The field of contemporary Neuroscience is engaged in exploring the nervous system at a multitude of scales, encompassing molecular and cellular, subsystems, and entire organism levels (Soe et al., 2012). Despite significant advances, several challenges remain. These include the complexity and heterogeneity of the brain, characterized by millions of interconnected neurons (Sim et al., 2017), the difficulty in studying its size and intricacy, the inaccessibility of parts located within the skull (Boyden, 2011), limited tools for in vivo study, and the high speed of operation, with electrical activity patterns relevant to behavior taking place at the millisecond scale (Gradinaru et al., 2007). Despite substantial progress in the study of the nervous system, the understanding of the mechanisms behind its natural behaviors and responses to disease states remains extremely limited. As a consequence, the prevalence and global burden of nervous system disorders – including Alzheimer’s Disease (AD), Parkinson’s, and epilepsy – continues to increase, affecting millions of people worldwide. Despite considerable advances in medical treatments, including pharmacotherapy and electrical stimulation devices, their effectiveness is often limited by non-specificity and the occurrence of side effects (Rivnay et al., 2017). Therefore, addressing these disorders is a key challenge in neuroengineering, medicine, and science. Advances in this field are contingent upon technological progress toward the development of innovative tools for cell-type specific manipulation and observation.
Neural engineering research has produced a multitude of electrical, optical, chemical, and genetic tools for examining and controlling neural activity with increasingly high temporal and spatial resolution (Chen et al., 2017). These efforts strive to uncover fundamental neural functions, by manipulating and recording neuronal activity in vivo (Sim et al., 2017). Electrical stimulation and electrode-mediated recording have been the gold-standard methodologies applied to investigate neural circuits since these provide outstanding sensitivity and temporal resolution. However, some important constraints include limited in vivo spatial resolution and lack of efficiency to target multiple cells (Emiliani et al., 2015). Light-based techniques present clear advantages for monitoring and modulating neural activity. They are non-invasive, allowing for precise and flexible targeting of specific groups of neurons, and are capable of performing multiple tasks through the use of different wavelengths (Emiliani et al., 2015). Besides, optical tools hold the potential to offer a high spatial and temporal resolution, facilitating simultaneous interaction with numerous neurons (Doronina-Amitonova et al., 2015). A remarkable revolutionary advance was the introduction of optogenetics that, through the use of light-sensitive proteins, allows the manipulation of neurons and circuits with groundbreaking sub-microsecond precision (Boyden, 2011; Emiliani et al., 2015). Recent powerful trends include technologies that integrate multiple modes of interaction with neurons into a single device and allow for bidirectional communication with neural circuits with greater spatiotemporal accuracy (Frank et al., 2019). These advancements are not only aiding at a more extensive understanding of the brain, spinal cord, and peripheral circuits in the context of health and disease but also guiding the development of future, closed-loop therapies for neurological conditions (Canales et al., 2018; Frank et al., 2019).
Optical technologies are, therefore, at the forefront of various informative approaches to brain research, including high-resolution nonlinear optical and chemically selective microscopy and the widely used fluorescent biomarkers (Doronina-Amitonova et al., 2015; Cohen and Farhi, 2018). The ability to control light in space and time has recently been merged with traditional neuroscience approaches, resulting in the field of neurophotonics – a rapidly evolving cross-disciplinary area of natural sciences. This field encompasses the development of cutting-edge tools that use light to precisely and non-invasively perform manipulation and monitoring of individual and/or multiple brain cells and synapses (Ruthazer et al., 2022), with a wide range of neurobiology applications, from brain functional diagnostics, stimulation of neural networks, and molecular engineering, toward effective and selective diagnosis and therapy solutions for neurodegenerative and psychiatric diseases (Doronina-Amitonova et al., 2015; Ruthazer et al., 2022).
This review is focused on discussing the progress of the last decade in neurophotonics research. The work presented here aims to systematize the advances in photonic tools and methodologies, as well as identify new research trends, challenges and potential directions in this promising area. From a total of 166 screened articles, 56 representative works from the past 10 years, depicting innovative photonic approaches applied to Neuroscience case studies, were carefully selected by a systematic methodology [PRISMA 2020 (Page et al., 2021)]. The discussion is organized into six categories, concerning the main applications identified: (1) Advanced optogenetics, (2) Multimodal neural interfaces, (3) Imaging devices and probes, (4) Targeted therapeutics, (5) Remote operations, and (6) Microfluidic platforms. For each topic, the main challenges and motivations are debated, followed by a discussion on purposed innovative solutions. We intend not to evaluate in full detail each technology selected, but rather provide a broad perspective on the recent state-of-the-art of Neurophotonics. Although other reviews on the topic already exist, they focus on specific brain areas (Suter et al., 2014) or merely on imaging operations (Abdelfattah et al., 2022). Besides, some were published over 7 years ago (Doronina-Amitonova et al., 2015; Cho et al., 2016). Therefore, this work complements the existing literature by providing not only diverse examples of the most recent, innovative and impactful technologies under the Neurophotonics umbrella, but also by comparing and analyzing the evolution of paradigms, techniques and materials used, the challenges that remain in need of innovative approaches (light delivery, physical constraints, data management) and an insightful reflection on future directions for effective innovation and translation in this promising area.
MethodologyThe main goal of the present review is to understand the advances in neurophotonics research, regarding methodologies, tools and tackled scientific problems. Preceding the systematic analysis conducted, the following research questions were selected as guidance: (1) What are the main photonic tools applied in the context of neuroscience research? (2) What are the main neuroscience challenges/problematics tackled by photonic technologies? and (3) What gaps exist and what challenges remain for future research? In order to select the relevant scientific articles for the proposed systematic review, a multi-source research was conducted using three major electronic databases: PubMed, Web of Science and Scopus. For each database, a similar set of keywords was applied, based on the terms “Neurophotonics,” “Photonic devices” and “Optics and Photonics” applied to the “Neuroscience” and “Brain” research fields. A more detailed description of the search terms and retrieved results for each database is presented in Table 1. Notes, letters, reviews and erratum were excluded. A temporal period of 10 years (2013 – present) was selected. Each database was last consulted in August 2023.
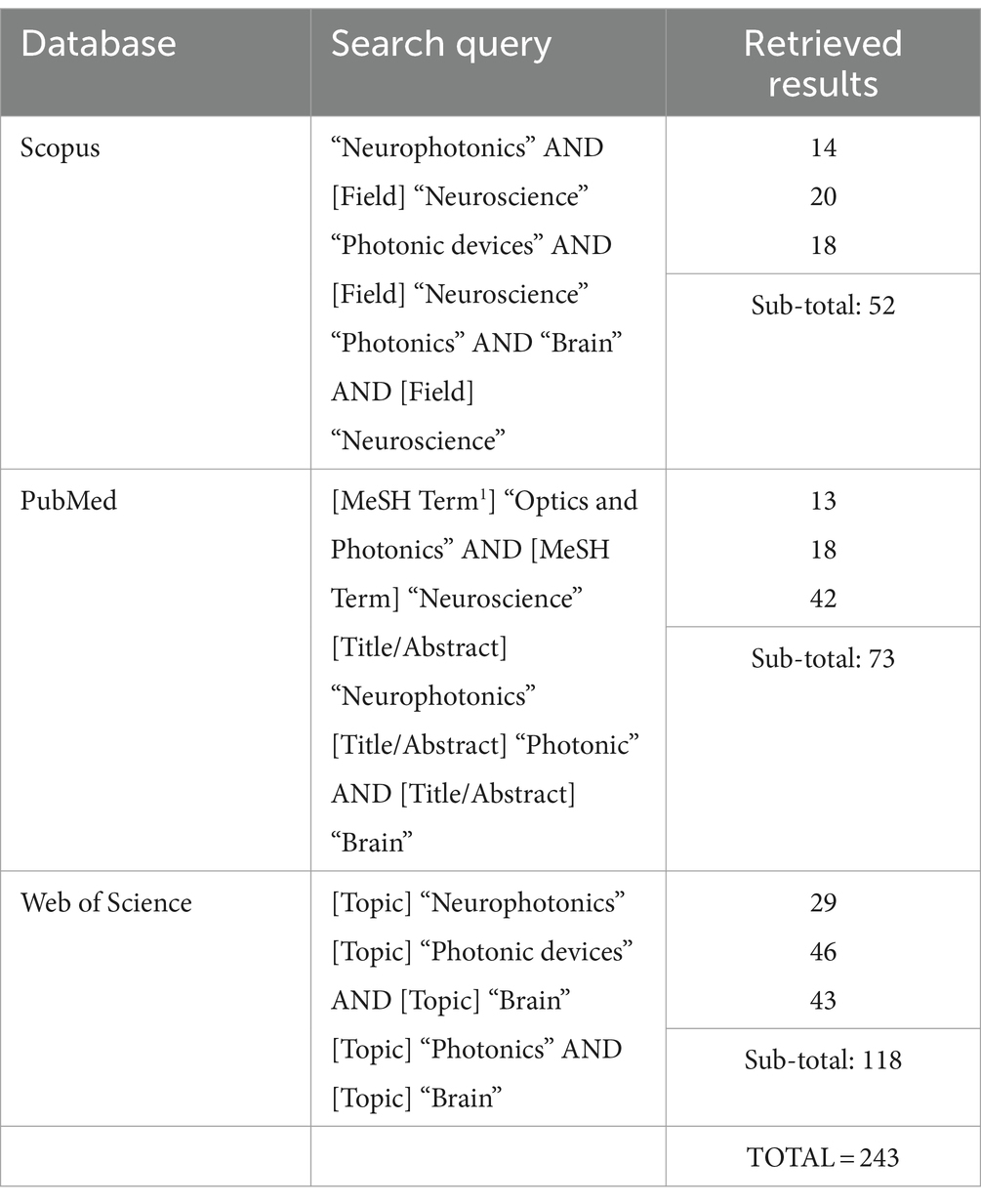
Table 1. Electronic databases used for the conducted review, with the corresponding search query applied and number of total retrieved results.
