Bipolar disorder (BD) is a chronic and recurrent disease characterized by depressive and manic or hypomanic mood episodes alternated with periods of euthymia (1). It is associated with reduced functionality and medical comorbidities (1), especially metabolic disorders that impact physical and mental health prognosis (2). Individuals with BD face an elevated risk of premature cardiovascular-related death, attributed in part to a reduced exercise capacity (3).
Exercise tolerance has been widely studied in somatic diseases. Endurance time during constant work rate cycle ergometry (CWRCE), known as the total time the individual maintains exercise at a constant work rate, has been associated with patients’ experience of physical functioning in daily life, and considered a useful efficacy endpoint in clinical intervention trials (4). Poor aerobic endurance and muscle strength are associated in mental illnesses, including BD, with impaired physical function, increased risk of lifestyle-related diseases, and early mortality (5). In BD, longer illness duration, higher body mass index (BMI), higher levels of depression and lower physical activity levels have been associated with lower physical fitness, emerging as an eminent modifiable risk factor for somatic comorbidity (3).
Cardiorespiratory fitness, assessed through maximum oxygen uptake (VO2peak), is considered a predictive measure for cardiovascular disease and premature mortality, demonstrating the potential of exercise to counteract compromised physical health in BD (3).
Exercise-induced changes in mitochondrial function, crucial for determining exercise capacity (6), have been explored in various contexts but remain understudied in BD, despite suggestions of mitochondrial dysfunction as a potential marker in the disorder’s pathophysiology (7, 8).
No biomarkers have yet been implemented in clinical practice to support diagnostic and therapeutic processes. This study addresses a gap in research by longitudinally assessing intra-individual differences in aerobic capacity and mitochondrial respiration during different mood states in BD patients. We primarily hypothesized a positive correlation between aerobic capacity and mitochondrial respiration in BD, and secondarily an improvement in both after clinical remission compared to the acute episode. Therefore, we aimed to study the association between respiratory capacity during physical activity and mitochondrial respiration, measured after the extraction of peripheral blood mononuclear cells (PBMC), across the different states of the illness, and also longitudinally determine differences in the aerobic capacity and oxygen uptake between the acute mood episodes and remission. Finally, we studied the association between aerobic capacity with BMI and physical activity.
MethodsStudy design and populationThe current work, derived from a financed longitudinal study (PI21/00169), aimed to assess intra-individual differences in oxygen consumption, exercise capacity and mitochondrial function longitudinally in patients with BD (9).
Adult inpatients admitted to our acute psychiatric unit with BD type I with an acute manic or depressive episode, according to DSM-5 criteria (10), were eligible for this study. Assessments occurred during the acute episode (T0) and after symptomatic remission (T1), defined as standardized clinical scores ≤7 at the Young Mania Rating Scale (YMRS) (11) or the 17-item Hamilton Depression Rating Scale (HDRS) (12) (i.e. symptoms absent or nearly absent), before hospital discharge. A disease course shorter than ten years was necessary for patient recruitment. Patients with intellectual quotient lower than 80, with substance use disorders other than tobacco and cannabis, and with any cardiac, respiratory, auto-immune, inflammatory illness or with an acute infectious illness were excluded from this study, as well as those with known history of familial mitochondrial disease.
The capacity to provide informed consent was assessed before entering the study and re-assessed after remission. This study was approved by the Hospital Clínic Research Ethics Committee (HCB/2021/0358).
Clinical evaluationSocio-demographic and clinical data were collected. Lung function was assessed with forced spirometry and diffusing lung capacity for carbon monoxide (DLCO), to exclude any respiratory limitations (13). Manic and depressive symptoms were assessed respectively using standardized psychometric scales: YMRS (11) and HDRS (12). Functioning was assessed with Functioning Assessment Short Test (FAST) (14), disease severity with Clinical Global Impression Scale – Severity (CGI-S) (15), and physical activity with International Physical Activity Questionnaire (IPAQ) (16), and adherence to Mediterranean diet with a 17-score scale (PREDIMED-17) (17). At T1, CGI-Improvement (CGI-I) scale was obtained, and HDRS, YMRS, FAST, IPAQ, CGI-S were re-administered. Clinical variables were assessed at T0 and T1.
