Neisseria gonorrhoeae (NG) is one of the most common bacterial STIs worldwide. When left untreated, NG may lead to severe consequences such as pelvic inflammatory disease, infertility and an increased risk of ectopic pregnancy. Therefore, current policies favour screening for gonorrhoea on a large scale to quickly diagnose and treat patients. However, antimicrobial resistance (AMR) to ceftriaxone, the first-choice treatment for NG, is on the rise worldwide.1–3 If treatment with ceftriaxone becomes ineffective, other options are currently limited and gonorrhoea may become an untreatable disease. Research is being conducted regarding the effectiveness of new and existing antibiotics, such as the New AntiBiotic treatment Options for uncomplicated GOnorrhoea (NABOGO) trial. In this study, intramuscular ertapenem 1000 mg was found to be non-inferior to ceftriaxone.4
Spontaneous clearance of asymptomatic anal, pharyngeal and urogenital NG infections has been described.5–7 A recent study found spontaneous clearance at any anatomical site of infection in 20.5% of patients over a median of 10 days.8 Spontaneous clearance of pharyngeal NG infections has been reported in 11.0%–27.3% of infections over a median of 10–11 days,5–7 9 whereas 33.3% of urogenital and 0.0%–20.0% of anorectal NG infections cleared spontaneously over a median of 8–9 and 7–11 days, respectively.5 7 Previous studies found that older patients were more likely to clear pharyngeal NG spontaneously,6 whereas individuals with symptoms (dysuria) and Chlamydia trachomatis (CT) coinfection were less likely to clear any NG infection.8
Given the rise of AMR to ceftriaxone, antibiotic stewardship will become increasingly important. More insight into spontaneous clearance of NG could guide clinical management and the development of screening guidelines regarding the start of antibiotic treatment in asymptomatic patients. Using data of patients participating in the NABOGO trial, we assessed spontaneous clearance of anogenital and pharyngeal NG. In addition, we assessed the time between two testing points before treatment administration in which spontaneous clearance could occur and the determinants associated with spontaneous clearance.
MethodsStudy design and participantsFor this study we used observational data of NABOGO trial participants collected before randomisation. The NABOGO trial was a randomised controlled, double-blind, single-centre, non-inferiority trial that assessed non-inferiority of ertapenem, gentamicin and fosfomycin to ceftriaxone for treatment of uncomplicated gonorrhoea. Full study procedures have been published previously.4 In brief, enrolment took place between 18 September 2017 and 5 June 2020 at the Public Health Service of Amsterdam, the Netherlands. Individuals seeking care at the Centre of Sexual Health in Amsterdam were eligible if they were at least 18 years old and had an anorectal or urogenital NG infection confirmed with a positive nucleic acid amplification test (NAAT) (Aptima Combo 2, Hologic, Massachusetts, USA). Symptomatic patients suspected of anogenital gonorrhoea could be included based on a positive Gram-stained smear, after which study treatment was administered; diagnosis was confirmed with a NAAT-positive result. Asymptomatic patients were screened for NG with NAAT at a pre-enrolment visit (T−1 visit) and were invited to participate if the NAAT was positive, after which a secondary NAAT was collected at the enrolment visit (T0 visit). An asymptomatic infection was defined as absence of mucopurulent discharge for urogenital and anal NG and absence of sore throat for pharyngeal NG. Patients could participate only once in the NABOGO trial. The study was registered at ClinicalTrials.gov (NCT03294395). All patients provided written informed consent.
ProceduresRandomisation and administration of trial medication took place after the second sample was collected at the enrolment visit. The anatomical location of the collected samples at the T0 visit was based on the sexual preference of the patient. From men who have sex with men (MSM), anal and pharyngeal swabs were collected, as well as urine; from women, anal, vaginal and pharyngeal swabs were collected; and from men who exclusively have sex with women (MSW), urine was collected. For the T−1 visit, the anatomical location of the collected samples was based on routine procedures. The routine procedures for MSM and for MSW were the same as during the T0 visit, whereas for women the routine practice for the anatomical location of the collected samples was dependent on anamnesis and presence of symptoms. We collected sociodemographic and clinical characteristics during the enrolment visit. Sexual behavioural characteristics were collected during patients’ initial consultations.
Statistical analysisIn this analysis, we included patients who (1) had a T−1 visit, (2) had a positive NAAT result at the T−1 visit at any site, (3) had a second NAAT result at the T0 visit at the same anatomical location(s) of the positive NAAT result(s) of the T−1 visit, and (4) did not receive any antibiotics before NAAT samples were collected at T0. We compared sociodemographic, clinical and sexual behavioural characteristics of patients who were included and excluded from the analysis using Pearson’s χ2 or Fisher’s exact test for categorical variables and t-test and Wilcoxon rank-sum test for continuous variables.
