Microorganisms added to the standard CLSI M45 that causes infection less frequently than those covered by CLSI M02, M07, and M100 are defined as “Infrequently Isolated” or “Fastidious Bacteria.” According to CLSI M45, “Infrequently Isolated” or “Fastidious Bacteria” include coryneform bacteria, Bacillus spp., Granulicatella spp., Aeromonas spp. and potential bacterial agents of bioterrorism, etc.
These bacteria are widely distributed in the environment and food, and can affect human health. For example, Aeromonas spp. are distributed in aquatic environments (Majeed et al., 2023), and the pathogenic bacteria Bacillus spp. (Özdemir and Arslan, 2019) and Micrococcus spp. (Tizabi and Hill, 2023) in food. Due to the widespread use of antimicrobial drugs, antimicrobial resistance levels are increasing worldwide (World Health Organization, 2022). It is worth noting that as the concentration of antibacterial drugs increases in environments such as in water and in agriculture farms, multi-drug-resistant (MDR: resistant to ≥ one agent in ≥3 antimicrobial classes) genes are emerging in Infrequently Isolated or Fastidious Bacteria (Zhang et al., 2022; Yang and Wu, 2023). Many reports have demonstrated that antimicrobial-resistant Infrequently Isolated or Fastidious Bacteria are increasingly occurring in the environment (Gonzalez-Avila et al., 2021; Algammal et al., 2022a; Wu et al., 2023). Therefore, we believe that it is necessary to monitor the resistance levels of Infrequently Isolated or Fastidious Bacteria isolated from clinical patients. The CLSI M45 standard defines methods for susceptibility testing and interpretive criteria for Infrequently Isolated or Fastidious Bacteria is an important reference standard for clinical microbiological testing.
However, antimicrobial susceptibility testing standardization of Infrequently Isolated or Fastidious Bacteria in clinical work has not been fully studied. This study collected Infrequently Isolated or Fastidious Bacteria isolated from blood samples from 70 hospitals in Guangdong Province, and analyzed the epidemiology and antimicrobial susceptibility of Infrequently Isolated or Fastidious Bacteria. This study provided a basis for standardized testing of Infrequently Isolated or Fastidious Bacteria in clinical laboratories and provided data for monitoring resistance levels of Infrequently Isolated or Fastidious Bacteria.
Methods StrainsThe study included Infrequently Isolated or Fastidious Bacteria isolated from blood samples in 70 hospitals in Guangdong Province between 2017 and 2021. Identical strains isolated from the same site in the same patients were eliminated. Strains were identified using BD Phoenix M50, BD Phoenix 100 Incubator Bioreactor Colony Microbiology Culture, Sensititre ARIS HiQ AST, and VITEK® MS IND MALDI TOF and VITEK®2 Compact. The definition of Infrequently Isolated or Fastidious Bacteria referred to CLSI M45 A3. According to the definition of CLSI M45 A3, Bacillus anthracis was not included in Bacillus spp. in this study. Potential Bacterial Agents of Bioterrorism included Bacillus anthracis, Yersinia pestis, Burkholderia mallei, Burkholderia pseudomallei, Francisella tularensis and Brucella spp. This study was approved by the ethical review committee of the First Affiliated Hospital of Guangzhou Medical University (GMU).
Antimicrobial susceptibility testingReferred to the antimicrobial susceptibility testing methods recommended by CLSI M45-A3 (2015) (CLSI, 2015) and the 2022 CLSI M100 standard (CLSI, 2022). The quality control bacteria for antimicrobial susceptibility testing were Escherichia coli ATCC25922, Staphylococcus aureus ATCC25923, and Pseudomonas aeruginosa ATCC27853.
Susceptibility interpretationThe interpretation of antimicrobial susceptibility testing results of quality control bacteria referred to the 2022 CLSI M100 judgment standard (CLSI, 2022). CLSI M45 A3 recommends the broth microdilution method for antimicrobial susceptibility testing of Infrequently Isolated or Fastidious Bacteria, however, some hospitals used the disk diffusion method. Therefore, the antimicrobial susceptibility testing results in this study originated from both the broth microdilution method and the disk diffusion method. The results of the disk diffusion method were interpreted using WHONET 5.6 based on the 2022 CLSI M100 resistance breakpoints for common bacteria. The breakpoint standards for the disk diffusion method are the standards being used by the clinical laboratories of the hospitals, and the source is no longer available. Antimicrobial susceptibility testing results were not included when the number of strains analyzed were less than ten. The breakpoints of the broth microdilution method referred to CLSI M45 A3 and the 2022 CLSI M100 standard (CLSI, 2022). The breakpoints of the disc diffusion method and E-test method referred as follows (Supplementary Tables S1–S8).
Data statistical analysisWHONET 5.6 was used for data analysis. Data were processed through SPSS 26.0. The results were subjected to chi-square test, and the difference was considered statistically significant at p < 0.05.
Results Epidemiological dataOur member hospital number has changed between 2017 and 2021, namely from 69 hospitals in 2017, 79 hospitals in 2018 to 70 hospitals from 2019 to 2021. The isolation rates of Infrequently Isolated or Fastidious Bacteria from 2017 to 2021 were 1.5% (401/27631), 1.4% (415/28858), 1.8% (530/29710), 1.7% (472/28134), 2.1% (694/32218), respectively, and a total of 2,512 strains were isolated. The isolation rate of Infrequently Isolated or Fastidious Bacteria in blood samples increased significantly between 2017 and 2021 (p < 0.0001). The proportion of Infrequently Isolated or Fastidious Bacteria isolated were, Aeromonas spp. 37.1% (933/2512), Coryneberium spp. 19.4% (488/2512), Micrococcus spp. 9.7% (244/2512), Potential Agents of Bioterrorism 6.7% (168/2512), Abiotrophia spp. and Granulicatella spp. 6.6% (165/2512), Bacillus spp. 5.7% (144/2512) and other Infrequently Isolated or Fastidious Bacteria 14.7% (370/2512). Aeromonas spp. was isolated in 98.5% (69/70) of the participant hospitals.