Assessment of aerobic capacityAt T0, patients were evaluated with an incremental CPET, and then with a CWRCE (18), both conducted on a cycle ergometer (Lode Corival CEPT mod:960900, Groningen, The Netherlands). During the CWRCE, patients performed the test at the 80% of the peak work-load achieved in the incremental CPET. The CWRCE was also conducted at T1.
In the CWRCE, endurance time in seconds, an indicator of aerobic capacity (6), VO2peak and oxygen uptake at the anaerobic threshold in L/min were measured. The last was obtained as an expression of the exercise intensity indicating the transition from mild to moderate exercise and from aerobic to anaerobic work intensity. The association between the aerobic capacity with BMI and physical activity was studied to assess the influence of non-psychiatric factors, such as physical fitness and exercise routines, in exercise performance.
Assessment of mitochondrial respirationMitochondrial oxygen consumption rates (OCRs) were assessed at T0 and T1, after the isolation of peripheral blood mononuclear cells (PBMC) obtained by a Ficoll density gradient centrifugation procedure. To determine OCRs at T0 and T1, a million living PBMCs resuspended in PBS1x were used. High-resolution respirometry was conducted in fresh cells at 37°C by polarographic oxygen sensors in a two-chamber Oxygraph-2k system (OROBOROS Instruments, Innsbruck, Austria). Specific OCRs were obtained (Routine: basal oxygen consumption with no exogenous substrates; Leak: oxygen consumption not coupled to ATP synthesis; ETC: maximal capacity of the electron transport chain; and Rox: oxygen consumption not linked to mitochondrial activity). Routine, Leak and ETC OCRs were registered by subtracting the rates from Rox as it is considered unspecific and non-mitochondrial oxygen