The methodology followed to select the relevant studies from the retrieved results was based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021), divided into three main steps, represented in Figure 1. The first step was the databases research presented above (Table 1), that retrieved a total of 243 references. A total of 77 duplicate records were removed in this step. Following that, 166 records were then screened by title and abstract. Articles presenting a methodology based on computational techniques, focused on photonic neuromorphic technology, a subject of computational sciences (use of neurophotonic paradigms for computational neural networks), and with no application or test conducted with a neuroscience case-study were excluded. Besides, articles in the format of reviews, perspectives, protocols and letters were also excluded. The remaining 98 articles were then evaluated for eligibility through full-text reading, where 42 were excluded by either only presenting theoretically and simulation results or by not including a clear innovation in Photonic technologies and methods. These included studies based on widely used microscopy tools, evaluation of physiological effects, case-studies, additional tests or small improvements to existing technologies. In case of encountering more than one study of the same group on a new technology, the paper where the method was completely described and properly tested for the first time was selected. The remaining 56 articles were considered for the intended discussion of recent advances in Neurophotonics, with the following information extracted: Year, photonic technology applied, brain target, aim and operations performed (sensing, imaging/recording, stimulation and optogenetics). The corresponding extracted data is synthesized and organized in Table 2. This methodology applies a restrictive selection process by considering simultaneously a moderately reduced timeframe (last 10 years), a clearly stated and fundamented photonic innovation, applied in a Neuroscience case study. This justifies the high number of records excluded. However, every requirement is essential to focus on the motivation questions stated previously and assure an accurate report of the most recent, innovative and impactful research in Neurophotonics.
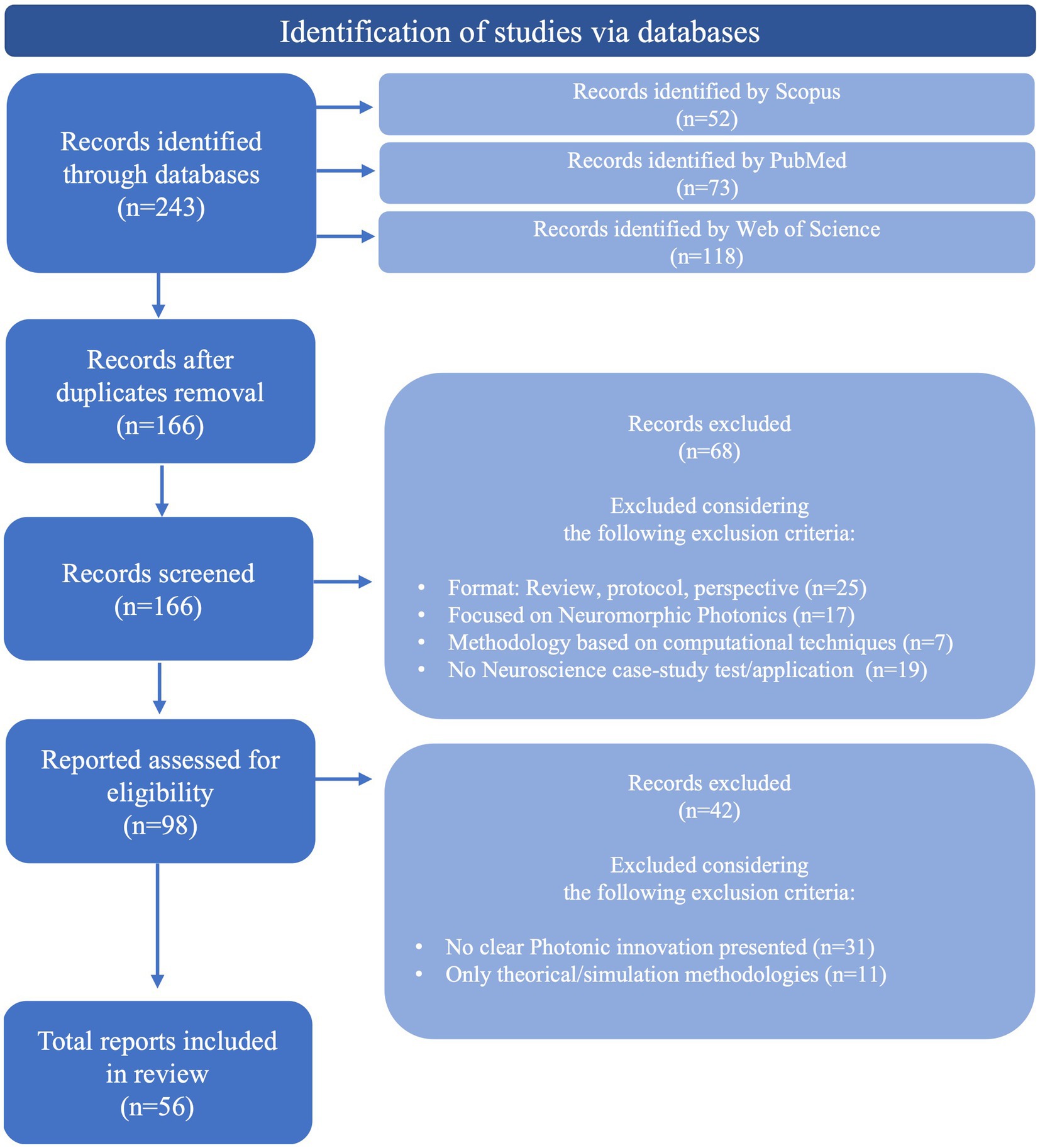
Figure 1. Methodology followed to select relevant studies from the retrieved results, based on PRISMA guidelines (Page et al., 2021).
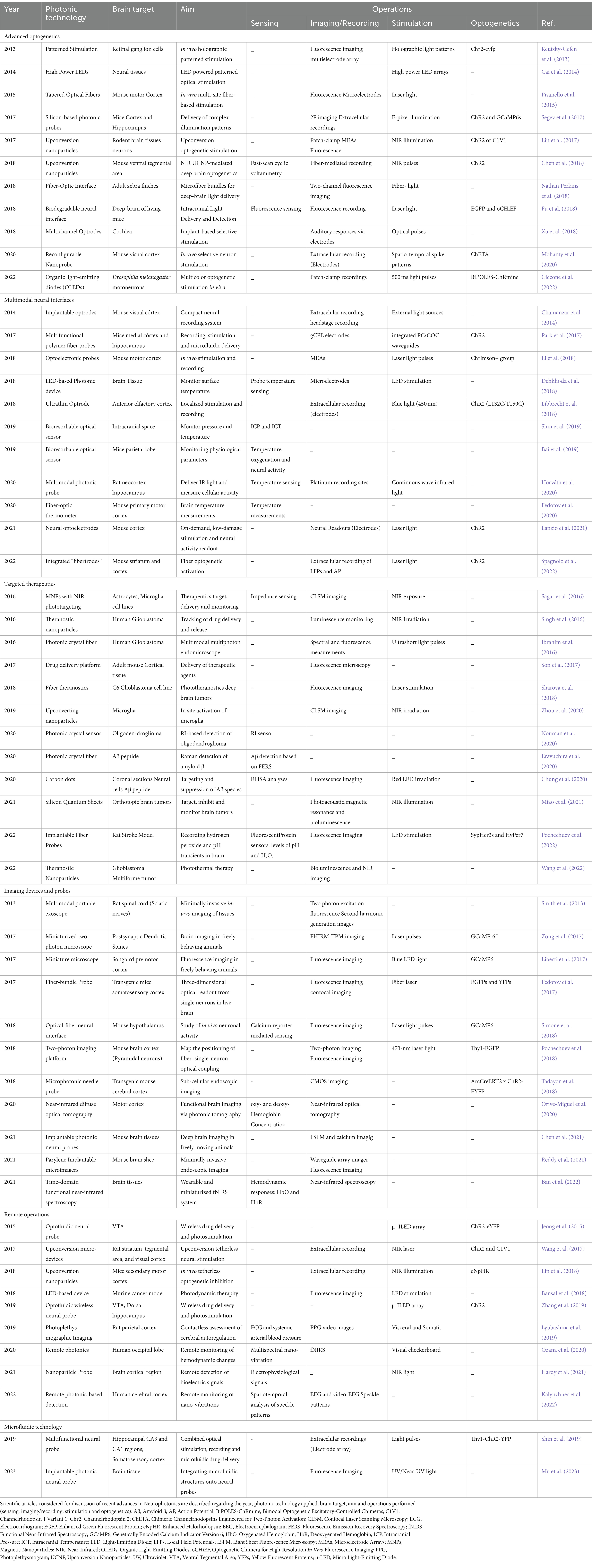
Table 2. Results table obtained after the application of the complete literature search methodology.