The proportion of isolation of Aeromonas spp. decreased significantly [44.4% (2017) vs. 27.5% (2021), p < 0.0001]. In particular, the proportions of isolation of Aeromonas hydrophila [28.2% (2017) vs. 16.0% (2021), p < 0.0001] and Aeromonas sobria [6.5% (2017) vs. 3.6% (2021), p < 0.05] among Aeromonas spp. decreased significantly. There was no significant difference in the proportions of Aeromonas caviae [6.2% (2017) vs. 5.0% (2021), p = 0.404] and other Aeromonas [3.5% (2017) vs. 2.9% (2021), p = 0.575].
The proportion of Corynebacterium spp. increased significantly during the study period [12.2% (2017) vs. 27.0% (2021), p < 0.0001] was isolated from 58.6% (41/70) of the hospitals. The proportion of Corynebacterium striatum [3.7% (2017) vs. 19.0% (2021), p < 0.0001], Corynebacterium jeikeium [0.5% (2017) vs. 2.0% (2021), p < 0.05] and Corynebacterium afermentans [0.3% (2017) vs. 2.3% (2021), p < 0.01] increased significantly. The proportion of other coryneform bacteria decreased significantly [7.7% (2017) vs. 3.6% (2021), p < 0.005].
Micrococcus spp. was isolated from 53.0% (37/70) of participant hospitals. The proportion of Micrococcus spp. decreased significantly [13.2% (2017) vs. 7.9% (2021), p < 0.01]. The proportion of Micrococcus luteus decreased significantly [11.2% (2017) vs. 6.9% (2021), p < 0.05], there was no significant difference for other Micrococci [2.0% (2017) vs. 1.0% (2021), p = 0.176].
Brucella spp. was isolated from 25.7% (18/70) of participant hospitals, and Burkholderia pseudomallei was isolated from 25.7% (18/70) of member hospitals. The proportion of Potential Bacterial Agents of Bioterrorism increased significantly [5.5% (2017) vs. 9.4% (2021), p < 0.05]. The proportion of Burkholderia pseudomallei [1.3% (2017) vs. 3.2% (2021), p < 0.05] increased significantly. There was no significant difference in Brucella spp. isolation [4.0% (2017) vs. 6.0% (2021), p = 0.142].
Abiotrophia spp. and Granulicatella spp. were isolated from 51.4% (36/70) of participant hospitals. There was no significant change in the proportions of Abiotrophia spp. and Granulicatella spp. [5.5% (2017) vs. 5.9% (2021), p = 0.773], including Granulicatella adiacens [3.5% (2017) vs. 4.0% (2021), p = 0.652], Abiotrophia defectiva [1.5% (2017) vs. 1.4% (2021), p = 0.941] and others [0.5% vs. 0.4% (2021), p = 1].
Bacillus spp. was isolated from 40.0% (28/70) participant hospitals. The proportion of Bacillus spp. increased significantly [4.0% (2017) vs. 7.6% (2021), p < 0.05]. The proportion of Bacillus cereus increased significantly [1.0% (2017) vs. 5.9% (2021), p < 0.0001]. The proportion of Bacillus subtilis decreased significantly [2.5% (2017) vs. 0.3% (2021), p < 0.005]. There was no significant change in the proportion of other Bacillus spp. [0.5% (2017) vs. 1.4% (2021), p = 0.254].
There was no significant change in the proportion of other Infrequently Isolated or Fastidious Bacteria [15.2% (2017) vs. 14.7% (2021), p = 0.818] (Supplementary Figure S1 and Supplementary Table S8).
Antimicrobial susceptibility testing Aeromonas spp.Antimicrobial susceptibility testing was performed for 27.0% (252/933) of Aeromonas spp. isolates, 26.6% (153/575) of Aeromonas hydrophila isolates, and 26.9% (39/145) of Aeromonas caviae isolates.
In the antimicrobial susceptibility testing of Aeromonas spp. for cefotaxime, the resistance rate using broth microdilution method (93.7% 151/161) was 19.2%, the resistance rate using the disk diffusion method (6.2% 10/161) was 30.0%. All other antimicrobial susceptibility testing used the disk diffusion method. And in most cases, that was performed for a small number of isolates with resistance rates of 46.2% for cefuroxime, 46.2% for cefoxitin, 26.1% for ceftazidime, 34.1% for imipenem, 21.1% for aztreonam, 8.3% for amikacin, 4.3% for gentamycin, 0.0% for ciprofloxacin.
In the antimicrobial susceptibility testing of Aeromonas hydrophila for cefotaxime, the resistance rate using broth microdilution method (92.4% 85/92) was 17.6%. The resistance rate using the disk diffusion method (7.6% 7/92) was 42.9%.
All other antimicrobial susceptibility testing used the disk diffusion method, with resistance rates of 25.0% for ceftazidime, 27.3% for imipenem, 16.7% for aztreonam, 0.0% for gentamycin (Supplementary Table S9).
In the antimicrobial susceptibility testing of Aeromonas caviae to cefotaxime, the resistance rate using broth microdilution method was 33.3%.
Corynebacterium spp.Antimicrobial susceptibility testing was performed for 84.1% (410/488) of Corynebacterium spp. isolates and 77.7% (206/265) of Corynebacterium striatum isolates.