consumption. The following inhibitors and uncouplers were manually injected: (i) oligomycin (1.5 mM), an ATP synthase inhibitor, (ii) carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (1 mM), a mitochondrial uncoupler, and (iii-iv) rotenone (2 mM) and antimycin A (0.2 mM), which are complex I and complex III inhibitors, respectively.
Oxygen uptake was normalized per million cells. Results are expressed as picomoles of oxygen per million cells (pmol O2/million).
Statistical analysisStatistical analyses were computed with ‘IBM SPSS Statistics 25’ and GraphPad Prism. Quantitative variables were summarized as median and interquartile range (IQR), and categorical variables as frequencies. For intra-subjects’ comparisons, Wilcoxon matched-pairs signed rank tests were used. Spearman test was used for correlation analyses. Results were controlled for pharmacological treatment. Statistical significance level was set at p<0.05.
ResultsTable 1 outlines the key characteristics of the 8 participants (6 manic, 2 depressed) included.
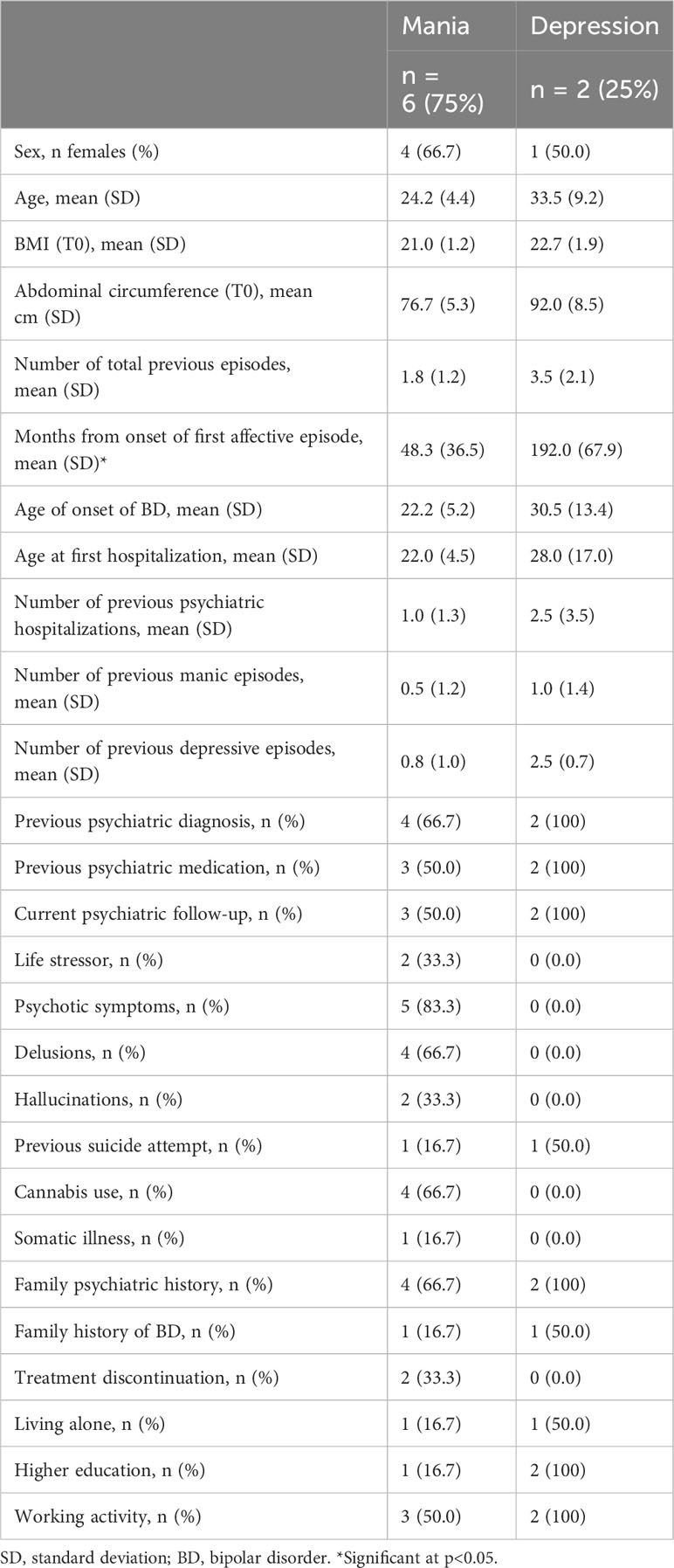
Table 1 Socio-demographic and clinical characteristics of the sample.
Compared to patients with a manic episode, those with a depressive episode presented higher HDRS total score at admission (25.0 ± 11.3 vs 4.0 ± 2.7), with no major differences at endpoint. As expected, those with a manic episode displayed higher YMRS total score at admission compared to depressive patients (23.5 ± 10.5 vs 1.5 ± 2.1), which partially reduced at discharge (4.8 ± 2.0 vs 0.0 ± 0.0). In addition, relevant differences were found between groups in FAST total score at admission (13.3 ± 8.5 in mania vs 37.5 ± 3.5 in depression) and after clinical remission (6.0 ± 7.1 in mania vs 29.5 ± 4.9 in depression), with no major differences in CGI, IPAQ or PREDIMED-17 scales scores.
