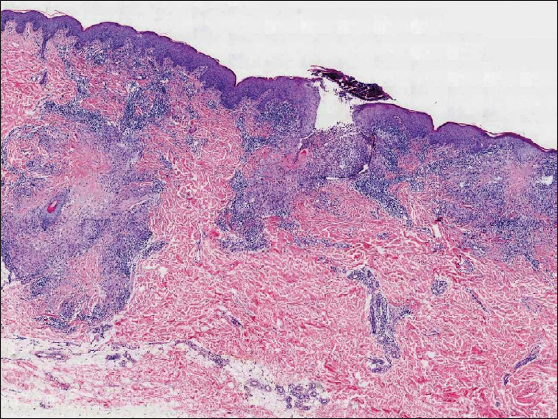
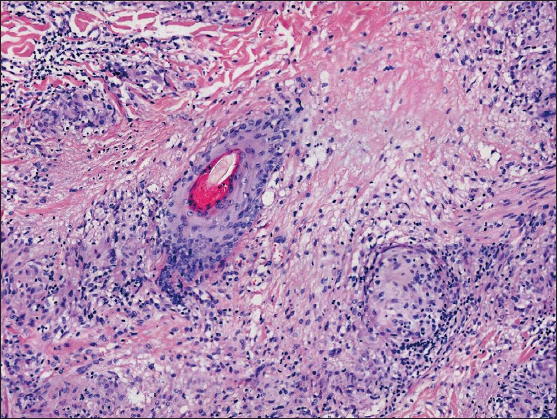
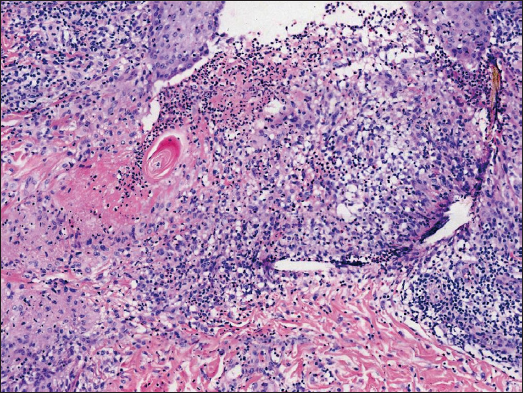
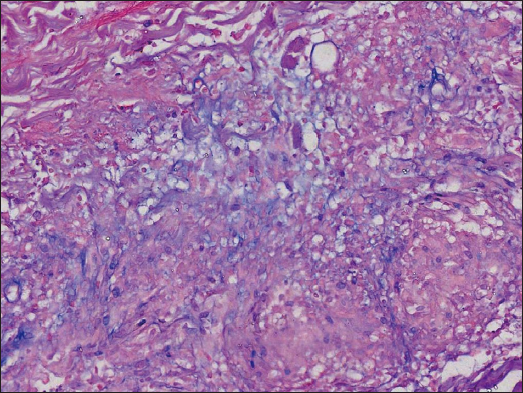
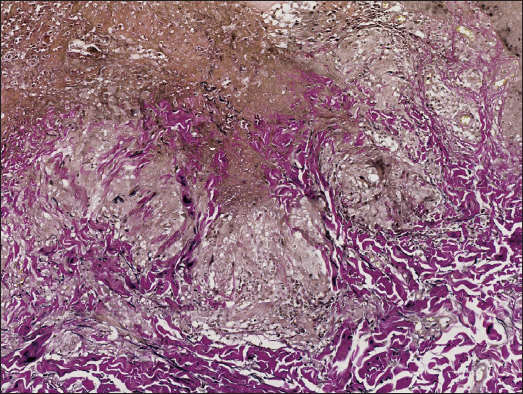
A 34-year-old female presented with persistent and mildly itchy papules and pustules on her abdomen for 2 months. There was no evidence of systemic disease, drug abuse or family history. Physical examination revealed a cluster of red papules, 2–5 mm in diameter, exhibiting a central pustule surrounded by an erythematous halo on the lower abdomen [Figure 1]. Histopathology showed epithelial histiocyte granulomas in palisade arrangement in the dermis. These granulomas showed a central core of a follicular structure, encircled by lymphocytes, scattered eosinophils and neutrophils. The superficial granuloma was transepidermally eliminated through the follicular infundibulum with dense neutrophil aggregation and nuclear dust [Figures 2a–2c]. Alcian blue staining displayed abundant mucin in granulomas’ focus [Figure 2d]. Elastic-van Gieson revealed degeneracy and loss of elastic fibres and loss of elastic fibres within the granulomas [Figure 2e]. Periodic acid-Schiff and acid-fast staining, bacterial and fungal tissue cultures, mycobacterial polymerase chain reaction detection, T-SPOT.TB test, as well as chest computed tomography imaging, yielded negative results.