In antimicrobial susceptibility testing of Corynebacterium spp. for penicillin, the resistance rate was 53.0% using the broth microdilution method (35.1% 115/328) and the resistance rate was 82.2% using the disk diffusion method (64.9% 213/328). In antimicrobial susceptibility testing for cefotaxime, the resistance rate was 70.0% using the broth microdilution method (25.9% 30/116) and the resistance rate was 58.1% using the disk diffusion method (74.1% 86/116). In antimicrobial susceptibility testing for vancomycin, the resistance rate using broth microdilution method (30.5% 120/394) and disk diffusion method (69.5% 274/394) was 0.0%. All other antimicrobial susceptibility testing used the disk diffusion method, with resistance rate of 26.7% for gentamycin, 67.8% for erythromycin, 84% for ciprofloxacin, 4.3% for doxycycline, 13.0% for tetracycline, 86.4% for clindamycin, 55.6% for trimethoprim-sulfamethoxazolel, 22.6% for rifampin. In the antimicrobial susceptibility testing of Corynebacterium striatum for penicillin, the resistance rate was 61.4% using the broth microdilution method (44.3% 70/158) and the resistance rate was 94.3% using the disk diffusion method (55.7% 88/158). In antimicrobial susceptibility testing for cefotaxime, the resistance rate was 88.9% using the broth microdilution method (36.0% 18/50) and the resistance rate was 81.2% using the disk diffusion method (64.0% 32/50). In antimicrobial susceptibility testing for vancomycin, the resistance rate was 0.0% using the broth microdilution method (38.8% 78/201) and the disk diffusion method (61.2% 123/201). All other antimicrobial susceptibility testing used the disk diffusion method. With resistance rate of 27.4% for gentamycin, 74.0% for erythromycin, 95.6% for ciprofloxacin, 14.1% for tetracycline, 92.6% for clindamycin, 59.3% for trimethoprim-sulfamethoxazole, 1.6% for rifampin (Supplementary Table S10).
Micrococcus spp.Antimicrobial susceptibility testing was performed for 86.1% (210/244) of Micrococcus spp. isolates and 86.7% (189/218) of Micrococcus luteus isolates.
In antimicrobial susceptibility testing of Micrococcus spp. for penicillin, the resistance rate using the broth microdilution method (48.3% 72/149) was 18.1%, the resistance rate using disk diffusion method (51.7% 77/149) was 15.6%. In antimicrobial susceptibility testing for vancomycin, the resistance rate using broth microdilution method (45.6% 73/160) and disk diffusion method (54.4% 87/160) was 0.0%. All other antimicrobial susceptibility testing used the disk diffusion method, with resistance rate of 36.9% for erythromycin and 18.3% for clindamycin. In the antimicrobial susceptibility testing of Micrococcus luteus for penicillin, the resistance rate was 18.8% using the broth microdilution method (49.6% 64/129) and the resistance rate was 13.8% using the disk diffusion method (50.4% 65/129). In antimicrobial susceptibility testing for vancomycin, the resistance rate using broth microdilution method (47.4% 64/135) and disk diffusion method (52.6% 71/135) was 0.0%. All other antimicrobial susceptibility testing used the disk diffusion method, with resistance rates of 36.3% for erythromycin and 17.5% for clindamycin (Supplementary Table S11).
Abiotrophia spp. and Granulicatella spp.Antimicrobial susceptibility testing was performed for 84.2% (139/165) of Abiotrophia spp. and Granulicatella spp. isolates and 83.2% (99/119) of Granulicatella adiacens isolates.
In the antimicrobial susceptibility testing of Abiotrophia spp. and Granulicatella spp. for penicillin, the resistance rate using the broth microdilution method (47.5% 47/99) was 2.1%, the resistance rate using the disk diffusion method (49.5% 49/99) was 44.9%, and the resistance rate using the E-test method (3.0% 3/99) was 0.0%. In antimicrobial susceptibility testing for cefotaxime, the resistance rate was 0.0% using the broth microdilution method (11.9% 7/59) and the resistance rate was 11.5% using the disk diffusion method (88.1% 52/59). In antimicrobial susceptibility testing for vancomycin, the resistance rate of broth microdilution method (13.2% 16/121), disk diffusion method (86.0% 104/121) and E-test method (0.8% 1/121) was 0.0%. All other antimicrobial susceptibility testing used the disk diffusion method, with resistance rates of 2.4% for ampicillin, 58.8% for erythromycin, 51.3% for clindamycin, 3.1% for chloramphenicol. In antimicrobial susceptibility testing of Granulicatella adiacens for penicillin, the resistance rate using the broth microdilution method (49.3% 35/71) was 2.9%, the resistance rate using the disk diffusion method (49.3% 35/71) was 45.7%, and the resistance rate using the E-test method (1.4% 1/71) was 0.0%. In antimicrobial susceptibility testing for cefotaxime, the resistance rate using the broth microdilution method (15.0% 6/40) was 0.0%, the resistance rate using the disk diffusion method (85.0% 34/40) was 14. 7%. In antimicrobial susceptibility testing for vancomycin, the resistance rate using broth microdilution method (11.4% 10/88) and disk diffusion method (84.1% 74/88) was 0.0%. All other antimicrobial susceptibility testing used the disk diffusion method, with the resistance rate of 3.8% for ampicillin, 59.0% for erythromycin, 56.2% for clindamycin, 4.5% for chloramphenicol (Supplementary Table S12).
Bacillus spp.Antimicrobial susceptibility testing was performed for 72.2% (104/144) of Bacillus spp. isolates and 68.5% (63/92) of Bacillus cereus isolates.