Spirometry was performed in all patients to assess resting pulmonary capacity, which was within reference values in all cases.
Intra-subject longitudinal comparisons between acute mood episodes (T0) and clinical remission (T1) for the overall patients’ sample are shown in Figure 1.
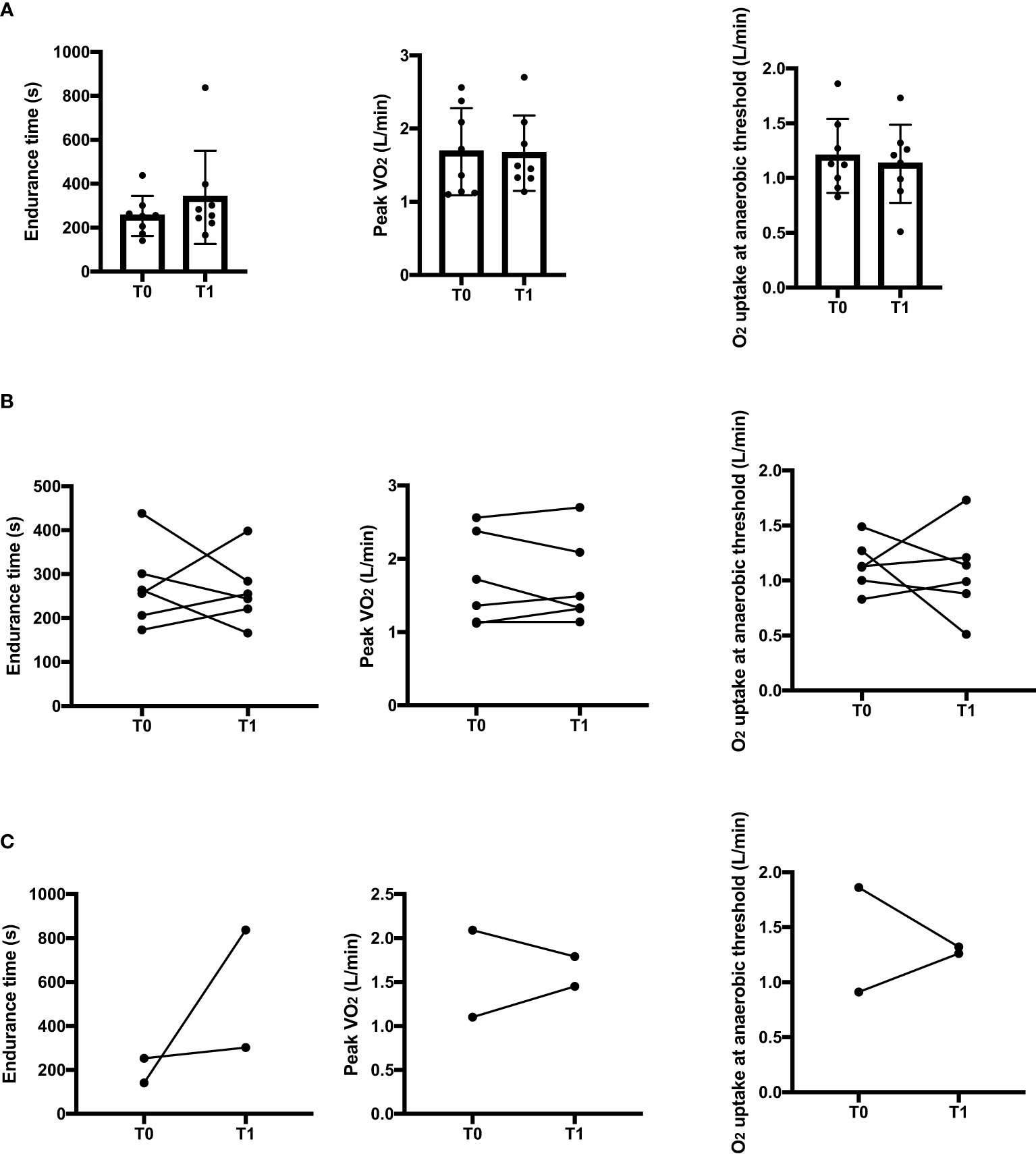
Figure 1 Intra-subject comparisons in endurance time, peak oxygen uptake and oxygen uptake at the anaerobic threshold between the acute state (T0) and clinical remission (T1) for the overall sample (A), for manic patients (B) and for depressive patients (C) in the constant work rate cycle ergometry. s, seconds; L, liters.
No significant differences between T0 and T1 were found in the endurance (p=0.779), VO2peak, (p=0.779) or oxygen consumption at the anaerobic threshold (p=0.726).
All patients included had normal weight (median: 21.20 kg/m2, IQR: 20.39-22.61). However, patients’ BMI tended to be directly correlated with endurance time at T1 (r=0.667, p=0.071). IPAQ total score at T0 was associated with more prolonged endurance time (r=0.905, p=0.005).
Despite mitochondrial respiratory capacity showed a tendency to increase at T1 compared to T0, differences in the different OCRs were not significant.
Endurance time at T0 or T1 did not show any association with mitochondrial respiratory capacity. Likewise, at T0, no relevant correlations were observed between oxygen uptake during the effort test and mitochondrial oxygen consumption. Nevertheless, at T1, basal oxygen consumption (before starting CWRCE) tended to be inversely correlated with maximum mitochondrial respiratory capacity (ETC) (r=-0.690, p=0.058). In addition, VO2peak at T1 was inversely correlated with Routine (r=-0.810, p=0.015) and Leak (r=-0.786, p=0.021) OCRs.
