1Department of Gastroenterology, Ishikawa Prefectural Central Hospital, Ishikawa, Japan
2Department of Gastrointestinal Oncology, Osaka International Cancer Institute, Osaka, Japan
3Department of Gastroenterology and Hepatology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan
4Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
5Department of Gastroenterology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
6Department of Endoscopy, Fukuoka University Chikushi Hospital, Fukuoka, Japan
7Department of Gastroenterology, Fukui Prefectural Hospital, Fukui, Japan
8Department of Gastroenterology, Kochi Red Cross Hospital, Kochi, Japan
9Department of Preventive Medicine and Public Health, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
Abstract Background/AimsThe etiology of superficial non-ampullary duodenal epithelial tumors (SNADETs) remains unclear. Recent studies have reported conflicting associations between duodenal tumor development and Helicobacter pylori infection or endoscopic gastric mucosal atrophy. As such, the present study aimed to clarify the relationship between SNADETs and H. pylori infection and/or endoscopic gastric mucosal atrophy.
MethodsThis retrospective case-control study reviewed data from 177 consecutive patients with SNADETs who underwent endoscopic or surgical resection at seven institutions in Japan over a three-year period. The prevalence of endoscopic gastric mucosal atrophy and the status of H. pylori infection were compared in 531 sex- and age-matched controls selected from screening endoscopies at two of the seven participating institutions.
ResultsFor H. pylori infection, 85 of 177 (48.0%) patients exhibited SNADETs and 112 of 531 (21.1%) control patients were non-infected (p<0.001). Non-atrophic mucosa (C0 to C1) was observed in 96 of 177 (54.2%) patients with SNADETs and 112 of 531 (21.1%) control patients (p<0.001). Conditional logistic regression analysis revealed that non-atrophic gastric mucosa was an independent risk factor for SNADETs (odds ratio, 5.10; 95% confidence interval, 2.44–8.40; p<0.001).
ConclusionsNon-atrophic gastric mucosa, regardless of H. pylori infection status, was a factor independently associated with SNADETs.
Keywords: Duodenal neoplasms; Gastritis; Helicobacter pylori
INTRODUCTION Superficial non-ampullary duodenal epithelial tumors (SNADETs), which can manifest as either adenomas or carcinomas, can be defined as lesions that are limited to the mucosa and/or submucosa and do not arise from the papilla of Vater. The incidence of non-ampullary primary duodenal tumors is low (0.02%–0.50%) according to autopsy findings,1-3 with primary duodenal cancer accounting for only approximately 0.5% of all gastrointestinal cancers.4 Furthermore, the incidence of duodenal adenoma detected by endoscopic screening is 5 Due to the low incidence of duodenal tumors, there are no established guidelines for the treatment and surveillance of duodenal carcinoma. Despite its low incidence in general, duodenal carcinoma is the most common of all small intestinal carcinomas.6 Furthermore, the incidence of small intestinal carcinoma (together with colorectal carcinoma) has been increasing since the 1970s,6 contributing to the increasing number of duodenal tumors reported each year.7 A further problem is that duodenal adenocarcinoma has the lowest five-year survival rate of all small intestinal carcinomas (8,9 This dismal prognosis is explained by the fact that most tumors are detected at an advanced stage.10 Some recent reports have attributed the increased incidence of duodenal tumors to the standardization of esophagogastroduodenoscopy and to the aging society in Japan.7 The adenoma–carcinoma model is believed to be applicable to both the small and large intestine.6 Therefore, the progression to advanced cancer can be prevented if it can be detected at the adenoma or early adenocarcinoma stage. This may result in improved prognosis for those with duodenal cancer and a better quality of life. As such, we believe that identifying the etiology of duodenal tumors is critical for early detection. Recent studies in Japan have suggested a relationship between duodenal tumors and either Helicobacter pylori infection or endoscopic gastric mucosal atrophy. One case series suggested a relationship between SNADETs and non-atrophic gastric mucosa,11 whereas another reported that H. pylori infection was a risk factor for non-ampullary duodenal adenocarcinoma (NADAC).12 NADAC can be defined as a carcinoma that has not originated from the papilla of Vater. However, it remains controversial whether duodenal tumors are caused by non-atrophic gastric mucosa or H. pylori positivity, and the relationship between duodenal tumors and these two conditions has not been fully substantiated. Furthermore, no large-scale study has been undertaken due to the low incidence of duodenal tumors.As such, the present multicenter study, conducted in Japan, aimed to examine and clarify the association between SNADETs and H. pylori infection and/or endoscopic gastric mucosal atrophy.