In antimicrobial susceptibility testing of Bacillus spp. for penicillin, the resistance rate using the broth microdilution method (58.8% 47/80) was 38.3%, the resistance rate using the disk diffusion method (36.3% 29/80) was 86.2%, and the resistance rate using the E-test method (5.0% 4/80) was 0.0%. In antimicrobial susceptibility testing for vancomycin, the resistance rate using broth microdilution method (46.8% 44/94), disk diffusion method (48.9% 46/94) and E-test method (4.3% 4/94) was 0.0%. All other antimicrobial susceptibility testing used the disk diffusion method, with resistance rates of 81.8% for ampicillin, 71.4% for trimethoprim-sulfamethoxazole, 65.2% for rifampin. 3.6% for imipenem, 14.0% for erythromycin, 15.6% for clindamycin, 3.8% for tetracycline, 15.2% for ciprofloxacin, 3.8% for chloramphenicol, 0.0% for amikacin and gentamycin.
In the antimicrobial susceptibility testing of Bacillus cereus for penicillin, the resistance rate using the broth microdilution method (73.5% 36/49) was 88.9%, the resistance rate using the disk diffusion method (18.4% 9/49) was 100%, and the resistance rate using the E-test method (8.2% 4/49) was 0.0%. In antimicrobial susceptibility testing for vancomycin, the resistance rate using broth microdilution method (57.6% 34/59), disk diffusion method (35.6% 21/59) and E-test method (6.8% 4/59) was 0.0%. All other antimicrobial susceptibility testing used the disk diffusion method, with resistance rates of 78.6% for ampicillin, 61.5% for trimethoprim-sulfamethoxazole, 7.1% for imipenem.9.1% for erythromycin, 5.0% for clindamycin, 8.3% for tetracycline, 20.0% for ciprofloxacin, 0.0% for chloramphenicol, amikacin, and gentamycin (Supplementary Table S13).
Potential bacterial agents of bioterrorismAntimicrobial susceptibility testing was performed for 46.5% (47/101) of Brucella spp. isolates and 36.5% (23/63) of Burkholderia pseudomallei isolates by disk diffusion only.
In antimicrobial susceptibility testing of Brucella spp., the resistance rate for gentamycin was 0.0%. In the antimicrobial susceptibility testing of Burkholderia pseudomallei, the resistance rate for trimethoprim-sulfamethoxazole was 45.5%, and the resistance rate to ceftazidime and imipenem was 0.0% (Supplementary Table S14).
Standardization of antimicrobial susceptibility testingAccording to CLSI M45 A3, antimicrobial susceptibility testing of Aeromonas spp. can be performed using disk diffusion and broth microdilution method. For other Infrequently Isolated or Fastidious Bacteria, only the broth microdilution method and is recommended. We defined not recommended by CLSI M45 A3 as non-standard method. The proportion of broth microdilution method in antimicrobial susceptibility testing results of Corynebacterium spp. strains increased significantly throughout the study period (2017 vs. 2021) for cefotaxime (0.0% vs. 45.2% p < 0.05), penicillin (17.4% vs. 50.0% p < 0.05), and vancomycin (17.4% vs. 51.7% p < 0.001). The proportion of broth microdilution method in antimicrobial susceptibility testing results of Micrococcus spp. strains increased significantly for penicillin (50.0% vs. 77.8% p < 0.05), whereas no significant change was observed for vancomycin (46.2% vs. 64.3% p = 0.142). The proportion of broth microdilution method in antimicrobial susceptibility testing results of Abiotrophia spp. and Granulicatella spp. strains increased significantly for penicillin (14.3% vs. 86.4% p < 0.001), and no significant change were observed for cefotaxime (0.0% vs. 14.3% p = 0.515) and vancomycin (10.5% vs. 16.0% p = 0.684). The proportion of broth microdilution method in the antimicrobial susceptibility testing results of Bacillus spp. strains showed no significant change for penicillin (88.9% vs. 82.3% p = 1) and vancomycin (60.0% vs. 45.0% p = 1) (Figure 1). Total antimicrobial susceptibility test results n < 10 were not included.
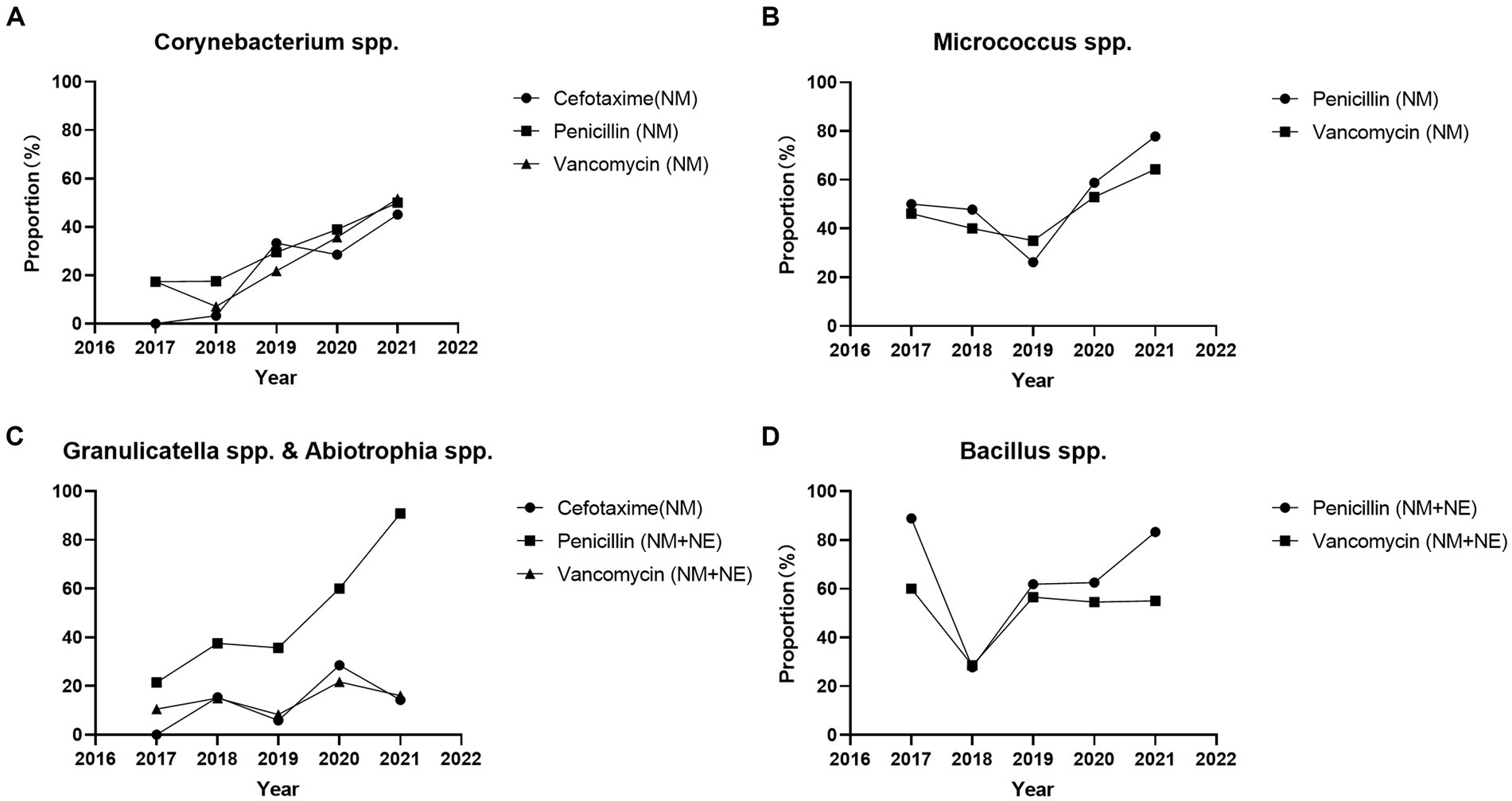