DiscussionIn this preliminary study, no significant differences were found in aerobic capacity, including endurance time, VO2peak or oxygen consumption at the anaerobic threshold during CWRCE, or mitochondrial respiration between severe acute mood states and after clinical remission, although the second showed a tendency to increase in clinical remission compared to the acute states, which is supported by recent results from our group (9). An inverse association was noted between basal oxygen consumption and maximum mitochondrial respiratory capacity after remission, suggesting individuals with increased mitochondrial capacity might have lower basal oxygen requirements and higher mitochondrial efficiency, which should be confirmed in larger samples.
In addition, the maximum oxygen uptake during CWRCE at clinical remission was inversely correlated with basal mitochondrial respiration, suggesting physical fitter individuals may exhibit lower resting mitochondrial oxygen requirements. Our results suggest an association between an electron transport chain dysfunction and an impaired aerobic respiration, which could be a risk factor for an increased anaerobic respiration and oxidative stress.
Higher BMI tended to be correlated with longer endurance time after clinical remission, hinting a better physical fitness in this cohort. Also, IPAQ total score was associated with more prolonged endurance time during the acute state, revealing a stronger association of endurance capacity with physical fitness rather than with the severity of the acute episode.
To the authors’ knowledge, this is the first study reporting intra-subject longitudinal differences in BD between acute states and clinical remission and aiming to find a potential association between oxygen consumption capacity during an effort test and mitochondrial respiration. Other studies assessing mitochondrial OCRs in mood disorders have been conducted with smaller samples (19, 20).
The study strengths include intra-individual comparisons between clinical states in patients with short course of illness and severe episodes, larger samples compared to previous studies, and in vivo mitochondrial respiratory capacity assessment. Also, laboratory measurements were performed at the same time as the clinical evaluation and the effort tests in the cycle ergometer were obtained. Limitations include the small sample size, explained by the severity of mood episodes, which hindered recruitment. The small sample size did not allow differentiation from participants at index mania and index depression, which might be expected to differ. Finally, even though the inpatient unit ensures a lower variability in different environmental conditions, since illicit substances and tobacco are strictly forbidden, and a balanced diet is provided in all cases, other factors, such as some individual characteristics, might have influenced mitochondrial respiration.
In conclusion, our results suggest that impaired mitochondrial oxygen consumption capacity may be reflected by exercise performance, and that physical fitness might predict a better exercise performance over the illness’ state, whereas mitochondrial respiratory capacity might increase in clinical remission compared to the acute states. Further studies should elucidate aerobic exercise could enhance mitochondrial respiratory capacity, whether this could be used as a state-dependent marker in the assessment of clinical response, and if the enhancement of physical activity might be a potential strategy to prevent not only metabolic comorbidities, but also mitochondrial dysfunction, which might pave the way for personalized interventions in BD.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by Hospital Clinic Research Ethics Committee (HCB/2021/0358). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsAG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. MG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. GR: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. ES: Investigation, Supervision, Visualization, Writing – review & editing. RB: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AM: Investigation, Methodology, Project administration, Resources, Writing – review & editing. FG: Conceptualization, Data curation, Investigation, Resources, Visualization, Writing – review & editing. ET: Investigation, Methodology, Resources, Supervision, Writing – review & editing. LV: Resources, Supervision, Visualization, Writing – review & editing. GA: Conceptualization, Methodology, Software, Supervision, Writing – review & editing. MV: Conceptualization, Methodology, Visualization, Writing – review & editing. LO: Project administration, Visualization, Writing – review & editing. Od: Project administration, Visualization, Writing – review & editing. IO: Project administration, Visualization, Writing – review & editing. HA: Project administration, Visualization, Writing – review & editing. JR: Conceptualization, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. NV: Conceptualization, Supervision, Visualization, Writing – review & editing. MB: Conceptualization, Data curation, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. EV: Conceptualization, Supervision, Visualization, Writing – review & editing. GG: Writing – review & editing, Writing – original draft. JR: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization. XA: Conceptualization, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing, Project administration, Resources. IP: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing, Funding acquisition, Writing – original draft.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. AG-P is supported by a Rio Hortega 2021 grant (CM21/00094) and M-AES mobility fellowship (MV22/00057), from the Spanish Ministry of Health financed by ISCIII and cofinanced by Fondo Social Europeo Plus (FSE+). GA is supported by a Rio Hortega 2021 grant (CM21/00017) and M-AES mobility fellowship (MV22/00058), from the Spanish Ministry of Health financed by the Instituto de Salud Carlos III (ISCIII) and cofinanced by Fondo Social Europeo Plus (FSE+). EV thanks the support of the Spanish Ministry of Science and Innovation (PI18/00805, PI21/00787) integrated into the Plan Nacional de I + D+I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the Instituto de Salud Carlos III; the CIBER of Mental Health (CIBERSAM); the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017 SGR 1365), the CERCA Programme, and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357, and the European Union Horizon 2020 research and innovation program (EU.3.1.1. Understanding health, wellbeing and disease: Grant No 754907 and EU.3.1.3. Treating and managing disease: Grant No 945151). This study has been funded by Instituto de Salud Carlos III (ISCIII) through the project PI21/00169 and co-funded by the European Union. MB is supported by a NHMRC Senior Principal Research Fellowship and Leadership 3 Investigator grant (1156072 and 2017131). MG-M is supported by a Sara Borrell grant (CD21/00019) from the Spanish Ministry of Health financed by ISCIII and cofinanced by Fondo Social Europeo Plus (FSE+).