ResultsThe corresponding extracted data is synthesized and organized in Table 2. Since Neurophotonics is a rapidly evolving cross-disciplinary area, several distinct applications are described in the 56 articles selected. In order to provide a complete overview of the field, such applications are used to segment the discussion and highlight the different technology innovations and corresponding challenges of each main neuroscience target operation. Therefore, results are categorized and discussed into six main areas: (1) advanced optogenetics, (2) multimodal neural interfaces, (3) innovative therapeutics, (4) imaging devices and probes, (5) remote operations, and (6) microfluidic platforms.
The 6 main areas of research, with the corresponding technologies included in this review, are presented in Figure 2 and will be detailed in specific sections of this review. Advanced optogenetics area comprises technologies that use optogenetic methods for stimulation of neuronal components. In this area the aim is to perform localized delivery of light for precise stimulation of the signaling molecules. In this section, different optical stimulators, patterned and multi-spectral solutions to deliver light, and new methods for tetherless light delivery will be discussed. In the Multimodal neural interfaces area, we aggregated platforms that integrate two or more operations – stimulation, recording and/or sensing – simultaneously. These include optrode and fibertrode designs, micro and nanophotonic implantable probes and new sensing mechanisms to monitor physiological parameters. In the Imaging devices and probes section, we present various technologies to observe brain activity, from low-cost, portable imaging devices to implantable imaging probes and photonic-based innovative imaging techniques. The Targeted therapeutics category contains technologies developed for three main goals: Tumor-targeting, drug-delivery and detection of disease biomarkers. A brief description of each main disease is provided, followed by a discussion of the different solutions presented for precise, efficient, and personalized therapies. The Remote operations area includes innovative technologies for tether-free optogenetics, wireless, miniaturized, and lightweight platforms to monitor brain activity and in-vivo detection of biosignals. Finally, in the category of Microfluidic Technology, different methodologies are discussed regarding photonic probe-embedded microfluidics for drug-delivery and microfluidic chip devices to study neural networks connectivity.
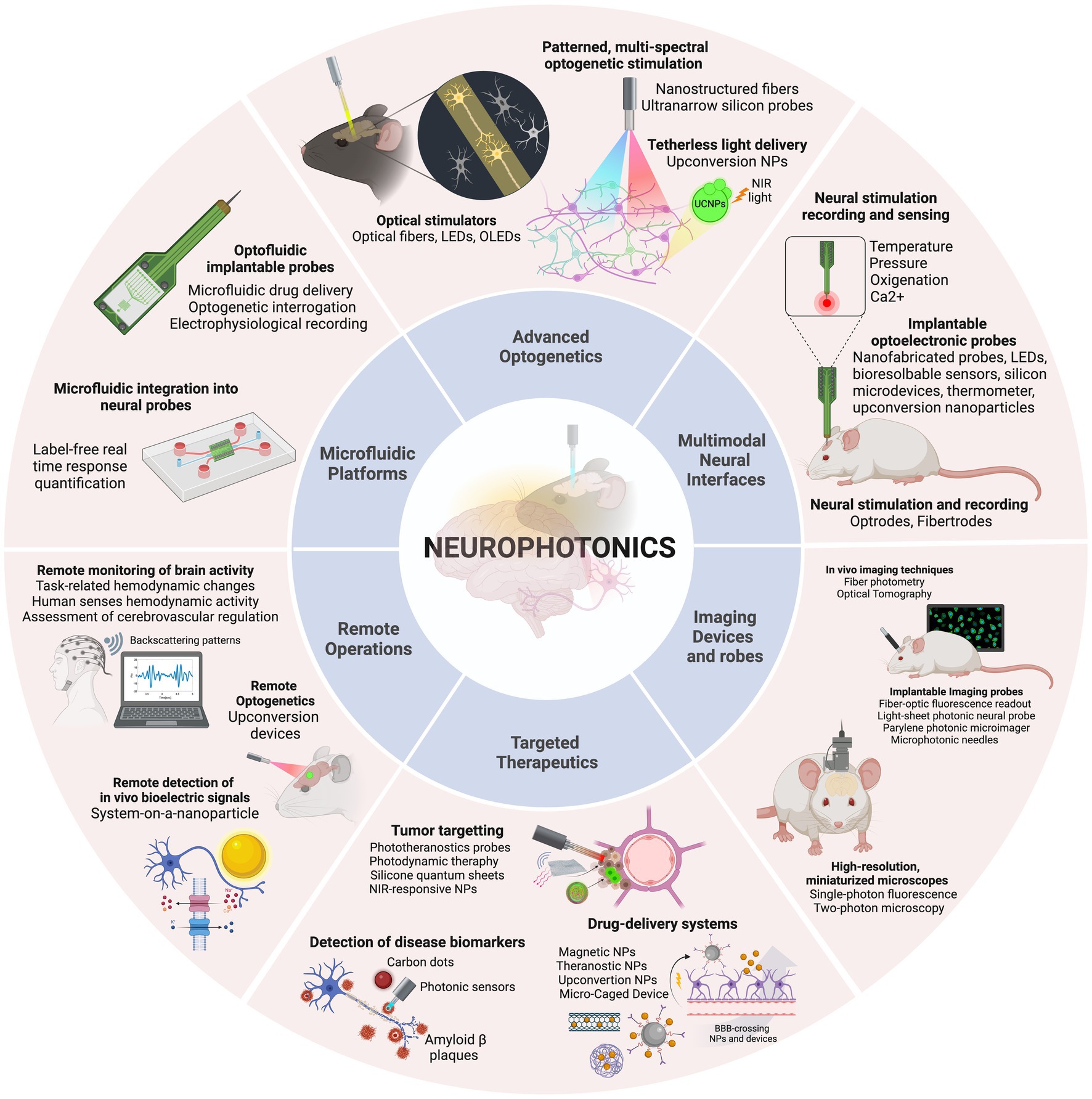
Figure 2. The discussion is organized into six categories, concerning the main applications identified: Advanced optogenetics, Multimodal neural interfaces, Imaging devices and probes, Targeted therapeutics, Remote operations, and Microfluidic platforms. Created with BioRender.com.
Advanced optogeneticsThe complexity of the brain, with billions of neurons highly interconnected through a sophisticated network of electrical and chemical signals, is a source of tremendous challenges in neuroscience research. Understanding the intricacies of this network is a major goal as it provides insights into the functioning of neuronal circuits and how they are related to the physiopathology of living systems (Yizhar et al., 2011). Recent advances in genetics and optics have revolutionized the investigation of functional connectivity in the brain by allowing simultaneous control and monitoring of neural activity. The combination of these two fields – known as optogenetics – has opened up new avenues for neuroscientists to study neural microcircuits and their functional connectivity in awake and behaving animal models (Deisseroth, 2011). The technique involves expressing light-sensitive proteins such as microbial opsins in neurons, intracellular organelles and molecules, performing labeling of the target structures, that can then be controlled by illumination (Boyden, 2011). The origin of optogenetics was prompted by the discovery of light-gated channels in the form of channelrhodopsins ChR1 (Nagel et al., 2002) and ChR2 (Nagel et al., 2003) and the application of ChR2 for exciting mammalian neurons with light (Boyden et al., 2005; Li et al., 2005). Since this revolutionary approach, optogenetics has become a versatile tool for precise manipulation of brain circuits, with the research efforts of several interdisciplinary groups originating the diverse toolbox of optogenetic tools available nowadays. Examples of advanced optogenetic stimulation techniques included in this study are presented in Figure 3.
