Deidentified unit-record data from 1982 to 2015 were extracted from the state cancer registries and linked through the Australian Institute of Health and Welfare (www.aihw.gov.au). In Australia, it is a statutory requirement to report all new malignancies to the Department of Health. International Classification of Disease for Oncology version 3 (ICD-O-3.1, ICD-O-3)13 codes for melanoma (8720–8790) and site (C69.0–C69.9) were used to obtain unit data with the following information: state, sex, country of birth, year of diagnosis, age at diagnosis (rounded down to nearest whole year), ICD-O-3 topography, ICD-O-3.1 histology, vital status at 31/12/15, cause of death and survival time. The following topographies were analysed as UM: choroid, ciliary body and iris codes (C69.2–C69.4); retinal melanomas were also analysed as choroidal due to probable miscoding4 14; and we also included the C69.9 ‘Eye, not otherwise specified (NOS)’ non-specific code, assuming that the majority would be UM based on previous literatures showing conjunctival melanomas account for only ~5% of all ocular melanoma cases.6 15 Data from New South Wales (NSW) and the Australian Capital Territory (ACT) were combined to obfuscate values n≤5 as per the request of the data custodians. Both NSW and ACT cancer database data were missing for the year 2015, thus calculation of incidence was performed for years 1982–2014.
Data analysisAustralian Census and annual population data were downloaded from the Australian Bureau of Statistics (https://www.abs.gov.au/) from 1981 to 2015 and used to calculate direct age-standardised incidence rates (ASRs) and 95% CIs for all states and territories except for the Northern Territory (NT) due to the low case numbers. Rates were standardised to the 2001 Australian standard population (https://www.abs.gov.au/). Incidence was plotted using R (R core team, V.4.0.4). Indirect age-standardised incidence ratios (SIRs) were calculated for the NT, and regions of birth based on the major groups of the Standard Australian Classification of Countries, using population data from the 1993 Estimated Resident Population by country of birth, age, and sex, and the 2001, 2006, 2011 and 2016 Australian Census (https://www.abs.gov.au/) with direct age-standardised rates calculated from Australian-born persons used as the reference group.
Changes in incidence from 1982 to 2014 were calculated using the JoinPoint Regression Program (V.4.8.0.1, April 2020; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute).16 We fit a maximum of three joinpoints, using the program-selected model with the fewest joinpoints that best fitted the data. The change in incidence in these segments was reported as the APC per trendline, and average APC (AAPC) from 1982 to 2014. A significant change in the APC or AAPC was reported if p≤0.05. A map of Australia was generated using cartopy (V.0.18.0), geopandas (V.0.9.0) and matplotlib in Python (V.3.8.10). Calculated direct ASRs were plotted to each state or territory except for the NT. The 2017–2018 population was overlayed to give readers estimates of populous areas.
Assessment of mortality includes years 1982–2015. Five-year disease-specific overall survival17 was calculated. Disease-specific survival was also calculated using survival time between date of diagnosis and date of death coded to UM, or censored in case of unrelated death or if alive by end of 2015. Disease-specific survival was compared between states, sex and histology using a univariate Kaplan-Meier estimate with a log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon post hoc tests to measure the significance between survival curves in R (R core team, V.4.0.2) with the survival (V.3.2–3)18 19 and survminer (V.0.4.8)20 packages. Univariate and multivariate Cox proportional-hazards model was used to measure subgroup HRs to determine predictors of survival, including state, sex, age (<60 years vs ≥60 years), topography and histology using R with survival (V.3.2–7) and survminer (V.0.4.9) packages. For the Cox regression, only tumours with ICD-O-3.1 codes ‘C69.2 and C69.3 Choroidal melanoma’, ‘C69.4 Iris and ciliary body melanoma’ and ‘C69.9 Eye, NOS’ were included for survival analysis. Furthermore, only histology codes ‘8720—Malignant melanoma, NOS’, ‘8770—Mixed epithelioid and spindle cell melanoma’, ‘8771—Epithelioid cell melanoma’, ‘8772—Spindle cell melanoma, NOS’, ‘8773—Spindle melanoma, type A’, and ‘8774—Spindle cell melanoma, type B’ were included for analysis. Lastly, cumulative incidence of mortality was calculated comparing the cumulative risk of death to UM or other causes using the cmprsk (V.2.2-10) package for R (V.4.1).21 22
ResultsPopulation characteristicsFrom 1982 to 2014, there were a total of 5087 cases of ocular melanoma in Australia, of these, 4617 were classified as UM. From these, 3230 (70%) were classified as choroidal, 577 (12.5%) as iris or ciliary body, and the remaining 810 (17.5%) as eye, NOS (online supplemental table 1).