Conflict of interestAG-P has received CME-related honoraria, or consulting fees from Janssen-Cilag, Lundbeck, Casen Recordati, LCN, Rovi and Angelini. GA has received CME-related honoraria, or consulting fees from Angelini, Casen Recordati, Janssen-Cilag, Lundbeck, Lundbeck/Otsuka, and Rovi, with no financial or other relationship relevant to the subject of this article. EV has received grants and served as consultant, advisor, or CME speaker for the following entities: AB-Biotics, AbbVie, Angelini, Biogen, Biohaven, Boehringer-Ingelheim, Celon Pharma, Compass, Dainippon Sumitomo Pharma, Ethypharm, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, Idorsia, Janssen, Lundbeck, Medincell, Novartis, Orion Corporation, Organon, Otsuka, Rovi, Sage, Sanofi-Aventis, Sunovion, Takeda, and Viatris, outside the submitted work.
IP has received CME-related honoraria, or consulting fees from ADAMED, Janssen-Cilag, and Lundbeck. IG has received grants and served as consultant, advisor or CME speaker for the following identities: Angelini, Casen Recordati, Ferrer, Janssen Cilag, and Lundbeck, Lundbeck-Otsuka, Luye, SEI Healthcare.
MB Grant/Research Support: MRFF, NHMRC, Congressionally Directed Medical Research Programs CDMRP USA, AEDRTC Australian Eating Disorders Research and Translation Centre, Patient-Centered Outcomes Research Institute PCORI, Baszucki Brain Research Fund, Danmarks Frie Forskningsfond. Psykiatrisk Center Kobenhavn, Stanley Medical Research Institute, Victorian Government Department of Jobs, Precincts and Regions, Wellcome Trust, Victorian Medical Research Acceleration Fund, Controversias Psiquiatria Barcelona, CRE, Victorian COVID-19 Research Fund, Consultancies: Lundbeck, Sandoz, Servier, Medisquire, HealthEd, ANZJP, EPA, Janssen, Medplan, RANZCP, Abbott India, ASCP, International Society of Bipolar Disorder, Precision Psychiatry, Penn State College of Medicine, Shanghai Mental Health Centre. Last 3 years – all unrelated to this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Primers. (2018) 4:1–16. doi: 10.1038/nrdp.2018.8
CrossRef Full Text | Google Scholar
2. Giménez-Palomo A, Gomes-da-Costa S, Dodd S, Pachiarotti I, Verdolini N, Vieta E, et al. Does metabolic syndrome or its component factors alter the course of bipolar disorder? A systematic review. Neurosci Biobehav Rev. (2022) 132:142–53. doi: 10.1016/j.neubiorev.2021.11.026
CrossRef Full Text | Google Scholar
3. Vancampfort D, Hagemann N, Wyckaert S, Rosenbaum S, Stubbs B, Firth J, et al. Higher cardio-respiratory fitness is associated with increased mental and physical quality of life in people with bipolar disorder: A controlled pilot study. Psychiatry Res. (2017) 256:219–24. doi: 10.1016/j.psychres.2017.06.066
CrossRef Full Text | Google Scholar
4. Casaburi R, Merrill D, Dolmage TE, Garcia-Aymerich J, Fageras M, Goldstein R, et al. Endurance time during constant work rate cycle ergometry in COPD: development of an integrated database from interventional studies. Chronic Obstructive Pulmonary Diseases. (2022) 9:520–37. doi: 10.15326/jcopdf.2022.0331
CrossRef Full Text | Google Scholar
5. Brobakken MF, Nygård M, Wang E. Physical health impairment and exercise as medicine in severe mental disorders: A narrative review. Sports Med - Open. (2022) 8:115. Springer Science and Business Media Deutschland GmbH. doi: 10.1186/s40798-022-00490-3