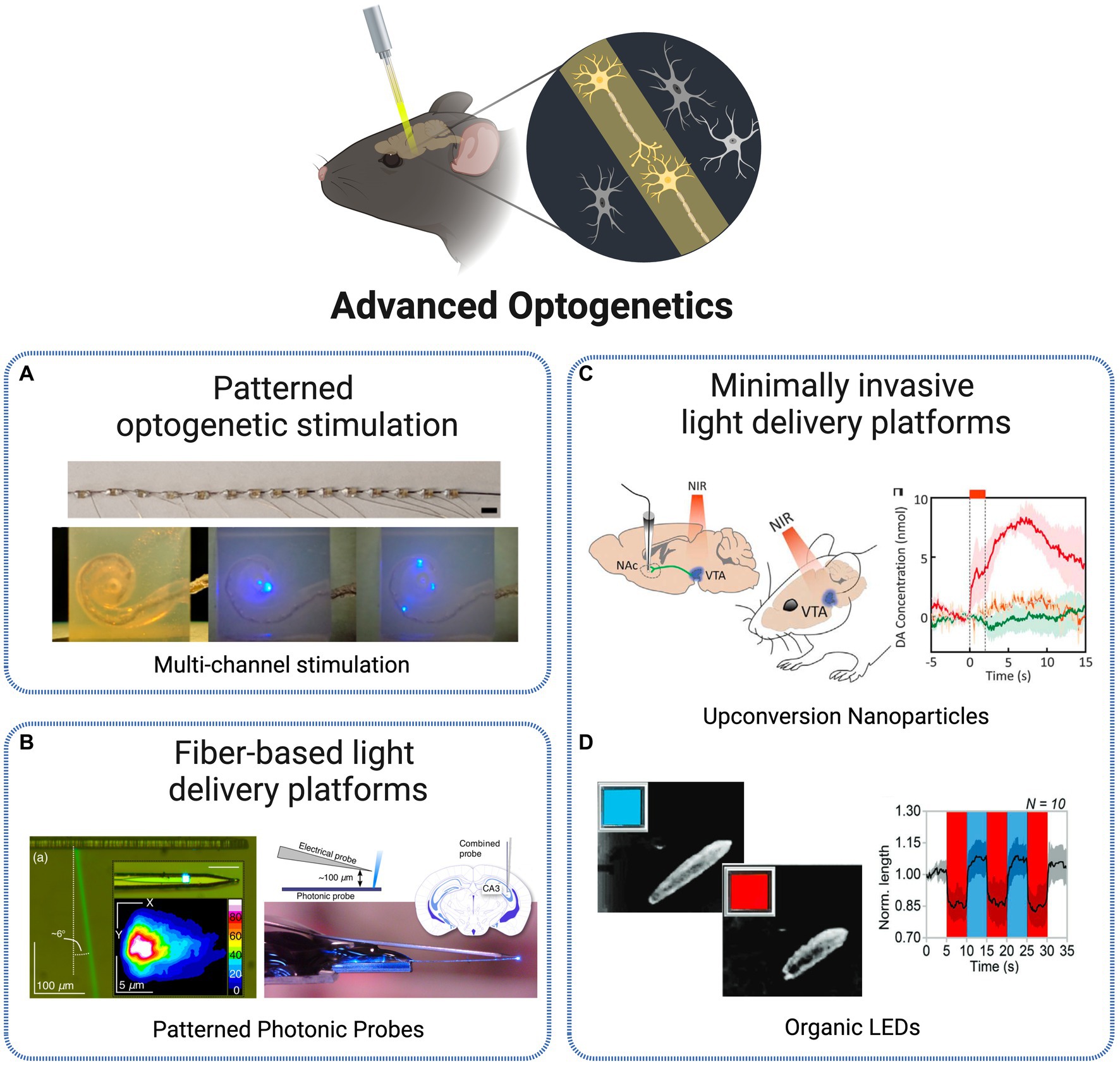
Figure 3. Technologies for optogenetic stimulation. (A) Multi-channel optrode-mediated stimulation constituted by an array of 15 μLEDs, inserted into a human scala tympani model (Xu et al., 2018). Licensed under CC BY 4.0. (B) Photonic probe with recording electrode tip and E-pixel optical stimulation (Segev et al., 2017). Licensed under CC BY 4.0. (C) In vivo transcranial NIR stimulation of the VTA using upconversion nanoparticles, with DA transient measurement in ventral striatum (Chen et al., 2018). Reprinted with permission from AAAS. (D) Blue-red OLEDs for Drosophila larvae motoneurons activation and inhibition (Ciccone et al., 2022). Licensed under CC BY 4.0. Created with BioRender.com.
One of the first critical aspects limiting optogenetic research was the ability to achieve control at a single-cell level. By having precise control over individual neurons, researchers could investigate the role of specific cells within a circuit and gain a better understanding of how circuits function. Significant progress has been continually made in the development of excitatory and inhibitory opsins that allow for direct optical control of cellular processes (Deisseroth, 2015). In order to achieve higher levels of precision in optogenetic tools, several complementary methods for delivering light with cellular specificity in vivo were proposed over the last decade. The initial conventional approaches for optical excitation of genetically targeted populations were based on light delivery tools such as optical fiber coupled illumination, LEDs and μ-LEDs, digital micromirror arrays or wide-field flashes, that deliver light directed to the entire population rather than in a specific manner (Reutsky-Gefen et al., 2013).
One solution adopted to tackle single-cell specificity was the use of patterned optogenetic stimulation. Reutsky-Gefen et al. demonstrated the first example of using a holographic pattern for optogenetic stimulation (Reutsky-Gefen et al., 2013). With this method, retinal ganglion cells expressing ChR2-eYFP were stimulated in vivo with a temporal precision in the millisecond timescale, with high cellular resolution through parallel multi-point stimulation delivered by the complex pattern. Another approach presents a patterned stimulation strategy based on high-power LEDs, where each frame can display flexible stimulation patterns (Cai et al., 2014). The 1,024 channels optical stimulation platform offers the possibility to manipulate the light delivery angle in each individual LED, producing flexible stimulation patterns refreshed at above 20 Hz. In Xu et al. (2018) present different methodologies to create multichannel optrodes with arrays of small optical sources such as side-emitting laser diodes (SELDs), vertical cavity surface emitting lasers (VCSELs) and μLEDs, that allow spatially selective stimulation through infrared neural stimulation. As an example, the authors depict a flexible optrode made with 15 connected μLEDs and show its potential to be applied in cochlear implants by a successful implantation into a human scala tympani model (Figure 3A). Another approach, at a smaller scale, is presented in Mohanty et al. (2020) based on nanophotonic optical technology. The authors present an implantable, 8-beam silicon-based nanoprobe, capable to stimulate specific sets of neurons with exceptional precision and simultaneously record their activity. This was achieved through a nanoscale switching network within the probe, controlling the flow of light dynamically. They demonstrated the ability to generate various neuron spike patterns in vivo, including sequential bursts and random pulses, all with sub-millisecond temporal precision. Their experiments highlighted the probe’s high-speed and reliable switching capabilities, suggesting its potential for precise control of neuronal populations.
Despite the advances in manipulating the transmission of light, important limitations arise at the time of its delivery to the brain. Optical stimulation and detection techniques, to be applied in the deep neural tissue, need to cope with scattering and absorption effects, demanding the use of relatively large optical probes and fibers to be implanted, which in turn cause significant damage to the tissue (Szarowski et al., 2003). Initial light delivery probe technologies such as fiber-based devices or large chip shank-like designs are highly effective in delivering light to deep tissues. However, these are characterized by large cross-sections that lead to tissue destruction, activating immunological response and disrupting the biological system. Multiphoton stimulation and microfabricated multi-site light delivery probes are among the most notable methods attempting to address invasiveness issues while achieving deep tissue light delivery. These methods aim to reduce tissue damage and immunological response while maintaining high precision and resolution, paving the way for non-destructive and minimally invasive optical stimulation and detection in deep tissue (Fain et al., 2017).
Pisanello et al. (2015) elaborated a technology tackling such challenges, through the use of tapered, nanostructured optical fibers as light delivery platforms for optical control of neural activity in vivo. The properties of the fibers – flexibility, small size and high spatial resolution – allow for a minimally invasive operation within the brain in a highly controlled manner. The authors present an advanced multi-site optogenetic stimulation achieved through the ability to switch, along the length of the taper, optical excitation to different regions of neural tissue. This allows for dynamic and selective illumination of different regions, providing optogenetic control of multiple brain sites. The device was tested in combination with MEAs-mediated extracellular recording. When tested in awake mice, it showed a remarkable in vivo suitability for layer-selective optical control of neural activity.
