatment user satisfaction and tool usageIn terms of user satisfaction with AI-delivered online psychotherapy tools, several studies (n=10) reported that their study tool was well received and perceived as helpful and encouraging by around 60% to 90% of users. These studies highlighted the number of interactions with the tool, a sense of empathy and understanding, and the appropriateness of the dialogue as important positive factors determining treatment outcomes and satisfaction. They also reported that a minority of users, around 30% or less, thought that the tool did not understand them or found their interactions unhelpful or bothersome (73, 74, 76–78, 80–82, 84, 90).
In terms of tool usage and outcomes, Anthony et al. (2020) found that their AI-delivered online ACT intervention for postoperative pain, resulted in users consuming 36.5% fewer opioid tablets than the control group and significantly lower postoperative pain (41). Binik et al. (1988) reported that users of their AI-delivered virtual sexual dysfunction therapy tool (Sexpert) appeared comfortable discussing issues with their sexuality and other intimate topics with the tool (82). Danieli et al. (2022) found that when added to traditional in-person CBT, their AI-delivered online psychotherapy tool (TEO), promoted a greater improvement in stress levels and overall well-being compared to either in-person CBT or TEO by itself (73). Frias et al. (2021) determined that younger patients, those with a higher level of emotional dysregulation, and those with a higher level of education reported a high level of tool (B·RIGHT) usability (81).
3.5 AI improvements and feature detection algorithmsAI technology has been used to find ways to improve the features or the delivery of previously validated online psychotherapy treatments. In terms of improvement to the delivery of online therapy, the included studies [n=4 (30, 75, 79, 80)] have reported on the implementation of intelligent notifications, momentary sensing, monitoring of treatment outcomes, and triage decision support. Burns et al. (2011) implemented an AI momentary sensing tool (Mobilize) which used mobile device sensor data, to determine the appropriate time to deliver a treatment notification based on a participant’s mood and location. This tool demonstrated a 60% to 91% accuracy in location prediction (no significant results in predicting mood state) and significant effectiveness improving symptoms of depression (80). Morrison et al. (2017) implemented a similar momentary sensing approach to develop intelligent notifications to support treatment engagement. They found that compared to occasional notifications, intelligent notifications and daily notifications encouraged a higher exposure to the therapeutic content while maintaining engagement levels. However, they noted that the timing of the notification did not seem to support treatment engagement, when comparing intelligent notifications against daily notifications (79). Gonzalez Salas Duhne et al. (2022), implemented an AI algorithm which used demographic and clinical factors to determine whether participants would benefit from face-to-face guided self-help or eCBT retrospectively. Participants who received the appropriate intervention determined by AI experienced improved treatment outcomes (OR=2.10) and a lower risk of dropping out (OR=1.12) compared to the other participants. Notably this study reported that despite its availability, 98% of the sample received face-to-face therapy, while according to their AI algorithm 96% of participants with mild to moderate depression symptoms would have benefited from eCBT (30). Forsell et al. (2019) implemented an AI monitoring tool to detect participants at risk of dropping out of their eCBT program for insomnia. At-risk participants were randomized to either continue with their program or receive an adapted version including additional therapist interaction (around 14 minutes per week). This adaptation supported higher symptomatic improvement and a lower risk of dropping out (OR=0.33), compared to at-risk participants that continued with their standard eCBT program (75).
In terms of feature detection algorithms to improve online therapy delivery, the relevant studies [n=3 (85, 86, 91)] focused on the content of the therapy program and the utterances of the participants and found several factors that could promote and predict higher effectiveness and engagement. Ewbank et al. (2020) and Ewbank et al. (2021) applied a deep learning approach to label large datasets of patients and therapy transcripts obtained from a variety of eCBT programs for mental health symptoms (85, 86). Ewbank et al. (2020) determined that for therapy structure, the time spent on cognitive and behavioral techniques (changed methods content) was associated with higher odds of symptomatic improvement (OR=1.11) and treatment engagement (OR=1.12) compared to non-therapy-related content. Though they recognize that some non-therapy-related content such as greetings can be important for the session, excessive amounts of it can be distracting and ultimately detrimental. They also reported that the model had a precision of 50% to 100%, and human-level accuracy when labeling therapy transcripts (86). Ewbank et al. (2021) found that patients’ statements showing a desire or commitment to change (Change-talk active: OR=1.38 and Change-talk explore: OR=1.14) were associated with increased odds of symptomatic improvement and engagement. Comparatively, statements that moved away from the target behavior (Counter-change talk: OR=0.80) or that were not related to change (Neutral/follow: OR=0.94), were associated with poorer symptomatic improvement and adherence. They also reported that the model had human-level accuracy when labelling therapy transcripts, with a precision of around 50% to 80% for most labels (human assessor was better at labelling “Counter-Change talk”: 62% vs 18% than the model, and the model was better at labelling “Describing problems”: 57% vs 22% than the human assessor) (91). Myllari et al. (2022) applied a text-mining approach to identify common topics in the written assignments of patients participating in an eCBT program for generalized anxiety disorder. They reported that patients who wrote about the well-being of their family and loved ones showed faster and better symptomatic improvement, compared to those who wrote about monitoring thoughts, worries, and concerns about internet therapy (91).