CrossRef Full Text | Google Scholar
6. Schaefer PM, Rathi K, Butic A, Tan W, Mitchell K, Wallace DC, et al. Mitochondrial mutations alter endurance exercise response and determinants in mice. Proc Natl Acad Sci USA. (2022) 119(18):e2200549119. doi: 10.1073/pnas.2200549119
CrossRef Full Text | Google Scholar
7. Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, et al. A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev. (2017) 74:1–20. doi: 10.1016/j.neubiorev.2017.01.014
CrossRef Full Text | Google Scholar
8. Giménez-Palomo A, Dodd S, Anmella G, Carvalho AF, Scaini G, Quevedo J, et al. The role of mitochondria in mood disorders: from physiology to pathophysiology and to treatment. Front Psychiatry. (2021) 12. doi: 10.3389/fpsyt.2021.546801
CrossRef Full Text | Google Scholar
9. Giménez-Palomo A, Guitart-Mampel M, Meseguer A, Borràs R, García-García F, Tobías E, et al. Reduced mitochondrial respiratory capacity in patients with acute episodes of bipolar disorder: Could bipolar disorder be a state-dependent mitochondrial disease? Acta Psychiatrica Scandinavica. (2024) 149(1):52–64. doi: 10.1111/acps.13635
CrossRef Full Text | Google Scholar
10. American Psychiatric Association. DSM-5: Diagnostic and Statistical Manual of Mental Disorders. 5th Editio. Washington DC: APA (2013). doi: 10.1176/appi.books.9780890425596
CrossRef Full Text | Google Scholar
11. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
CrossRef Full Text | Google Scholar
13. Neder JA, Berton DC, Muller PT, O’Donnell DE. Incorporating lung diffusing capacity for carbon monoxide in clinical decision making in chest medicine. Clin Chest Med. (2019) 40:285–305. doi: 10.1016/j.ccm.2019.02.005
CrossRef Full Text | Google Scholar
14. Rosa AR, Sánchez-Moreno J, Martínez-Aran A, Salamero M, Torrent C, Reinares M, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. (2007) 3:5. doi: 10.1037/t69921-000
CrossRef Full Text | Google Scholar
15. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). (2007) 4(7):28–37.
16. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
CrossRef Full Text | Google Scholar
17. Galilea-Zabalza I, Buil-Cosiales P, Salas-Salvadó J, Toledo E, Ortega-Azorín C, Díez-Espino J, et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PloS One. (2018) 13(6):e0198974. doi: 10.1371/journal.pone.0198974
CrossRef Full Text | Google Scholar
18. Blanco I, Valeiro B, Torres-Castro R, Barberán-García A, Torralba Y, Moisés J, et al. Effects of pulmonary hypertension on exercise capacity in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. (2020) 56:499–505. doi: 10.1016/j.arbres.2019.10.015
CrossRef Full Text | Google Scholar
19. Kathuria A, Lopez-Lengowski K, McPhie D, Cohen BM, Karmacharya R. Disease-specific differences in gene expression, mitochondrial function and mitochondria-endoplasmic reticulum interactions in iPSC-derived cerebral organoids and cortical neurons in schizophrenia and bipolar disorder. Discover Ment Health. (2023) 3(1):8. doi: 10.1007/s44192-023-00031-8
CrossRef Full Text | Google Scholar
20. Marques AP, Resende R, Silva DF, Batista M, Pereira D, Wildenberg B, et al. Mitochondrial alterations in fibroblasts of early stage bipolar disorder patients. Biomedicines. (2021) 9(4):522. doi: 10.3390/biomedicines9050522
留言 (0)