Besides optical fibers, silicon has become over the years a suitable and conventionally selected material for optical neural implants due to a range of beneficial characteristics including optical transparency, mature fabrication methods, cost-effectiveness, high stability and reduced loss in biological environments (Fu et al., 2018). Several approaches were thus developed centered on this material for implantable photonic devices for deep-brain complex light delivery. One example is proposed in Segev et al. (2017), based on ultranarrow probes with embedded silicon-based nanophotonic components for the delivery of complex illumination patterns within brain tissues (Figure 3B). Local optogenetic neural activation was observed in the cortex of mice expressing GCaMP6, through both extracellular electrical recordings and two-photon functional imaging.
Another trending solution over the recent years is the miniaturization of tools used to deliver, manipulate and collect light within deep brain regions. The use of microfibers is an example of such paradigm. In Nathan Perkins et al. (2018), the authors suggest the implantation of numerous (hundreds to thousands) 8 μm multimode optical microfibers into the brain, as stimulation light sources. As the implanted fibers spread gradually through the brain, a detailed surface fluorescence image is obtained for the deep 3D neuronal volume. With this approach, optical delivery is extended to targeted deeper regions of the brain while tissue displacement, immunological response and local network disruption are minimized due to the small diameter of the fibers. Extending the miniaturization to an even lower scale, Lin et al. (2017) suggest an approach based on a specific type of nanoparticles designated UCNPs. The authors designed an implantable optrode based on UCNPs with the goal to achieve remote activation of brain tissues. The nanoparticles, based on NaYF4, emit visible light as a response to tissue penetrating NIR light. As an outcome, neurons expressing different ChR proteins (ChR2 or C1V1), when close or in direct contact with the nanoparticle, are stimulated. Another example is presented in Chen et al. (2018) where transcranial NIR UCNP-mediated optogenetics was applied to evoke dopamine release from genetically tagged neurons in the ventral tegmental area (Figure 3C). The activation of inhibitory neurons in the medial septum, silenced seizure by inhibition of hippocampal excitatory cells and triggered memory recall. This innovative strategy allows for light delivery deep within the brain, in a tetherless way and without requiring any type of electronic components, paving the way for new all-optical wireless optogenetic stimulation techniques able to control brain activity not only at the single-cell levels but also in a network perspective.
The research and implementation of new materials is resulting in the integration of very interesting properties into technologies for deep-brain optogenetic control. For instance, to address the compatibility and stability challenges of common silica fibers, biocompatible and biodegradable materials have been explored for the investigation of deep-brain neural activity. In Fu et al. (2018), PLLA fibers have been implemented as a biodegradable optical neural interface for intracranial light delivery and detection, enabling deep brain fluorescence sensing and optogenetic interrogation. The in vivo study involving fluorescence signal collection and optogenetic stimulation in freely moving animals demonstrates the feasibility of biodegradable photonic devices and systems for both fundamental biological studies and clinical applications within the human body. In device fabrication, an alternative to LEDs has been gaining popularity. Although commonly used as a light source for optogenetic experiments, these devices are characterized by a low biocompatibility, rigidity and bulk design, which prompted the interest for alternative OLEDs. These operate at a reduced voltage, allow fabrication on flexible substrates and offer more tunable optical properties. Ciccone et al. (2022) present an implementation and characterization of such devices for switchable multi-color emission. This property allows for bidirectional optogenetic control – the OLEDs are able to shift between blue and red/green light emission, triggering both optogenetic excitation and inhibition (Figure 3D). This operation was tested in ND7/23 cells and Drosophila melanogaster larvae expressing bidirectional optogenetic proteins, showing high capability for a precise control of neuronal activity through innovative bicolor optical brain stimulation in vivo. Another approach is presented in Hillebrandt et al. (2023) where authors suggest the use of ultra-high brightness OLEDs for application in vision loss and, more specifically, restoration of visual perception, by providing a technology able to perform high-resolution optogenetic control of parallel retinal cells able to be adapted and, in the future, implemented into light-amplifying prosthetics. Lastly, nanomaterials are a class holding great promise for neural activity research, by functioning as transducers for neural stimulation via thermal conversion or light upconversion, as implemented in the UCNPs-based implantable optrode discussed previously.
Multimodal neural interfacesThe demand for a multi-scale, mechanistic comprehension of brain function that accounts for local and whole-brain circuits remains a challenging task. One of the primary hurdles is the need for suitable tools to perform high-resolution monitoring of local neuron ensembles concurrently in various brain regions in awake and freely moving animals (Chamanzar et al., 2014). Given the crescent evidence that numerous cognitive behaviors implicate neural circuits spanned across multiple brain regions, the adoption of distributed recording and stimulation instruments is of utmost importance. A continuous challenge is the required trade-off between the range of stimulation and optical power, which ultimately results in an inherently low spatial resolution (Yizhar et al., 2011). This limitation directed research efforts toward the use of optical light guides to deliver light to target locations beneath the surface of the brain. More specifically, to enable light delivery and electrographic recording simultaneously from the intact central nervous system, researchers have designed dual optical and electrical probes, which are known as optrodes, combining both optical and electrical elements in a single device (Petrovic et al., 2022). The multimodal neural interfaces included and discussed are presented in Figure 4.
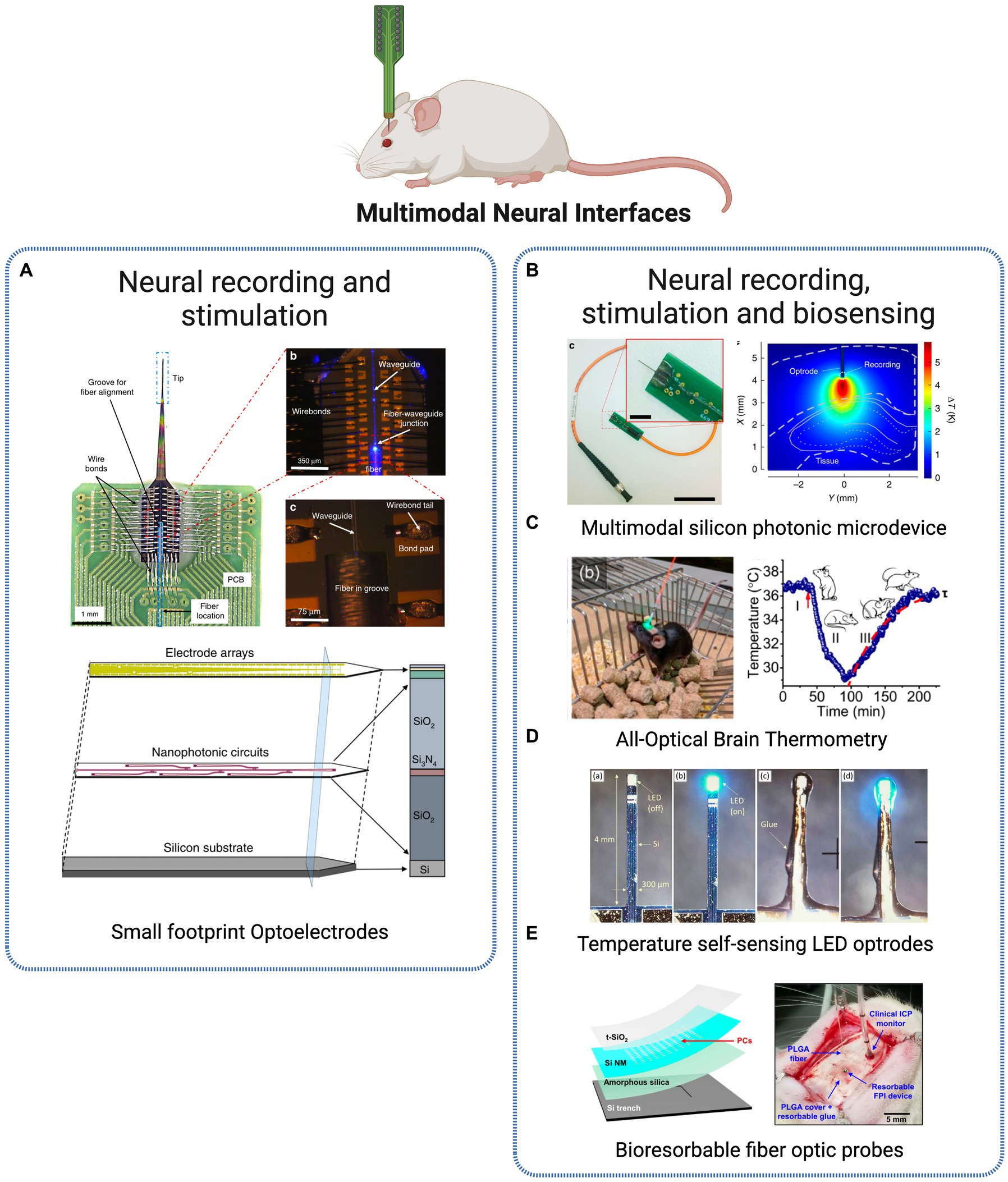
Figure 4. Multimodal Neural Interfaces. (A) Optoelectrode probe combining miniaturized arrays of sensors and nanophotonic circuits, combined for electrode-based neural readout and light delivery for optogenetic stimulation (Lanzio et al., 2021). Licensed under CC BY 4.0. (B) Silicon neural microprobe for infrared stimulation/inhibition and temperature sensing (Horváth et al., 2020). Licensed under CC BY 4.0. (C) Implanted fiber-optic thermometer in freely behaving mice (Fedotov et al., 2020). Reprinted with permission from American Chemical Society. Copyright 2020 American Chemical Society (D) LED-based optrodes mini-LED mounted on a silicon shank for temperature sensing (Dehkhoda et al., 2018). Licensed under CC BY 3.0. (E) Implantable bioresorbable sensor for pressure and temperature monitoring (Shin et al., 2019). Licensed under CC BY 4. Created with BioRender.com.