There were slightly more males diagnosed with UM (n=2432, 53%) than females (n=2185, 47%). The mean (±SD) and median age of diagnosis were 61 (±15) and 63 years (range 9–97 years), respectively. From ages 0–54, there were fewer cases of UM (n=1442, 31%) compared with those ≥55 years of age (n=3175, 69%). People born in Australia composed most cases of UM (n=3242, 70%), followed by those born in North-West Europe (n=607, 13%), Southern and Eastern Europe (n=306, 7%), and other regions (n=462, 10%).
The number of UM cases was highest in the most populous state of NSW (combined with ACT) (n=1667, 36%), followed by Victoria (VIC, n=1016, 22%), Queensland (QLD, n=912, 20%), South Australia (SA, n=513, 11%), Western Australia (WA, n=391, 9%), Tasmania (TAS, n=100, 2%) and the NT (n=18, 0.4%).
Incidence ratesThe average ASR of UM was 7.6 (95% CI 7.3 to 7.9) per million. Males had a higher ASR at 8.4 (95% CI 8.0 to 8.8) per million compared with females (ASR 6.9; 95% CI 6.5 to 7.3 per million) (figure 1A). While there was no overall change in incidence from 1982 to 2014 (AAPC 0.0%; 95% CI −0.9% to 1.0%), JoinPoint analysis of UM revealed an increase in cases from 1982 to 1993 (APC 2.5%; 95% CI 0.0% to 5.0%), followed by a significant (p≤0.05) decrease from 1993 to 2014 (APC −1.2%; 95% CI −2.0% to −0.4%).
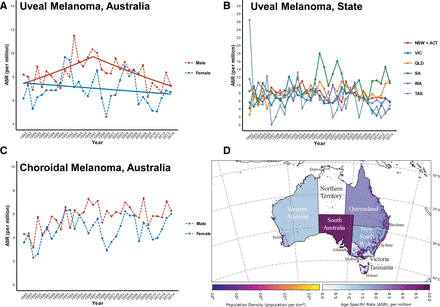
 Figure 1
Figure 1 Incidence of uveal melanoma. Age-standardised incidence rates (ASRs) from 1982 to 2014 per million population standardised to the 2001 Australian standard population of (A) total uveal melanoma with superimposed joinpoints; (B) New South Wales and Australian Capital Territory (NSW+ACT, red), Victoria (VIC, blue), Queensland (QLD, orange), South Australia (SA, green), Western Australia (WA, purple) and Tasmania (TAS, gun metal); (C) choroidal uveal melanoma. (D) Map of Australia indicating states, capital cities, population density and average ASRs of uveal melanoma.
Analysis of males with UM showed a significant increase in incidence from 1982, peaking in 1997 (APC 1.8%; 95% CI 0.4% to 3.3%). From 1997 to 2014, there was a significant decrease in cases (APC −1.7%; 95% CI −2.7% to −0.8%). There was no change in UM incidence in males from 1982 to 2014 (AAPC −0.1%; 95% CI −0.9% to 0.7%). Analysis of females with UM revealed a best joinpoint result with zero join points. Similar to males, there was no significant change in the incidence from 1982 to 2014 in females (AAPC 0.4%; 95% CI −1.1 to 0.3%).
Males had a higher incidence of UM in each Australian state when compared with females. SA had the highest overall average ASR of UM, at 9.7 (95% CI 8.6 to 10.7) per million, followed by QLD at 8.1 (95% CI 7.4 to 8.7), NSW and the ACT at 7.7 (95% CI 7.3 to 8.2), WA at 7.2 (95% CI 6.3 to 8.1), VIC at 6.6 (95% CI 6.1 to 7.1) and lastly TAS at 6.3 (95% CI 4.9 to 7.8) cases per million population (figure 1B). Furthermore, due to the low case numbers, an indirect SIR calculation was performed for the NT. This revealed a 35% lower incidence than expected based on the average age-specific rate of the country (SIR 0.65; 95% CI 0.39 to 1.03). Given that the NT has a large population of Indigenous Australians (~25% of the Territory population) that do not harbour the known risk factors for development of UM, we further assessed the indirect SIR after filtering out the Indigenous Australian population numbers which only marginally increased the calculated incidence ratio (SIR 0.80; 95% CI 0.47 to 1.27). Sex-specific ASRs per state and SIR for the NT are shown in table 1.
Table 1Age-standardised incidence of uveal melanoma by sex, state and territory
The analysis of age-standardised rates by age strata in UM revealed that from ages 0–54 years, male and female patients with UM had similar incidence rates. UM incidence increased with age in both males and females but began to decline after the age of 74 in both sexes. From the age of 55 years and above, males had a higher incidence than females (online supplemental figure 1).
Choroidal melanoma incidence had an average age-standardised incidence of 5.2 (95% CI 5.0 to 5.4, figure 1D) per million. Males had a choroidal melanoma incidence 23.4% higher than females (table 2). Due to the combination of ciliary body and iris UM, their incidence rate was not assessed. The incidence rate for choroidal UM is likely to be underestimated due to removal of C69.9 coding.