Spontaneous clearance was defined as having NAAT-positive anal, urethral, vaginal or pharyngeal result at the pre-enrolment visit, followed by NAAT-negative result at that same location at the enrolment visit. We calculated the proportion of patients with spontaneous clearance, per anatomical location, by dividing the sum of patients who spontaneously cleared an NG infection by the sum of all patients with an NG infection at the pre-enrolment visit.
We computed the median time between the pre-enrolment and enrolment visit, together with its IQR, for patients who cleared their NG infections spontaneously and those who did not clear, and compared the median times using Wilcoxon rank-sum test.
To analyse the determinants associated with any spontaneous clearance, we performed univariable and multivariable logistic regression with generalised estimating equation (GEE) to account for clustered data. We included all determinants from univariable analysis with a p value <0.20 in the multivariable analysis. Determinants that were a priori determined to have a plausible association with spontaneous clearance (ie, days between the pre-enrolment and enrolment visit, location of NG infection, age, HIV status and pre-exposure prophylaxis (PrEP) use) were forced into the multivariable model. We assessed the presence of multicollinearity between variables in the multivariable model using variance inflation factor (VIF). Multicollinearity was assumed present when variables had a VIF higher than 10. We performed backward selection to achieve the multivariable model with the best fit; variables that did not significantly improve the model (p>0.05) based on the likelihood ratio test were removed from the multivariable model. We assessed the determinants of spontaneous clearance of anal and pharyngeal infections separately using logistic regression models (without GEE) in a similar manner.
Statistical analyses were performed in Stata V.15.1. Violin plots were generated using the ggplot2 package in R (V.3.6.3; Vienna, Austria).10
ResultsBetween 18 September 2017 and 5 June 2020, 8099 patients with NG were seen at the STI clinic, of whom 2160 were invited to participate in the NABOGO trial (online supplemental figure 1). Of these, 253 were ineligible for participation in the NABOGO trial (see footnote to online supplemental figure 1 for exclusion criteria) and 1561 declined participation. In total, 346 patients were included in NABOGO and tested on T0 visit.4 For this analysis, we excluded 105 patients: 103 did have a symptomatic NG infection and 2 had a negative NAAT at the T−1 visit. Thus, 241 patients were included in the analysis, all of whom were asymptomatic. The included patients had a median age of 32 years (IQR 26–41) and were more often female than the excluded patients (7.1% vs 0.0%, p<0.001) (table 1). Compared with the excluded patients, the included patients were more likely to be a college/university graduate (p=0.040), had a higher median number of sexual partners in the 6 months before the enrolment visit (median 10 (IQR 5–20) vs 6 (IQR 3–15), p=0.018) and were less likely to have a coinfection with chlamydia (p<0.001). Included HIV-negative patients were more likely to use PrEP (p=0.005).
Table 1Baseline sociodemographic and behavioural characteristics of patients included and excluded from analysis of the NABOGO trial, Amsterdam, the Netherlands, from 18 September 2017 to 5 June 2020
The 241 included patients had a total of 354 NG infections at T−1: 222 (62.7%) were anal NG infections, 91 (25.7%) pharyngeal, 13 (3.7%) vaginal and 28 (7.9%) urethral (online supplemental figure 1). Of the patients, 92 (38.2%) had an NG infection at two anatomical locations, of whom 80 (86.9%) had an anal and pharyngeal NG infection, 2 (2.2%) had an anal and vaginal NG infection, and 10 (10.9%) had an anal and urethral NG infection. Ten patients (4.1%) had an NG infection at three anatomical locations; all 10 had NG infections at the anus, urethra and pharynx. Spontaneous clearance could not be assessed for one patient with an anal NG infection at the pre-enrolment visit as the NAAT result was invalid at the enrolment visit. Spontaneous clearance was observed in 32 of 221 patients (14.5%) with anal NG, 17 of 91 (18.7%) patients with pharyngeal NG, 3 of 13 (23.1%) patients with vaginal NG, and 9 of 28 (32.1%) patients with urethral NG (figure 1). Of 241 patients, 32 (13.3%) spontaneously cleared all anatomical locations.
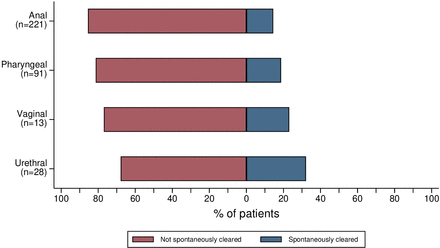
 Figure 1
Figure 1 Percentage of patients with spontaneous clearance of NG infections, NABOGO trial, Amsterdam, the Netherlands, from 18 September 2017 to 5 June 2020. Spontaneous clearance could not be assessed in one anal NG infection due to an invalid NAAT result at T0 and was therefore excluded from this analysis. NAAT, nucleic acid amplification test; NABOGO, New AntiBiotic treatment Options for uncomplicated GOnorrhoea; NG, Neisseria gonorrhoeae.