Export to PPT
Export to PPT
Export to PPT
Export to PPT
Export to PPT
Export to PPT
QuestionWhat is your diagnosis?
DiagnosisLocalised perforating follicular granuloma annulare
DiscussionGranuloma annulare (GA) represents an inflammatory condition with uncertain pathogenesis, where factors like drugs, infections, tumours, diabetes and thyroid diseases have been linked or considered predisposing. Typical lesions present as asymptomatic or pruritic, red or flesh-coloured annular papules or plaques. The histopathology is characterised by palisading granulomas with central collagen degeneration and mucin deposition. Perforating granuloma annulare (PGA) is a rare subtype of GA, predominantly observed in children and young individuals. Localised PGA often occurs in extremities, whereas generalised PGA can affect the trunk and limbs. The clinical manifestations include papules with an umbilicated crust. Pathologically, the granulomas are superficial and the degenerated collagen is extruded to the surface through the epidermis. Serial sections typically demonstrate perforation occurring through the hair follicle. Notably, approximately 26% of PGA cases may exhibit pustular crusts instead of the real pustules, pathologically corresponding to excreted degenerative collagen rather than aggregated neutrophils.1
In the present case, the histopathology revealed three palisading granulomas, arranged from deep to shallow layers, with one superficial granuloma perforating through the follicular infundibulum, corroborating findings in serial sections. In addition, neutrophils were observed to aggregate in and around the hair follicles at the centre of these structures. The rare pathological changes led to a singular form of pustular folliculitis-like rash, resembling neutrophil microabscesses forming sterile pustules at the hair follicles. Specifically, the observed neutrophil aggregation may suggest the induction of necroptosis or phagocytosis during the disease’s progression. Nevertheless, pustular follicular PGA with neutrophil aggregation is extremely rare with only three reported cases of generalised nature.1–3 Whether the cases constitute a peculiar pustular variant of PGA or an individualised disease remains undefined.3 Interestingly, our case was the first localised case, occurring in an uncommon anatomical site.
The differential diagnoses include perforating folliculitis and Kyrle’s disease, both exhibiting clinical resemblances to folliculitis. Perforating folliculitis shows transepidermal elimination at the follicular infundibulum, while Kyrle’s disease involves downward perforation through the epidermis at the base of the keratotic plug, both lacking palisading granuloma. This case demonstrates unique pathological features that distinguish it from lupus miliaris disseminatus faciei, papulonecrotic tuberculid and perforating necrobiosis lipoidica. Lupus miliaris disseminatus faciei presents tuberculous granuloma with central caseous necrosis, while papulonecrotic tuberculid displays a V-shaped zone of epidermal and dermal coagulative necrosis with dense inflammatory cell infiltration. Perforating necrobiosis lipoidica manifests palisading granuloma involving the entire dermis layer in a stratified arrangement. Their characteristic rashes and lesion locations can also be the identification points.
Previous reports have described clinical improvement of the generalised pustular follicular PGA after treatment with acitretin, pentoxifylline, prednisone and PUVA phototherapy.1–3 However, due to the self-limiting nature of GA, the localised lesions may not always necessitate an aggressive treatment. In our case, satisfactory efficacy was achieved with topical halometasone cream. Subsequent treatment involved the use of fluticasone propionate cream followed by gradual withdrawal. No recurrence was detected over the 6-month follow-up period. The distinctive clinical manifestations of PGA pose a challenge for diagnosis, emphasising the necessity of enhancing awareness about this condition and increasing biopsy rates to prevent misdiagnosis and oversight.
留言 (0)