Optrodes were initially designed as a combination of commercial optical fibers and metal microelectrodes. Although this approach is relatively simple, it has its drawbacks, such as poor scalability, tissue damage when both fibers and electrodes are inserted in the same brain area, and limited control over the light emission geometry (Dufour and De Koninck, 2015). Over recent years, more advanced designs have emerged, featuring multiple sites for light delivery and electrical recording while attempting to minimize invasiveness and tissue damage. For such requirements, the use of micro and nanofabrication techniques is becoming a popular approach, providing new opportunities to produce compact, integrated and scalable optoelectronic probes. An example of microfabricated optrodes for high resolution electrophysiology and optogenetic stimulation is provided in Chamanzar et al. (2014). The authors describe the design and implementation of minimally invasive (50 μm × 20 μm) implantable optrodes with a silicon-parylene electrical layer for extracellular recording and an integrated photonic layer (microfabricated photonic polymer optical waveguides) for localized light delivery, with single-cell resolution. Another example is presented in Park et al. (2017) through a multifunctional polymer-based probe for opto-electrophysiological investigation of neural circuits in the mouse brain. The device includes an optical waveguide, six electrodes, and two microfluidic channels that allow for the injection of viral vectors carrying opsin genes, neural recording, and optical stimulation all in one. The miniature size of the device allows for multiple implantations in the brain, enabling investigations of brain circuits during behavioral experiments. The device composition – polymers and polymer composites – minimizes tissue response and allows for chronic, high-fidelity interrogation of brain circuits. Also focused on minimal size platforms, Libbrecht et al. present an ultrathin neural interface based on microchip technology, with 12 optical outputs (silicon nitride waveguides) and 24 electrodes (titanium nitride electrodes) (Libbrecht et al., 2018). The authors show the potential of the device for spatially confined optogenetic stimulation in vivo, measuring its effect in the anterior olfactory cortex of anesthetized and awake behaving mice. Due to the design of the device, it is possible to confine optical stimulation to small volumes with approximately single-cortical layer thickness, while recording simultaneously without measurable electrical artifacts.
Li et al. (2018) present an example of implantable optoelectronic probes manufactured using nanofabrication techniques for fully integrated electrical recording and optical stimulation. Electron beam lithography technique was used to fabricate 40 electrical recording and 6 optical stimulation sites within the optoelectronic probe. Nanofabrication offers an inherent advantage for device miniaturization and scalability, which are highly desired characteristics for the goal of scale up recording throughput and light delivery capabilities of optoelectronic probes. Examples of such type of fabrication are applied in Lanzio et al. (2021) and Lanzio et al. (2021) where the group presents advanced neural probes for electrode-based neural readout and light delivery for optogenetic stimulation, by combining micro- and nanofabrication techniques for high throughput and scalability. The authors present an innovative strategy based on the miniaturization of neural optoelectrodes by employing microfabrication techniques to create compact, versatile, and easily controllable neural probes (Figure 4A). These probes are designed with arrays of sensors and nanophotonic circuits that incorporate embedded ring resonators, thus creating a platform successfully brings together several desirable attributes for optoelectrodes, from reduced size and invasiveness, to expanded number of sensors and stimulation sites, while achieving precise light control without generating heat (Lanzio et al., 2021).
More recently, new approaches re-inventing and improving the optical fiber and electrodes pioneer combination of optoelectronic probes are rising. In Spagnolo et al. (2022), the authors introduce the concept of “fibertrodes” to describe the integration of microelectrodes on tapered optical fibers. Through microfabrication techniques (two-photon polymerization), multiple recording sides are fabricated around the tapered edge. Angled light delivery is used for optogenetic stimulation, while electrical recording is simultaneously performed in one up to three neurons, in vivo, with no photoelectric artifacts, demonstrating spatially confined optogenetic activation and simultaneous artifact-free extracellular recording of LFPs and action potentials.
Besides the stimulation and recording multimodal approaches presented above, a new optical dimension has been introduced into optical implantable devices – sensing of physiological parameters within the brain. One of the most commonly measured parameters is temperature, due to its relevance in tissue damage, cellular metabolism and response of neuronal populations in neurodegenerative disease stage development. In Horváth et al. (2020), authors present a multimodal photonic neural probe combining, beyond the optical and physiological operations, thermal sensing (Figure 4B). The three distinct functions are fully integrated into a single device, capable of performing electrical readout of individual cells at multiple locations along the probe shaft while simultaneously allowing temporal and spatial control of temperature within the deep-brain tissues. The highly multifunctional microsystem thus offers the possibility to measure cellular activity in deep neural tissues regarding thermally evoked responses.
For a similar purpose, another group presents an implantable, ultracompact thermometer based on optical fibers for high-resolution temperature measurements in the brain of freely behaving mice (Fedotov et al., 2020). Microcrystals coupled to the guided modes of a fiber-optic probe are used to generate a photoluminescence spectrum from which is possible to read out the local temperature within the brain (Figure 4C). The thermometer offers subcellular resolution with an accuracy within 0.15°C while also maintaining laser-induced brain heating below 0.1°C. Such approach presents a valuable alternative solution to invasive components such as thermocouples and thermoresistors for high-resolution brain temperature measurement in vivo. Beyond physiological processes, measurement and monitoring of temperature are also important when manipulating and introducing light delivery devices within the brain. Light sources require moderate to high-intensity irradiation at the target site, whose absorption can lead to localized tissue heating and damage. For this reason, a regulatory limit of 2°C is imposed to restrain the consequences of probe-derived temperature rising. In Dehkhoda et al. (2018), authors tackle precisely this challenge through the development of a method to calculate the surface temperature of implantable photonic devices. The approach is based on a LED, commonly used in optogenetic light delivery, capable of sensing and detecting changes in its temperature at the surface, thus preventing neural tissue damage actively during operation (Figure 4D). Another class of interesting multimodal tools to monitor physiological parameters is based on bioresorbable electronic sensors. Conventional implantable platforms require surgical removal after the operating period, with associated costs and risk of additional complications to the patient. Bioresorbable materials are thus being used for developing electronic technologies that dissolve at well-defined, programmable rates at the implantation site. Upon completion of the treatment, the bioresorbable technology will completely dissolve into harmless substances that are naturally cleared by the body, eliminating the need for additional surgeries. In Shin et al. (2019), the authors present a bioresorbable optical sensor for pressure and temperature monitoring and establish the feasibility of creating completely bioresorbable and Magnetic Resonance Imaging (MRI) compatible sensors (Figure 4E). Another approach introduces injectable multifunctional bioresorbable photonic devices for the analysis of biological tissues and fluids and continuous monitoring of critical physiological parameters such as tissue oxygenation, temperature, and neural activity, in freely moving animals (Bai et al., 2019). The device is designed for minimally invasive implantation and is constructed using materials that dissolve naturally through hydrolysis and metabolic clearance after a specified period of operation. Through continuous spectroscopic analysis, information on physiological status such as metabolic activity and tissue health is obtained from minimally invasive measurements into deep brain regions.
Imaging devices and probesThe complexity of synaptic circuits is a great challenge to comprehend the brain functioning and information storage. Efforts to understand the connectome or the mapping of brain circuits have been made through mesoscopic methods such as electro-physiological analysis (Libbrecht et al., 2018) and functional image techniques (Bai et al., 2019; Shin et al., 2019). Fluorescence imaging in freely behaving animals has become a standard method to study neural circuit function, with miniaturized wide-field microscopes being applied for several years to observe brain activity while animals engage in voluntary behaviors. Several options are widely available, often characterized by low-cost, portable, and customizable designs and consumer-grade components. Technologies for deep-brain imaging are depicted in Figure 5.