3.6 AI prediction algorithmsAbout half of the identified studies (41.4%) focused on the implementation and analysis of a variety of AI prediction algorithms for different outcomes in online mental healthcare, including treatment responses and symptom remission, treatment dropouts, and treatment engagement and adherence (28–32, 87–89, 91–94). The identified studies applied ML algorithms such as: DL, and ANN algorithms to process large amounts of treatment data to identify helpful prediction factors. The specific AI data analysis approaches used by the relevant studies and an assessment of study elements following research guidelines and standards for ML studies (i.e., adequacy of the data for the intended outcomes, model training and fine-tuning, features analyzed, validation, interpretability, and code and data availability) are presented in Table 1. All ML studies [n=15 (28–33, 85–89, 91–94)] presented a comprehensive description of their data, justification for the implementation of ML in their analysis, and a description of the features analyzed. 80% of studies reported the data or approach used to train their ML algorithms (28–30, 32, 33, 85–89, 92, 93), 53.33% of studies reported the use of model fine-tuning (28, 30, 32, 85–87, 91, 93, 94), 73.33% of studies reported the use of internal validation methods (28–32, 85, 88, 89, 92–94), 13.33% of studies reported the use of external validation methods (28, 30), 26.67% of studies reported that the data or the code used in the study can be made available upon reasonable request (28, 30, 33, 92, 93), 20% of studies included a link to the repository for their ML algorithm code (29, 88, 93), and 6.67% of studies included a link to the repository for the dataset used (87).
3.6.1 AI treatment response predictionFor treatment response prediction, the relevant studies [n=9 (28, 29, 31–33, 88, 89, 92, 93)] focused on the identification of demographic and clinical factors [n=8 (28, 29, 31–33, 88, 89, 92, 93)], and engagement styles [(33)]. The 8 studies that focused on demographic and clinical factors included: Flygare et al. (2020) identified depressive symptoms, treatment credibility, number of body areas of concern, duration of symptoms, and working alliance as predictors of symptom remission with 78% accuracy, in patients participating in an eCBT program for BDD. They noted that the level of BDD insight and demographic variables were not important predictors of remission, explaining that treatment response may be more directly influenced by treatment factors (29). Lenhard et al. (2018) identified younger age of symptom onset, and duration and severity of symptoms in patients participating in eCBT for OCD were predictors of poorer treatment response with 75% to 83% accuracy. Based on these results they suggested that eCBT for OCD may be better suited for individuals with mild to moderate symptoms (88). Mansson et al. (2015) used fMRI and a machine learning approach to identify patterns of brain activation associated with long-term treatment response. They identified that patterns of Blood-Oxygen-Level Dependent response in the dorsal part of the anterior cingulate cortex and the amygdala could predict treatment response with 92% accuracy 1 year after participation in an eCBT program for social anxiety disorder (89). Pearson et al. (2019) identified pre-treatment assessments, comorbid psychopathology, disability, treatment credibility, and module usage as predictors of treatment response in patients receiving online psychotherapy for depression (32). Rocha et al. (2018) found that an average of 66% predicate engagement (messages exchanged with the platform) was positively associated with treatment outcomes, and that adherence to the treatment platform (eCBT for depression) predicted treatment outcomes with about 60% accuracy. Because of the low prediction accuracy, they explained that treatment engagement alone may not be a strong predictor of clinical improvement (31). Rodrigo et al. (2021) and Rodrigo et al. (2022) applied different AI approaches to identify predictors of treatment response in an eCBT program for tinnitus (92, 93). Rodrigo et al. (2021) applied decision trees models and determined that education level (master’s degree or higher education level 88% chance of treatment success), baseline tinnitus severity, and depression and anxiety symptoms were predictors of treatment response with around 70% accuracy (92). Rodrigo et al. (2022) applied an ANN and machine learning approach and determined that higher education level, older age, employment status (i.e., no or fewer work restrictions), baseline tinnitus severity, and lower insomnia scores were predictors of treatment response with 78% accuracy. These studies noted that additional factors may be at play determining a lower response success for individuals with a lower education background and argued for more resources targeted at this patient population (93). Wallert et al. (2022) reported that polygenic risk factors for intelligence was the most important genetic predictor, pre-treatment MADRS-S was the most important clinical predictor, and the time of day when the patient completed their MADRS-S was the most important treatment process predictor of symptom remission after participation in an eCBT program for depression with around 65% accuracy. They recognized that this accuracy level did not outperform other models in the literature (28).