The median time between T−1 and T0 was longer for patients who cleared their pharyngeal infection spontaneously compared with those who did not (median 8 days (IQR 7–11) vs 6 days (IQR 4–8), p=0.012) (figure 2B). The median time between T−1 and T0 was also longer for patients who cleared their anal, vaginal and urethral infection spontaneously, although not significantly (figure 2A,C,D).

 Figure 2
Figure 2 Time (in days) between T−1 and T0 for patients who cleared and did not clear their NG infection spontaneously, NABOGO trial, Amsterdam, the Netherlands, from 18 September 2017 to 5 June 2020: (A) anal NG, (B) pharyngeal NG, (C) vaginal NG and (D) urethral NG. Spontaneous clearance could not be assessed in one anal NG infection due to an invalid NAAT result at T0. NAAT, nucleic acid amplification test; NABOGO, New AntiBiotic treatment Options for uncomplicated GOnorrhoea; NG, Neisseria gonorrhoeae.
In the multivariable analysis, the odds of clearing an NG infection spontaneously were higher for patients with a larger interval between T−1 and T0 (adjusted OR (aOR) 1.07 per additional day (95% CI 1.02 to 1.12), p=0.011) (table 2). The anatomical location of NG infection was not associated with spontaneous clearance, neither were HIV status, PrEP use and age.
Table 2Determinants of spontaneous clearance of NG in patients with an NG infection at the pre-enrolment visit: results of the univariable and multivariable logistic regression analyses, NABOGO trial, Amsterdam, the Netherlands, from 18 September 2017 to 5 June 2020
The odds of clearing anal NG spontaneously were lower for patients who had 10 or more sexual partners (aOR 0.23 (95% CI 0.09 to 0.58), p=0.002) in the 6 months before T−1 (online supplemental table 1). For patients with pharyngeal NG, the odds of clearing spontaneously were higher for patients with a larger interval between T−1 and T0 (aOR 1.17 per additional day (95% CI 1.03 to 1.34), p=0.014) (online supplemental table 2).
DiscussionIn this study, we found that 14.5% of patients with an anal NG infection, 18.7% of patients with a pharyngeal NG, 23.1% of patients with a vaginal NG and 32.1% of patients with a urethral NG infection spontaneously cleared their asymptomatic infection. Patients with a longer interval between T−1 and T0 were more likely to clear spontaneously, whereas patients with more sexual partners were less likely to clear spontaneously.
Previous studies found higher proportions of spontaneous clearance of pharyngeal NG (ie, 27%–27.3%).5 7 However, the number of patients with pharyngeal NG in these studies was lower than in our study, which could limit the interpretation of their results.5 7 Similar to a previous study,6 we found that patients who cleared their pharyngeal NG infection spontaneously had a longer median time between the two testing visits than those who did not clear spontaneously. Nevertheless, a previous study with a similar study design, but with a lower sample size, did not find an association between spontaneous clearance and the time between the two testing visits for any anatomical location.5 Ideally longer follow-up studies are needed. The immune system might decrease the bacterial load over time and thereby clear NG infections spontaneously over time.11 12 As previous studies found that symptomatic NG infections have a higher bacterial load than asymptomatic infections,13 14 it may be possible that asymptomatic NG infections might clear more easily. One study found that a lower bacterial load for CT infections was associated with spontaneous clearance, but did not find this association for NG infections.5 Future studies should determine whether there is an association between bacterial load and spontaneous clearance of NG and whether this is different for symptomatic and asymptomatic infections.
While current policies steer towards frequent testing for NG and immediate treatment after a one-time positive NAAT, we showed that NG infections can also clear spontaneously without treatment. Moreover, due to the high sensitivity of NAAT, it may also give false-positive results due to sample contamination and postinfectious phases,15 which could lead to overtreatment of some patients. This is especially relevant now that AMR emerges worldwide,1–3 presumably as a result of excessive use of antibiotics. High use of antibiotics is seen in PrEP cohorts with intensive screening and treatment of MSM for NG and CT infections16 17; one study found that the consumption of macrolides in PrEP cohorts was 52 times higher than in the general population.18 Moreover, frequent screening and treatment of MSM may result in higher minimum inhibitory concentrations for ceftriaxone compared with women.19 Less frequent STI screening in MSM may allow for spontaneous clearance, limit the use of antibiotics and thus curb AMR emergence.