Figure 1. Proportion of broth microdilution method and E-test method in the antimicrobial susceptibility testing results of Infrequently Isolated or Fastidious Bacteria strains isolated from blood specimens from 2017 to 2021. Total antimicrobial susceptibility test results n < 10 were not included. (A) The proportion of broth microdilution method in antimicrobial susceptibility testing results of Corynebacterium spp. strains were increased significantly for cefotaxime (0.0% vs. 45.2% p < 0.05), penicillin (17.4% vs. 50.0% p < 0.05), and vancomycin (17.4% vs. 51.7% p < 0.001). (B) The proportion of broth microdilution method in antimicrobial susceptibility testing results of Micrococcus spp. strains were increased significantly for penicillin (50.0% vs. 77.8% p < 0.05), and no significant change for vancomycin (46.2% vs. 64.3% p = 0.142). (C) The proportion of broth microdilution method in antimicrobial susceptibility testing results of Abiotrophia spp. and Granulicatella spp. strains were increased significantly for penicillin (14.3% vs. 86.4% p < 0.001), and no significant change for cefotaxime (0.0% vs. 14.3% p = 0.515) and vancomycin (10.5% vs. 16.0% p = 0.684). (D) The proportion of broth microdilution method in the antimicrobial susceptibility testing results of Bacillus spp. strains were no significant change for penicillin (88.9% vs. 82.2% p = 1) and vancomycin (60.0% vs. 45.0% p = 1). Total antimicrobial susceptibility test results n < 10 were not included. NM, Microbroth dilution method; NE, E-test method.
DiscussionInfrequently Isolated or Fastidious Bacteria are often ignored in clinical practice due to low detection rates. However, some species of Infrequently Isolated or Fastidious Bacteria are more pathogenic than common bacteria, such as Brucella spp. and Burkholderia pseudomallei, which are also considered as “Potential Bacterial Agents for Bioterrorism.”
Burkholderia pseudomallei was first reported in the early 20th century and is distributed in tropical and subtropical regions. The mortality rate of sepsis caused by Burkholderia pseudomallei is as high as 40.0% even after antimicrobial treatment. The report by Limmathurotsakul et al. showed that diabetes is an important risk factor for Burkholderia pseudomallei infection. The report by Syed et al. pointed out that the prevalence of Burkholderia pseudomallei infection is increasing (Limmathurotsakul et al., 2016; Syed and Wooten, 2021; Brangsch et al., 2022). Brucella spp. is a facultative intracellular infectious pathogen that can infect major organs such as the heart and lungs, leading to infective endocarditis and various respiratory diseases, ultimately leading to heart failure. According to bio-safety regulations, experiments on living Brucella spp. need to be conducted in a bio-safety level three laboratory. However, in a report of occupational exposure to air-contaminated Brucella spp. that occurred in a bio-safety level three laboratory, the infection rate was as high as 43.6% (Koruk et al., 2012; Zhou et al., 2022). Therefore, we believe that in clinical laboratories, which are usually bio-safety level two, the isolation of highly pathogenic Infrequently Isolated or Fastidious Bacteria such as Brucella spp. requires more attention. And it is necessary to improve the standardization of bacterial testing to prevent occupational exposure incidents.
In this study, Aeromonas hydrophila, Corynebacterium striatum, and Micrococcus luteus were the top three of most isolated bacterial genera. Aeromonas hydrophila was once considered an environmental contaminant strain in clinical testing. However, current research shows that Aeromonas hydrophila often causes gastroenteritis, soft tissue infection, and can lead to sepsis when immunity is insufficient (Jones and Wilcox, 1995). In our study, the isolation of Corynebacterium striatum and its proportion in Infrequently Isolated or Fastidious Bacteria increased significantly from 2017 to 2021. Corynebacterium striatum infection is common in patients with underlying medical conditions such as hematological malignancies, solid tumors, and diabetes. Nosocomial infections caused by Corynebacterium striatum often occur in patients undergoing catheter intervention (Abe et al., 2021). Micrococcus luteus infection can cause liver and brain abscesses, bacteremia, and septic arthritis, and is common in patients with malignant tumors and immunodeficiency (Zhu et al., 2021).