Table 2Age-standardised incidence of uveal melanoma by anatomical site
The indirect age-standardised incidence ratio of UM when compared by country of birth differed from Australian-born cases (online supplemental table 2). In comparison, Asian-born individuals such as those from Southeast Asia (SIR 0.12; 95% CI 0.07 to 0.19), Northeast Asia (SIR 0.10; 95% CI 0.05 to 0.19) and Southern and Central Asia (SIR 0.2; 95% CI 0.11 to 0.33) were found to have the lowest SIRs of UM. Other groups with countries of origin such as Southern and Eastern Europe (SIR 0.73; 95% CI 0.65 to 0.82), South America and the Caribbean (SIR 0.41; 95% CI 0.20 to 0.73), and South and East Africa (SIR 0.37; 95% CI 0.22 to 0.58) also had lower SIRs than Australian-born individuals but not to the extent as Asian countries. Patients with UM born in North-West Europe (SIR 0.96; 95% CI 0.88 to 1.04) and North America (SIR 1.01; 95% CI 0.66 to 1.48) had similar ratios to that of the Australian-born population.
MortalityFrom 1982–2015, there were 4770 cases of UM. Over the last 34 years, 2370 patients died, with 1175 (50%) dying to UM. The yearly 5-year disease-specific survival for patients with UM has remained stable at an average of 81% (95% CI 80% to 82%), with a non-significant AAPC of 0.1% (95% CI −0.3% to 0.4%) (figure 2A). Furthermore, analysis of the mortality (figure 2B) between states and territories (excluding the NT due to low case numbers) revealed that patients in Victoria (5-year survival, 86%) had a significantly higher disease-specific survival (figure 2B) than each other state or territory, whereas WA (5-year survival, 77%) had the lowest disease-specific survival. However, at 5 years, TAS had the lowest disease-specific survival of 70%. Given the disparity of mortality between states, we assessed the cumulative mortality. At around 10 years, the Australian cumulative mortality begins to plateau, whereas deaths to other causes continue to increase, as expected, with risk of death to other causes over taking UM by 15 years (online supplemental figure 2A). Interestingly, in WA, UM is the leading cause of death until 30 years unlike other states and territories (online supplemental figure 2B). Furthermore, there was a significant difference between the disease-specific survival between epithelioid, mixed, spindle A and spindle B cells, with epithelioid cells having the lowest overall survival and Spindle A the highest (online supplemental figure 3).

 Figure 2
Figure 2 Overall survival of uveal melanoma from 1982 to 2015. (A) The 5-year disease-specific survival for uveal melanoma. (B) Kaplan-Meier estimates for the disease-specific survival for uveal melanoma in New South Wales plus the Australian Capital Territory (NSW+ACT, n=1656, red), Victoria (VIC, n=1066, blue), Queensland (QLD, n=955, orange), South Australia (SA, n=535, green), Western Australia (WA, n=406, purple), Tasmania (TAS, n=99, gun metal) and NT (NT, n=20, yellow). Below the line graph, p values from log-rank (Mantel-Cox) above the diagonal line, and p values from Gehan-Breslow-Wilcoxon below the diagonal line. Significant values are bolded.
In the Cox multivariate model (table 3), predictors of survival were residence in Victoria (HR 0.74, 95% CI 0.62 to 0.88, p≤0.001) or SA (HR 0.74, 95% CI 0.58 to 0.96, p=0.021); or having a histological classification of spindle A cells (HR 0.43, 95% CI 0.20 to 0.91, p=0.027). Conversely, predictors of worse survival were residence in WA (HR 1.40, 95% CI 1.15 to 1.71, p=0.001); age ≥60 years (HR 1.67, 95% CI 1.48 to 1.89, p≤0.001); or histological classifications of mixed (HR 2.18, 95% CI 1.85 to 2.56, p≤0.001) or epithelioid cells (HR 2.47, 95% CI 1.85 to 3.31, p≤0.001).
Table 3Subgroup survival analysis
DiscussionOur analysis of whole-population data provided detailed information on the UM incidence and mortality in Australia over the last three decades. During this period, the age-standardised incidence was relatively stable overall from 1982 to 2014. However, there was a distinct significant increase in incidence in the 1990s, driven by an increase in male incidence, followed by a significant decrease until 2014, although no specific reason can be related to explain this peak in incidence.