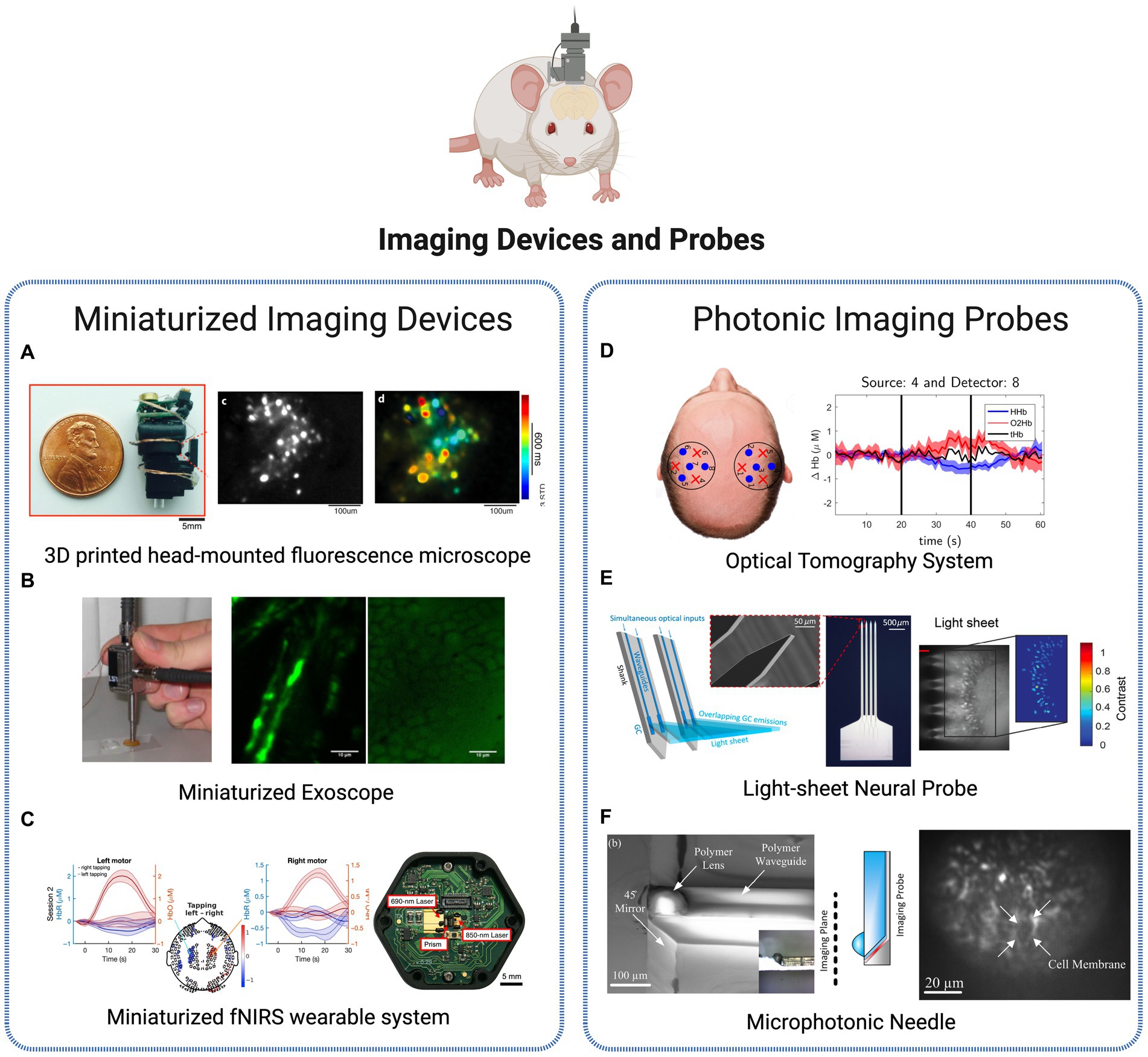
Figure 5. Brain imaging technologies. (A) Miniaturized imaging devices: 3D printed head-mounted fluorescence microscope with in vivo imaging (Liberti et al., 2017). Licensed under CC BY 3.0. (B) Portable, miniaturized exoscope for multimodal imaging of tissues. Images obtained demonstrating axons and surrounding fat cells in mouse sciatic nerve (Smith et al., 2013). Reprinted with permission of Optica Publishing Group. © Optical Society of America 2013. (C) Wearable time-domain fNIRS system offering whole-head covering through a miniaturized modular design. Brain activation is observed by measurement of hemodynamic changes (changes in the concentrations of HbO and HbR signals) in a finger tapping human experiment (Ban et al., 2022). Licensed under CC BY 4.0. (D) Diffuse Optical Tomography System for realtime oxygenated and deoxygenated hemoglobin concentration measurement (Orive-Miguel et al., 2020). Licensed under CC BY 4.0. (E) Light-sheet photonic neural probes for fluorescence imaging (Chen et al., 2021). Licensed under CC BY 4.0. (F) Microfabricated probe for sub-cellular level resolution endoscopic imaging (Tadayon et al., 2018). Licensed under CC BY 4.0. Created with BioRender.com.
Single-photon fluorescence imaging approaches, such as the system presented in Liberti et al. (2017), show to be a lightweight and inexpensive solution to longitudinal recordings of neural activity and structural dynamics in freely behaving animals (Figure 5A). More advanced designs as the two-photon high-resolution miniaturized microscope in Zong et al. (2017), now provide imaging of brain activity in freely moving animals at the single-dendritic-spine level, during extended periods of time, allowing the study of spatiotemporal dynamics in high detail and spatiotemporal resolution. Another application of two-photon imaging is presented in Pochechuev et al. (2018) where it is used to precisely determine the location of a fiber-optic probe in relation to individual neurons within the mouse brain cortex. This method allows for a detailed in situ assessment of the effectiveness of optical coupling between the fiber and individual neurons in the live brain of transgenic mice, which can support a selective stimulation and interrogation of individual neurons in vivo and be added to cranial-window imaging platforms and wearable miniature microscopes. Another application of in vivo imaging technology is presented by Smith et al. (2013) for minimally invasive imaging of tissues based on a multimodal, miniaturized exoscope. The device comprises a micro-electromechanical system scanning mirror and compact optics, and light delivery is achieved through the use of a photonic crystal fiber. The miniature objective lens is applied to observe the myelin surrounding the central axons in unstained, unfixed, fresh mouse sciatic nerves (Figure 5B). In Ban et al. (2022), authors present a wearable, whole-head coverage time-domain fNIRS where the innovative factor also relies on the miniaturization of components (Figure 5C). Contrary to the conventional large and complex NIRS systems, the suggested Kernel Flow device contains 52 small modules with dual wavelength (690 and 850 nm) laser source and a total of six detectors, mounted in a headset design that allows measurements over the frontal, parietal, temporal, and occipital cortices. Experiments based on finger-tapping task show the suitability of the system to accurately monitoring oxyhemoglobin and deoxyhemoglobin signals of human brain, with an equal or improved performance in comparison to traditional benchtop systems, thus representing a promising commercial approach for portable, miniaturized and non-invasive brain imaging.
The advances in fiber-optic components in Neuroscience have created numerous opportunities, including in-vivo brain imaging, functional studies of neurons in the brain, and the establishment of novel optical neural interfaces, through the control of neuronal activity through light. Additionally, the development of advanced fiber tools for high-speed fluorescence microscopy in freely moving animals has helped address long-standing challenges in the experimental study of the neuronal foundations of cognition and memory. A diversity of implantable microimagers have been purposed over recent years for functional brain imaging with cell-type specificity. Fedotov et al. (2017) presents a neurointerface composed of an optical fiber bundle for high-precision optical fluorescence readout and brain imaging formation. The analysis of individual fiber channels allows optogenetic recordings from single neurons at three dimensions, in vivo. In Simone et al. (2018), fibers are used to develop a low-cost photometry system to monitor in vivo calcium optical transients in neurons. The technique’s ability to measure calcium or other fluorescent indicators in target populations, coupled with its high-sensitivity recording, allows for the transformation of bulk activity interrogation into circuit-level neural encoding measurement. The customizable, low-light, fiber photometry system was applied in awake, freely moving mice to interrogate the behavioral correlates of the stress response. Another photonic-based imaging method – optical tomography – is also purposed using injectable fibers (silicon photomultipliers detectors) for dual-wavelength, real-time functional brain imaging (Orive-Miguel et al., 2020). The system allows to follow the brain activation variations over time through analysis of HbO and HbR concentration in the tissues (Figure 5D).