One study focused on engagement styles: Chien et al. (2020) classified the participants of an eCBT program for depression into 5 classes of treatment engagement which considered treatment platform usage (i.e., time spent on the platform, access to therapy sessions and tools, and therapy session completion) and rate of treatment disengagement. They found that lower platform usage was associated with a lower rate of symptom improvement and that higher platform usage and low rate of disengagement were associated with higher symptom reduction for depression and anxiety symptoms. However, they noted that this classification did not consider the impact of sociodemographic factors because the used dataset only contained de-identified information (33).
3.6.2 AI treatment dropout predictionFor treatment dropout prediction [n=2 (30, 83)], Bremer et al. (2020) focused on the analysis of user journeys and identified that time spent in earlier stages of the program, morning wake-up times (either before 4:30 am or later than 6:45 am), time to get out of bed (less than 9 minutes or more than 66 minutes), a greater wake after sleep onset, logging triggers for 18 days or more, receiving emails for 30 days or more, and no interaction with the treatment platform for over 67 days could predict dropout early in the treatment (eCBT for insomnia) with 60% to 90% accuracy (83). Gonzalez Salas Duhne et al. (2022), implemented a supervised ML approach to analyze data from a face-to-face and an online CBT program for depression, and identified five common variables that could predict a higher probably of early dropout from online CBT: younger age, belonging to an ethnic minority, lower socioeconomic status, medications, and higher baseline severity of depression symptoms (30).
3.6.3 AI treatment engagement and adherence predictionFor treatment engagement and adherence prediction [n=2 (87, 94)], Kim et al. (2021) determined that higher engagement in an eCBT program for obesity was associated with higher weight loss. The implemented machine learning approach identified self-esteem, in-app motivational measures, lower intake of high-calorie food, and higher interaction frequency with a healthcare mentor as predictors of higher treatment engagement. Because of the association found between treatment engagement and response, similar factors predicted short-term and long-term weight loss, with lower lunch and evening snack intake, lower fat intake, lower step count, higher will and higher confidence being additional predictors for long-term weight loss. They reported that around 59% of outcome variance was explained by their prediction model (87). Wallert et al. (2018) reported that for patients participating in an eCBT program for post-myocardial infarction depression and anxiety, self-assessed cardiac-related fear, female sex, number of words used to complete the first homework, and self-rated depression were predictors of adherence with 64% accuracy. They noted that contrary to the literature, education level and age did not show a strong predictive power in this study (94).
3.7 Risk of bias across studies and quality of the evidenceAnalysis of heterogeneity showed that for the meta-analysis and the NMA, the main effects outcome had the highest degree of heterogeneity (I2>50%). In the NMA, the depression outcome also had a significant degree of heterogeneity (I2>50%) (Table 2) (43, 59–62). Analysis of funnel plots (Supplementary Material 8) and Rosenthal’s fail-safe N (Table 2) showed that most reported outcomes presented a low degree of publication bias (p<0.05), except for anxiety and dropouts in the NMA (55). Analysis of inconsistency showed that the NMA model had a good fit for the analysis of study outcomes (p>0.05) (54) (Table 2). Analysis of imprecision showed that for main effects, the analysis model had a low level of imprecision when comparing AI-self-guided eCBT to AI chatbot, psychoeducation and AI-self-guided ACT. For depression, the analysis model had a low level of imprecision when comparing AI-self-guided eCBT to AI chatbot and psychoeducation. For anxiety all comparisons had a high level of imprecision, and for treatment dropout data, only the comparison between AI-self-guided eCBT and psychoeducation had a low level of imprecision (Figure 4) (54, 57, 58). Analyzing indirectness, shows that most head-to-head comparisons arose from indirect data comparisons, rather than comparisons presented in the literature (direct comparisons) (54) (Supplementary Material 7). In terms of risk of bias, most studies presented a high level of bias in several of the assessed domains (Supplementary Material 2). Therefore, applying the GRADE guidelines (54) the quality of the data was low to very low.
4 DiscussionThis mixed-methods review aimed to present a comprehensive assessment of the current uses of AI in online mental healthcare using meta-analytic and network meta-analytic approaches. Our systematic search strategy identified various applications including triage, psychotherapy delivery, therapy progress monitoring, therapy engagement support, identification of engagement subtypes, effective therapy features and themes in patients’ utterances, and prediction of short-term and long-term treatment response, treatment dropout and adherence, and symptom remission (28–33, 41, 73–94). Analyzing these applications, the results of this review suggested that despite the novelty of this technology, AI has already demonstrated promising benefits to the field of online menta
留言 (0)