Corynebacterium striatum was generally resistant to ciprofloxacin (95.6%) in our study, which is consistent with the result reported by Wang Y. et al. (2021) that 100.0% (410/410) of Corynebacterium striatum were resistant to ciprofloxacin. In our study, the resistance rate of Micrococcus luteus to erythromycin was 36.3%, and the resistance rate to clindamycin was 17.5%, which may indicate that a high number of infections caused by Micrococcus luteus resistant to ERY and CLI may fail therapy. The report by Bianco et al. in Italy showed that Bacillus cereus isolated from human bacteremia carries resistance genes to penicillin and trimethoprim, resulting in a 100% resistance rate to both of these antimicrobial agents (Bianco et al., 2021). We believe this might be the reason why the Bacillus cereus isolated in this study showed high levels of resistance to penicillin (88.0% for broth microdilution method, 100.0% for disk diffusion method) and trimethoprim-sulfamethoxazole (61.5% for disk diffusion method).
Due to the use of antimicrobial agents, the concentration of these agents in environments like water and food has increased rapidly (Jampani et al., 2024; Sun et al., 2024). This has led to a rapid increase in antimicrobial resistance levels in the environment, including among common bacteria and Infrequently Isolated or Fastidious Bacteria (Wyres and Holt, 2018; Silva-Santana et al., 2021; Algammal et al., 2022b; Denissen et al., 2022). Corynebacterium striatum appears to be an emerging MDR pathogen in among Corynebacterium spp. In the report of Yamamuro et al., Corynebacterium striatum and Corynebacterium jeikeium were less susceptible than other species to penicillin, ceftriaxone, meropenem, erythromycin, and ciprofloxacin (Yamamuro et al., 2021). In a survey in China, 96.2% of Corynebacterium striatum isolates were MDR strains (Wang et al., 2022). In the study by McMullen et al., the Corynebacterium striatum they isolated were highly resistant to antibacterial agents except vancomycin, linezolid and daptomycin. Surprisingly, all isolates became less susceptible to daptomycin and had elevated MIC50 and MIC90 to vancomycin after overnight incubation with daptomycin (McMullen et al., 2017). In the epidemiological investigation of Aeromonas spp., a low resistance levels were observed in our study. The studies by Nolla-Salas et al. and Sun et al. both showed that trimethoprim-sulfamethoxazole, ciprofloxacin and cefepime have good activity. However, Sun et al. found 9 MDR strains (15.5% 9/58) (Nolla-Salas et al., 2017; Sun et al., 2021). For Micrococcus luteus, Zhu et al. found low rates of resistance to linezolid, cefoxitin, rifampin, cefazolin and penicillin. However, resistance to gentamycin, cephalosporins, levofloxacin and carbapenems strains have also emerged. and blood stream infection (BSI) caused by Micrococcus luteus is increasing rapidly (Zhu et al., 2021). In short, Corynebacterium spp. is becoming a new MDR bacterium among Infrequently Isolated or Fastidious Bacteria. Although Aeromonas spp. and Micrococcus spp. were currently at a low resistance level, the emergence of MDR strains is still a threat worthy of attention.
Antimicrobial susceptibility testing was performed on less than 50.0% of the isolates in our study. We believe that some clinical laboratories send Potential Bacterial Agents of Bioterrorism strains to the Centers for Disease Control and Prevention for antimicrobial susceptibility testing due to insufficient bio-safety levels. However, this study did not include data from the Chinese Center for Disease Control and Prevention. Aeromonas spp. isolates antimicrobial susceptibility testing was performed on less than 30.0% of the isolates. Aeromonas spp. was once considered an environmental contaminant and in accordance with CLSI M45 A3, antimicrobial susceptibility testing is usually limited to isolates from extraintestinal sites.
Monitoring bacterial resistance levels is considered a necessary means to control bacterial resistance rate, so global and national bacterial resistance monitoring systems such as GLASS, CRASS, NARMS, and EUCAST have been established. Clinical laboratories are the important source of bacterial resistance data, so we believe that reference standards are crucial for antimicrobial susceptibility testing. Except for Aeromonas spp., the antimicrobial susceptibility testing method recommended by CLSI M45 A3 is the broth microdilution method. However, most hospitals in our study used the disk diffusion method for antimicrobial susceptibility testing. We attempted to analyze the difference in resistance rates between the broth microdilution method and the disk diffusion method. In our study, the resistance rate of Corynebacterium spp., Abiotrophia spp. and Granulicatella spp. and Bacillus spp. for penicillin by the broth dilution method was significantly lower than that by the disk diffusion method. There were no significant differences between the broth dilution and disk diffusion methods in the results of other antimicrobial susceptibility testing. In this study, no BSI strains of Infrequently Isolated or Fastidious Bacteria were obtained, so we were unable to verify whether the antimicrobial susceptibility testing results of the same strain by the broth microdilution method and the disk diffusion method were significantly different. Due to the small sample size and inconsistent breakpoints, we believe that this statistical result cannot prove whether there was a significant difference in antimicrobial susceptibility results between the broth microdilution method and the disk diffusion method. We will expand the sample size in future studies to evaluate the differences between broth microdilution and disk diffusion methods.