The research efforts are continuously converging to more flexible and compact implantable devices to reduce invasiveness and disruption in the brain. An innovative system presented in Chen et al. (2021) is the use of probe-enabled light-sheet fluorescence imaging. Implantable silicon photonic probes are able to generate multiple addressable light sheets in the targeted tissues at arbitrary brain depths, without requiring active micro-optical components on the probe, thus reducing the risk for tissue heating and displacement (Figure 5E). In Reddy et al. (2021), authors developed a micro-imager array with parylene photonic components for minimally invasive endoscopic brain imaging. The spatial resolution obtained show to be suitable for in-vivo fluorescence image studies, using the compact and minimalized device. Another example of a miniaturized, minimally invasive platform is provided in Tadayon et al. (2018) where authors describe a 100 μm cross-section probe for single cell resolution imaging (Figure 5F). The novelty of this work lies in the use of microfabrication methods (soft lithography, planar microfabrication) to fabricate 3D photonic elements (polymeric waveguide and polymeric micro-lens) with reduced cross-section while achieving high resolution. The potentialities of the probe were tested in combination with wide-field microscopy to image activated neural cells in brain slices, where neuron boundaries were clearly resolved at until 2 μm sized lithographic features. The authors present several options where the reduced size and sub-cellular resolution of these microphotonic needles can be a valuable advantage, namely minimally invasive imaging and excitation of deep brain tissues and diagnostics involving multiple modalities techniques emerging currently.
Targeted therapeuticsModern medicine faces a significant obstacle in developing therapies that can achieve precision, efficiency and personalization for individual patients. In particular, the demand for technologies with modulation, therapeutical and monitoring capabilities to be applied in the brain is vast and diverse and achieving successful outcomes in this field remains a formidable task, with the majority of neuroscience diseases being highly dependent on therapeutical approaches with lack of specificity and side-effects. Innovative therapeutics discussed in this review are represented in Figure 6 regarding drug-delivery, tumor-targeting and biomarker detection applications.
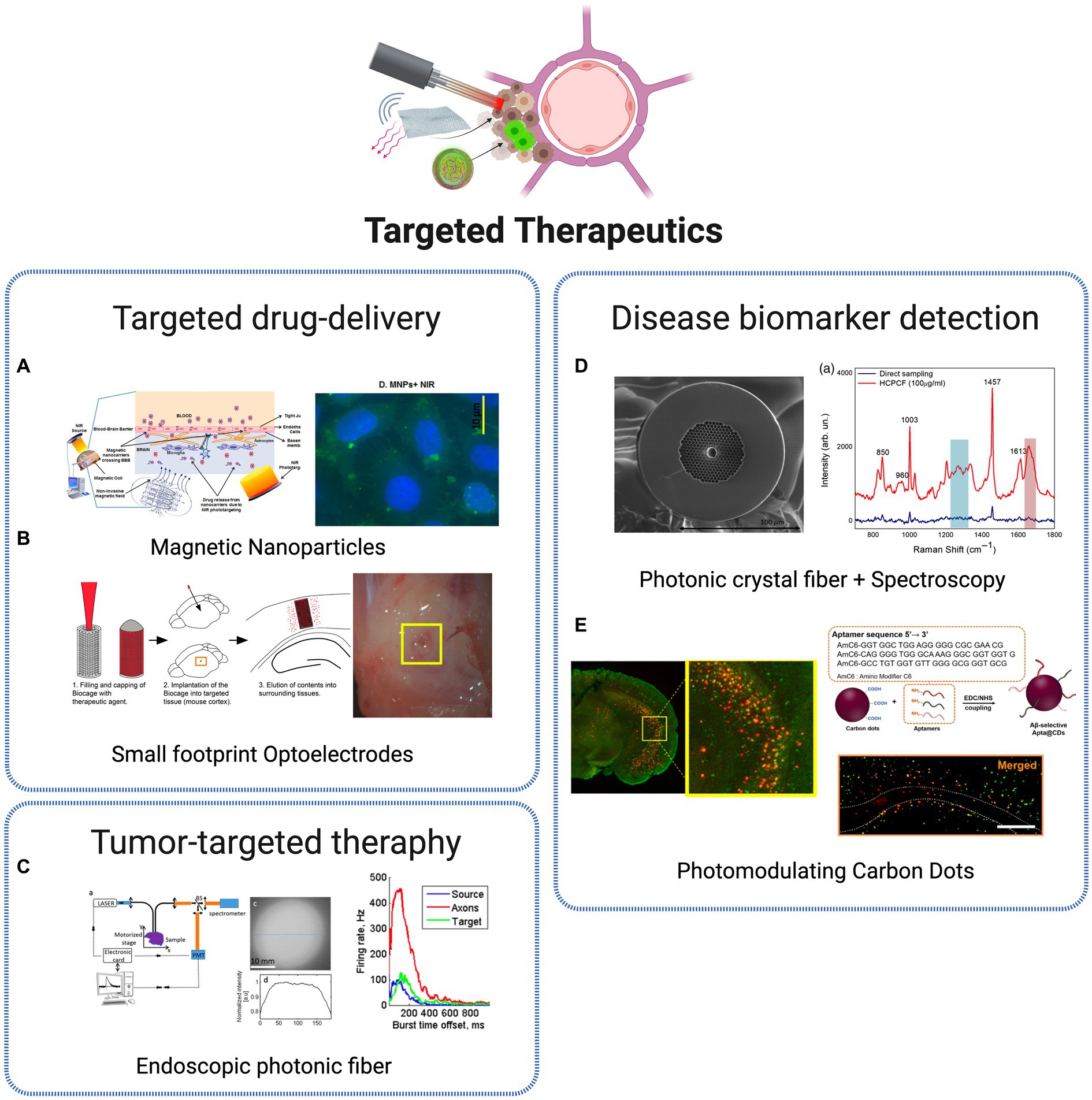
Figure 6. Targeted therapeutic technologies. (A) Non-invasive brain targeting for delivery and monitoring of therapeutics based on NIR phototargeting and Magnetic Nanoparticles (Sagar et al., 2016). Licensed under CC BY 4.0. (B) Implantable micro-caged device, implanted in a mouse cortex, for in vivo local delivery of agents (Son et al., 2017). Licensed under CC BY 4.0. (C) Photonic crystal fiber based endoscopic system, with excitation and fluorescence signal collection (Ibrahim et al., 2016). Reprinted with permission of Optica Publishing Group. © Optical Society of America 2016. (D) Photonic crystal fiber for AD biomarkers via Raman spectroscopy (Eravuchira et al., 2020). Licensed under CC BY 4.0. (E) Carbon Dots (CDs) for Alzheimer’s Aβ aggregation suppression by photomodulation (Chung et al., 2020). Reprinted with permission from Chung et al. (2020). Copyright 2020 American Chemical Society. Created with BioRender.com.
The transcranial delivery of central nervous system (CNS) drugs is a large challenge for translating the advances in drug development research to clinical practice, mainly related to the presence of the blood–brain barrier (BBB), with the role to protect the brain from harmful substances while regulating the entry of essential nutrients. As a consequence, the efficiency of drug delivery is commonly low, which requires high doses of most CNS-targeted drugs, applied with reduced specificity, commonly leading to severe side effects in peripheral organs (Obermeier et al., 2013). Therefore, efficient BBB-crossing brain delivery systems are crucial to enhance therapeutic outcomes while avoiding adverse systemic effects. Several nanoparticle-based approaches have been explored for localized and specific drug-delivery systems. A promising and crescently used NPs class for the goal of non-invasive targeting and monitoring strategies are MNPs. Due to its superparamagnetic properties, it became possible to target specific cells and tissues through the local application of non-invasive magnetic forces. The well-structured and rigid architecture of MNPs provides a stable binding site for various diagnostic or therapeutic agents. Besides, external control over the movement of MNPs increases their ability to reach the target site, reducing their peripheral circulation time compared to other nanocarriers (Sagar et al., 2016). With proper dose control, MNPs should pose few safety concerns and are highly suitable for in vivo applications. It was recently demonstrated that such type of nanoparticles, coupled with NIR biophotonic light delivery systems, provides a promising and safe approach for non-invasive therapeutics, through controllable drug delivery within the brain (Sagar et al., 2016; Figure 6A). The recent advances in optics and photonics have led to the development of nanoparticles combining both diagnostic and therapeutic properties – the commonly known theranostic nanoparticles – that have been exploited with great results in therapy, real-time monitoring and diagnosis. One example was the development of theranostic photonic nanoparticles (
留言 (0)