METHODS Study design This retrospective case-control study was conducted at seven Japanese institutions in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.13 Consent was obtained using the opt-out method and only anonymized data were used for analysis. All authors had access to the study data and reviewed and approved the final manuscript submitted for publication. PatientsData from 177 consecutive patients with SNADETs, who underwent endoscopic or surgical resection and histopathological examination between January 2016 and December 2018 at all participating hospitals, were retrospectively reviewed. Patients with hereditary diseases, such as familial adenomatous polyposis, medical history of gastrectomy, multiple lesions, and an unknown H. pylori infection status, were excluded.
Control groupThe control group included 2006 asymptomatic healthy patients who underwent screening endoscopy and were checked for H. pylori infection status at Ishikawa Prefectural Central Hospital (Ishikawa, Japan) and Fukui Prefectural Hospital (Fukui, Japan) between January 2016 and December 2018. Although it would have been ideal to recruit a control group from all participating institutions, five centers rarely performed screening endoscopy; as such, only two were selected. To minimize background differences, the SNADET and control groups were age- and sex-matched (within a 5-year difference), resulting in a SNADET patient and control subject ratio of 1:3. H. pylori infection status and endoscopic gastric mucosal atrophy were compared between the SNADET (n=177) and control (n=531) groups.
Definition of SNADETsThe duodenum runs from immediately below the gastric pylorus to the ligament of Treitz, and is the most proximal part of the small intestine. It is divided into four parts: bulbous, descending, horizontal, and ascending. The major duodenal papilla is located on the inner medial wall of the descending part, and the common bile and pancreatic ducts are opened. Additionally, the descending portion is divided between the oral and anal sides of the major papilla.
SNADET is defined as a solitary adenoma or adenocarcinoma that is limited to the mucosa or submucosa and is located in the non-ampullary region (Fig. 1). In accordance with the Japanese Classification of Gastric Carcinoma,14 the macroscopic types of SNADET are classified into basic types, 0–5, and 0 types are classified into subtypes I–III. Furthermore, low-grade adenomas are classified as category 3, high-grade adenomas and intramucosal carcinomas are classified as category 4, and submucosal invasive carcinomas are classified as category 5, in accordance with the revised Vienna classification.15 Definition of gastric mucosal atrophy The Kimura–Takemoto classification system was used for endoscopic diagnosis of gastric mucosal atrophy.16 Lesions were classified as closed type (C1, C2, or C3) when the atrophic demarcation was located on the lesser curvature and as open type (O1, O2, or O3) when the demarcation line was located on the anterior or posterior wall or on the greater curvature. Cases with no endoscopically observed atrophic inflammation in the entire gastric region, including the antrum, were categorized as non-atrophic (C0).17 In the Kyoto Classification of Gastritis, developed in 2015,18 gastric mucosal atrophy was divided into three groups: none (C0 to C1); mild (C2 to C3); and severe (O1 to O3). Accordingly, in this study, patients were divided into a non-atrophic group (C0 to C1) and a mildly and severely atrophic group (C2 to O3).Gastric mucosal atrophy was independently evaluated and classified by two gastroenterological endoscopists at each institution. The degree of atrophy was measured using several images. When the initial opinions of the two endoscopists differed, an additional gastroenterological endoscopist was consulted and a majority vote was used to determine the final classification. At the time of evaluation, the endoscopists were not informed of the presence or absence of SNADETs.