We have noticed that EUCAST Version 14.0 provides breakpoints for the disk diffusion method for most bacteria included in this study. For evaluation, we compared the breakpoints for the broth microdilution method in CLSI M45 A3 and EUCAST Version 14.0, and we found that the breakpoints specified in EUCAST Version 14.0 are lower than those in CLSI M45 A3. For example, the breakpoint of ceftazidime in Aeromonas spp. in CLSI is R ≥ 16, while in EUCAST it is R > 4. The same situation exists in other bacterial genera. For example, the breakpoint of ciprofloxacin in Corynebacterium spp. in CLSI is R ≥ 4, but in EUCAST it is R > 1. The breakpoint of rifampicin in CLSI is R ≥ 4, but in EUCAST it is R > 0.06. In view of the huge difference in breakpoints for the broth microdilution method in CLSI M45 A3 and EUCAST Version 14.0, we believe that it is inappropriate to use CLSI M45 for the broth microdilution method and EUCAST for the disk diffusion method. And since common bacteria usually use the CLSI M100 standard, we believe that it is inappropriate to use the EUCAST Version 14.0 standard for Infrequently Isolated or Fastidious Bacteria, which may lead to an abnormal increase in resistance rates. This is a challenge for bacterial resistance research and epidemiological investigations (The European Committee on Antimicrobial Susceptibility Testing, 2024).
Testing standards for common bacteria, such as CLSI M100, specify breakpoints for broth microdilution and disk diffusion methods. Clinical laboratories can choose appropriate testing methods based on economic and medical environments. The disk diffusion method is not recommended by CLSI M45 A3 as it has not been fully studied. However, some clinical laboratories do not have the ability to perform antimicrobial susceptibility testing using broth microdilution methods. We believe that the disk diffusion method is suitable for antimicrobial susceptibility testing of Infrequently Isolated or Fastidious Bacteria, but it lacks appropriate breakpoint criteria. Therefore, we hope that our research can provide a reference for the breakpoint standard of the disk diffusion method for Infrequently Isolated or Fastidious Bacteria. We look forward to CLSI launching the breakpoint of the Infrequently Isolated or Fastidious Bacteria disc diffusion method as soon as possible.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statementThe studies involving humans were approved by Ethics Committee of Scientific Research Project Review of the First Affiliated Hospital of Guangzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributionsNH: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. XY: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation, Data curation. AH: —. JC: Writing – review & editing, Visualization, Formal analysis. YG: Writing – review & editing, Supervision. JL: Writing – review & editing, Supervision. LY: Writing – review & editing, Supervision. ChuZ: Writing – review & editing, Supervision. JW: Writing – review & editing, Supervision. YW: Writing – review & editing, Supervision. ML: Writing – review & editing, Supervision. YL: Writing – review & editing, Investigation. SX: Writing – review & editing, Supervision. ChaZ: Writing – review & editing, Supervision.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Special Foundation for National Science and Technology Basic Research Program of China 2019FY101200. The funders had no role in the design, conduct, analysis or interpretation of the study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1335169/full#supplementary-material
ReferencesAbe, M., Kimura, M., Maruyama, H., Watari, T., Ogura, S., Takagi, S., et al. (2021). Clinical characteristics and drug susceptibility patterns of Corynebacterium species in bacteremic patients with hematological disorders. Eur. J. Clin. Microbiol. Infect. Dis. 40, 2095–2104. doi: 10.1007/s10096-021-04257-8
PubMed Abstract | Crossref Full Text | Google Scholar
Algammal, A. M., Alfifi, K. J., Mabrok, M., Alatawy, M., Abdel-Moneam, D. A., Alghamdi, S., et al. (2022a). Newly emerging MDR B. cereus in Mugil seheli as the first report Commonly Harbor nhe, hbl, cytK, and pc-plc virulence genes and bla1, bla2, tetA, and ermA resistance genes. Infection Drug Resistance 15, 2167–2185. doi: 10.2147/IDR.S365254
Crossref Full Text | Google Scholar
Algammal, A. M., Ibrahim, R. A., Alfifi, K. J., Ghabban, H., Alghamdi, S., Kabrah, A., et al. (2022b). A first report of molecular typing, virulence traits, and phenotypic and genotypic resistance patterns of newly emerging XDR and MDR Aeromonas veronii in Mugil seheli. Pathogens 11:1262. doi: 10.3390/pathogens11111262
Crossref Full Text | Google Scholar
Bianco, A., Capozzi, L., Monno, M. R., del Sambro, L., Manzulli, V., Pesole, G., et al. (2021). Characterization of Bacillus cereus group isolates from human Bacteremia by whole-genome sequencing. Front. Microbiol. 11:599524. doi: 10.3389/fmicb.2020.599524
PubMed Abstract | Crossref Full Text | Google Scholar
Brangsch, H., Singha, H., Laroucau, K., and Elschner, M. (2022). Sequence-based detection and typing procedures for Burkholderia mallei: assessment and prospects. Front. Vet. Sci. 9:1056996. doi: 10.3389/fvets.2022.1056996
Crossref Full Text | Google Scholar
CLSI. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 3rd ed. CLSI guideline M45. Wayne, PA: Clinical and Laboratory Standards Institute. (2015).