We found that the incidence of UM was higher in males, in agreement with previous studies in Australia, Europe, the USA and South Korea.3 4 6 7 Previous studies have shown that occupational exposures from solar radiation, welding UV or certain chemical carcinogens are associated with increased risk of developing UM,6 23 24 and as males are more likely to be exposed to chemical and solar carcinogens,25 26 may partially explain the differences between sex-specific incidences, especially given the sex-specific differences for ages ≥55 years. However, it should be noted that these factors are only weakly associated with UM, and there is no general consensus on whether they are causative. Interestingly, previous research has shown that the presentation of UM is different in males and females, with males presenting with larger27 and more posterior tumours than females,27 28 which may be due to different sex-related behaviours and exposures. However, further investigation between UM in males and females is required to properly answer these questions.
Interestingly, we found that SA had the highest levels of UM when compared with QLD, NSW plus the ACT, VIC, WA and TAS. This is interesting, as SA is an outlier in an otherwise gradual minor gradient reduction in incidence with decreasing latitude. Unfortunately, our data lacked precise geospatial data needed to precisely plot changes in incidence with latitude. However, given that 78% of the South Australian population resides in Adelaide (https://plan.sa.gov.au/state_snapshot/population) with a similar latitude to that of Sydney and Perth, further investigations should be performed to determine the cause of the observed increased incidence. Given previous research in Australia has shown that UM incidence is associated with rurality, latitude and lifetime solar exposure,6 29 it is notable that we observed an indirect SIR of 0.65 in the NT, which has also been shown to have the lowest incidence of cutaneous melanoma in Australia.30 This, however, may be due to the higher proportion of Indigenous Australians (~25%) in the NT compared with the rest of Australia. However, given that adjustment of the population to account for Indigenous Australians only marginally increased the indirect SIR, it may indicate that case ascertainment for UM in the NT is lower than that of the other states and territories. In fact, previous research has indicated that overall case ascertainment of UM in the NT registry is slightly lower than other Australian cancer registries.31
As expected, migrants from North-West Europe and North America had a similar indirect SIR of UM to that of Australian-born persons, likely due to similar host susceptibility factors within each population, such as light eye colour, fair skin colour or the inability to tan, which have all been shown to increase the risk of developing UM.5 In contrast, migrants from countries in Southern and Eastern Europe, South America and the Caribbean, North Africa and the Middle East, Southern and Eastern Africa and all Asian regions had a lower indirect SIR of UM, most likely due to a higher prevalence of protective host factors in these populations.32
The overall 5-year disease-specific survival of UM in Australia remained stable from 1982 to 2011, at an average of 81%, similar to a recent report of UM survival rates in the USA by Aronow et al, at 81% from 1973 to 2013.4 Despite the improvement and success of eye-sparing treatment,33 survival has not changed over the past three decades. The lack of effective treatments for metastatic UM33 may explain the persistent mortality observed. Previous reports have shown that patients with iris UM have better 5-year survival,34 whereas patients with choroidal and ciliary body melanoma have worse survival.34 Unfortunately, under the coding system used by the ACD, iris and ciliary body cannot be separated, and the 5-year disease-specific survival cannot be determined for either subtype. Although the 5-year survival has not shown to be improving worldwide in general, a recent study investigating conditional survival found that patients who had already survived past 5 years, their 10-year conditional survival rates were found to be high.35 Interestingly, we found significant differences between the disease-specific survival between each state, with Victoria having the highest overall survival rate and WA having the lowest. While this study cannot determine the cause, it may be due to a combination of factors, such as rural populations have a higher incidence of UM6; WA has many population centres classified as remote (rural) under the modified Monash model36; WA has a lower number of ophthalmologists compared with the national average37; and lastly, only a few ophthalmologists reside outside of the main population centre, Perth.36 Given that previous research has shown that larger UM tumours have worse survival,38 39 the higher incidence found in rural areas,6 coupled with fewer trained ophthalmologists located in rural areas that can detect the disease for referral to specialised ophthalmologists in Perth may potentially lead to delayed diagnosis of UM, and thus worse survival. Previous research on the mortality of UM in WA found that the largest basal diameter and treatment by enucleation were significant predictors of mortality,40 suggesting that delayed treatment is leading to poorer outcomes. A major limitation of the ACD dataset is the lack of staging information at the time of diagnosis. Nevertheless, in agreement with previous studies,41 epithelioid cell type was a predictor of lower disease-specific overall survival in UM.
Although cancer is a notifiable disease within Australia, and all cases are required to be reported to the Department of Health by law, we found a high level of non-specific site coding. This still allows for accurate quantification of the overall impact of UM within Australia, but calculation of subtype-specific incidence rates for UM is lacking. Furthermore, the ICD coding system lacks the ability to separate iris from ciliary body, and the true differences in both incidence and survival cannot be explored using the ACD. In this regard, as a rare disease, UM within Australia would benefit from a national database where detailed information, such as clinical, histological, genetic and metastatic disease monitoring, could be used for more detailed epidemiological analysis and would serve as a better baseline for future analysis on the long-term effectiveness of changes to treatments and clinical practice.
留言 (0)