Given that conventional NAAT results take a couple of days and NG infections can clear spontaneously in a relatively short time, an approach to decrease the use of antibiotics is to introduce a confirmational point-of-care (POC) NAAT test during the follow-up consultation where treatment is provided to asymptomatic patients.20 POC testing provides quick test results, often on the same day, without the interference of a clinical laboratory. In case of a negative test result during the follow-up consultation, meaning the NG infection cleared spontaneously or the previous NAAT result was a false-positive, the use of antibiotic treatment would be unnecessary. Two other solutions to false-positive results could be (1) to assess the viability of bacteria in positive NAAT results by new diagnostic tests and molecular targets (ie, molecular viability testing)21–23 or (2) to lower the cycle threshold cut-off values for NAAT. Lowering the cycle threshold cut-off values for NAAT could decrease false-positive test results, as contamination or non-viable bacteria will less likely result in a positive test result. Yet a lower cycle threshold cut-off value will unavoidably affect test sensitivity. Further research on the reliability and cost-effectiveness of POC NAAT and molecular viability testing is needed. In addition, the accuracy for correct test results when lowering the cycle threshold cut-off values for NAAT needs to be evaluated.
The strengths of this study were the prospective design of the NABOGO trial and the inclusion of four possible anatomical locations for an NG infection. Another strength was the use of sequential NAATs to determine whether spontaneous clearance occurred, as NAAT has higher sensitivity than diagnostic testing by culture.24 This study is not without limitations. First, we could not provide the exact time until spontaneous clearance of NG infections, but instead we based spontaneous clearance on NG results at two set time points (ie, pre-enrolment and enrolment visits). These time points likely do not reflect the exact day of infection and spontaneous clearance. Second, we only analysed spontaneous clearance in asymptomatic patients, as symptomatic patients were provided with treatment immediately, and hence we could not assess the difference in spontaneous clearance of NG between symptomatic and asymptomatic patients. Third, the proportions of spontaneous clearance of urethral NG could be biased by selection bias. Urethral NG infections are often symptomatic,25–27 and therefore most urethral NG infections in the NABOGO study were treated at the initial visit. Hence, only a small (selected) subset of urethral NG cases had both a pre-enrolment and an enrolment sample and could be included in the present analysis on spontaneous clearance. Fourth, in line with gonorrhoea epidemiology in Amsterdam, the numbers of MSW, women and transgenders were small. Therefore, our results may not be generalisable to all individuals with NG infections. Fifth, the proportion of spontaneous clearance of NG could be an underestimation, as theoretically patients could have cleared their NG infection spontaneously and got reinfected by resumed sexual contact with an untreated NG-positive sexual partner. However, as the time between the pre-enrolment and the enrolment visit was relatively short, with a median of 7 days (IQR 5–10), it is unlikely that patients cleared their NG infections spontaneously and got reinfected within the time frame of our study. Last, even though 18.5% of the included patients were living with HIV, we did not include any patients living with HIV who had a CD4 count of <350 cells/µL. A low CD4 count decreases immunity, which could affect the process of spontaneous clearance.28
In conclusion, in line with previous studies, we found that a considerable proportion of anal, urethral, vaginal and pharyngeal NG infections cleared spontaneously. The possibility for NG infections to clear spontaneously could indicate that some asymptomatic infections do not require treatment. Considering the emergence of AMR for NG, the use of antibiotics needs to be reduced. More research on the natural history of NG is needed in order to improve antibiotic stewardship for gonorrhoea.
Data availability statementData are available upon reasonable request. On request to the corresponding author (vjongen@ggd.amsterdam.nl), the following data will be available after publication: de-identified participant data and statistical analysis plan. Data will be shared after approval of an analysis proposal by the first, corresponding and other coauthors (BT, HdV, TH, AvD, MSvdL and VWJ).
Ethics statementsPatient consent for publicationObtained.
Ethics approvalThis study involves human participants and was approved by the Academic Medical Center Ethics Committee in Amsterdam (METC 2017_092, NL 60555.018.17). Participants gave informed consent to participate in the study before taking part.
AcknowledgmentsWe wish to thank all participants. Additionally, we thank Dr Anders Boyd, Dr Elske Hoornenborg, Dr Jan Prins, Dr Sylvia Bruisten, Myrthe de Laat, Carolien Wind, Jolinda de Korne-Elenbaas, Dr Yvonne van Duijnhoven, Dr Mirjam Knol, Dr Ron Mathôt, Myra van Leeuwen, Claudia Owusu, Luann Noordpool, Iris Deen, Marjo Broeren, Mark van den Elshout, Silvia Nieuwenburg, Ursa Gubensek, Michelle Kroone, Dominique Loomans and Ertan Ersan.
留言 (0)