CLSI. Performance standards for antimicrobial susceptibility testing. In: CLSI supplement M100. 32nd ed. Wayne, PA: Clinical and Laboratory Standards Institute. (2022)
Denissen, J., Reyneke, B., Waso-Reyneke, M., Havenga, B., Barnard, T., Khan, S., et al. (2022). Prevalence of ESKAPE pathogens in the environment: antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 244:114006. doi: 10.1016/j.ijheh.2022.114006
Crossref Full Text | Google Scholar
Gonzalez-Avila, L. U., Loyola-Cruz, M. A., Hernández-Cortez, C., Bello-López, J. M., and Castro-Escarpulli, G. (2021). Colistin resistance in Aeromonas spp. Int. J. Mol. Sci. 22:5974. doi: 10.3390/ijms22115974
Crossref Full Text | Google Scholar
Jampani, M., Mateo-Sagasta, J., Chandrasekar, A., Fatta-Kassinos, D., Graham, D. W., Gothwal, R., et al. (2024). Fate and transport modelling for evaluating antibiotic resistance in aquatic environments: current knowledge and research priorities. J. Hazard. Mater. 461:132527. doi: 10.1016/j.jhazmat.2023.132527
Crossref Full Text | Google Scholar
Jones, B. L., and Wilcox, M. H. (1995). Aeromonas infections and their treatment. J. Antimicrob. Chemother. 35, 453–461. doi: 10.1093/jac/35.4.453
Crossref Full Text | Google Scholar
Koruk, S. T., Erdem, H., Koruk, I., Erbay, A., Tezer-Tekce, Y., Erbay, A. R., et al. (2012). Management of Brucella endocarditis: results of the Gulhane study. Int. J. Antimicrob. Agents 40, 145–150. doi: 10.1016/j.ijantimicag.2012.04.009
Crossref Full Text | Google Scholar
Limmathurotsakul, D., Golding, N., Dance, D. A. B., Messina, J. P., Pigott, D. M., Moyes, C. L., et al. (2016). Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 1:15008. doi: 10.1038/nmicrobiol.2015.8
Crossref Full Text | Google Scholar
Majeed, S., De Silva, L. A. D. S., Kumarage, P. M., and Heo, G. J. (2023). Occurrence of potential virulence determinants in Aeromonas spp. isolated from different aquatic environments. J. Appl. Microbiol. 134:lxad031. doi: 10.1093/jambio/lxad031
Crossref Full Text | Google Scholar
McMullen, A. R., Anderson, N., Wallace, M. A., Shupe, A., and Burnham, C.-A. D. (2017). When Good Bugs Go Bad: Epidemiology and Antimicrobial Resistance Profiles of Corynebacterium striatum, an Emerging Multidrug-Resistant, Opportunistic Pathogen. Antimicrob. Agents Chemother. 61, e01111–17. doi: 10.1128/AAC.01111-17
Crossref Full Text | Google Scholar
Nolla-Salas, J., Codina-Calero, J., Vallés-Angulo, S., Sitges-Serra, A., Zapatero-Ferrándiz, A., Climent, M. C., et al. (2017). Clinical significance and outcome of Aeromonas spp. infections among 204 adult patients. Eur. J. Clin. Microbiol. Infect. Dis. 36, 1393–1403. doi: 10.1007/s10096-017-2945-4
Crossref Full Text | Google Scholar
Özdemir, F., and Arslan, S. (2019). Molecular characterization and toxin profiles of bacillus spp. isolated from retail fish and ground beef. J. Food Sci. 84, 548–556. doi: 10.1111/1750-3841.14445
Crossref Full Text | Google Scholar
Silva-Santana, G., Silva, C. M. F., Olivella, J. G. B., Silva, I. F., Fernandes, L. M. O., Sued-Karam, B. R., et al. (2021). Worldwide survey of Corynebacterium striatum increasingly associated with human invasive infections, nosocomial outbreak, and antimicrobial multidrug-resistance, 1976-2020. Arch. Microbiol. 203, 1863–1880. doi: 10.1007/s00203-021-02246-1
Crossref Full Text | Google Scholar
Sun, X., Wang, X., Han, Q., Yu, Q., Wanyan, R., and Li, H. (2024). Bibliometric analysis of papers on antibiotic resistance genes in aquatic environments on a global scale from 2012 to 2022: evidence from universality, development and harmfulness. Sci. Total Environ. 909:168597. doi: 10.1016/j.scitotenv.2023.168597
Crossref Full Text | Google Scholar
Sun, Y., Zhao, Y., Xu, W., Fang, R., Wu, Q., He, H., et al. (2021). Taxonomy, virulence determinants and antimicrobial susceptibility of Aeromonas spp. isolated from bacteremia in southeastern China. Antimicrob. Resist. Infect. Control 10:43. doi: 10.1186/s13756-021-00911-0
Crossref Full Text | Google Scholar
Syed, I., and Wooten, R. M. (2021). Interactions between pathogenic Burkholderia and the complement system: a review of potential immune evasion mechanisms. Front. Cell. Infect. Microbiol. 11:701362. doi: 10.3389/fcimb.2021.701362
Crossref Full Text | Google Scholar
The European Committee on Antimicrobial Susceptibility Testing (2024). Breakpoint tables for interpretation of MICs and zone diameters. Version 14.0. Available at: http://www.eucast.org.
Tizabi, D., and Hill, R. T. (2023). Micrococcus spp. as a promising source for drug discovery: a review. J. Ind. Microbiol. Biotechnol. 50:kuad017. doi: 10.1093/jimb/kuad017
Crossref Full Text | Google Scholar
Wang, J., Pei, J., Liu, M., Huang, R., Li, J., Liao, S., et al. (2022). Identification and evolutionary relationship of Corynebacterium striatum clinical isolates. Pathogens 11:1012. doi: 10.3390/pathogens11091012
Crossref Full Text | Google Scholar
Wang, Y., Shi, X., Zhang, J., Wang, Y., Lv, Y., Du, X., et al. (2021). Wide spread and diversity of mutation in the gyrA gene of quinolone-resistant Corynebacterium striatum strains isolated from three tertiary hospitals in China. Ann. Clin. Microbiol. Antimicrob. 20:71. doi: 10.1186/s12941-021-00477-0
Crossref Full Text | Google Scholar
Wu, Y., Dong, N., Cai, C., Zeng, Y., Lu, J., Liu, C., et al. (2023). Aeromonas spp. from hospital sewage act as a reservoir of genes resistant to last-line antibiotics. Drug Resist. Updat. 67:100925. doi: 10.1016/j.drup.2023.100925
Crossref Full Text | Google Scholar
Wyres, K. L., and Holt, K. E. (2018). Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol. 45, 131–139. doi: 10.1016/j.mib.2018.04.004
留言 (0)