Definition of H. pylori infection statusH. pylori infection was diagnosed according to the results of various H. pylori-related tests, endoscopic findings, and patient medical records documenting a history of H. pylori eradication therapy. H. pylori infection status was divided into three groups, current, non, and past infection. Current infection was defined as positive using any of the following approaches: rapid urease test; microscopic examination; culture method; serum immunoglobulin G antibody testing (≥10 U/mL, H. pylori antibody kit; Special Reference Laboratories, Tokyo, Japan); urea breath test; or fecal H. pylori antigen measurement.
Non-infection was defined as a negative result in all tests performed using serum immunoglobulin G antibody testing (H. pylori antigen measurement, and a case that was endoscopically considered non-infected using the Kyoto Classification of Gastritis.18 Finally, past infections were defined as those that did not fit the current infection or non-infection definitions. No distinction was made between patients who underwent H. pylori eradication therapy and those who experienced spontaneous elimination. Statistical analysis Continuous and discrete variables are expressed as median or percentage. The Mantel–Haenszel test and conditional logistic regression were applied, and differences with pwww.r-project.org). More precisely, it is a modified version of R Commander, designed to add statistical functions frequently used in biostatistics.19 Ethical statementsThe study protocol and consent forms were approved by the ethics committees of all participating hospitals: Ishikawa Prefectural Central Hospital (IRB No: 1402), Osaka International Cancer Institute (IRB No: 19146), Graduate School of Medical Sciences, Kumamoto University (IRB No: 1845), Graduate School of Medical Sciences, Kyushu University (IRB No: 2019-502), Fukuoka University Chikushi Hospital (IRB No: C20-05-008), Fukui Prefectural Hospital (IRB No: 19-21), and Kochi Red Cross Hospital (IRB No: 322).
RESULTS The characteristics of patients with SNADETs are summarized in Table 1. The median age of patients with SNADETs was 65 years (range, 25–88 years), and 133 (75.1%) were male. The median tumor size was 12 mm (range, 2–50 mm), and most SNADETs (80.2%) were located in the descending part of the duodenum. Ninety-three lesions (52.5%) were on the oral side of the major duodenal papilla, whereas 84 (47.5%) were on the anal side. Microscopic type revealed 32 (18.1%) type 0–I tumors, 120 (67.8%) type 0–IIa tumors, and 25 (14.1%) type 0–IIc tumors. Postoperative pathological diagnoses were categorized as category 3 (40.1%), 4 (59.9%), and 5 (0%). H. pylori infection status and degree of endoscopic gastric atrophy in the SNADET and control groups are shown in Table 2. In the SNADET group, 48.0% of patients had a non-infection status and 52.0% had a current or past infection status. In the control group, 21.1% of patients had a non-infection status and 78.9% had a current or past infection status. There was significantly less H. pylori infection in the SNADET group than in the control group (pppTable 3). Because the characteristics of SNADETs differ according to tumor location, the association between tumor location and H. pylori status and degree of endoscopic gastric mucosal atrophy was examined. However, no statistical difference was found for either (p=0.30 and p=0.23, respectively) (Table 4). DISCUSSION To our knowledge, the present multicenter study was the first to investigate the relationship between SNADETs and H. pylori infection or endoscopic gastric mucosal atrophy. Our results demonstrated that non-atrophic gastric mucosa was an independent factor associated with SNADETs, regardless of H. pylori infection status. Due to the low incidence of duodenal tumors, few large studies have been conducted, and much remains to be determined regarding their etiology. This investigation had two strengths. First, it was a multicenter, matched, case-control study. Second, H. pylori infection status was divided into current infection, non-infection, and past infection, which are most relevant to the disease state. Several previous reports have indicated a relationship between duodenal tumors and males of advanced age, the presence of colorectal polyps, and high-fat and high-protein diets.7,20-22 However, with regard to the relationship between duodenal tumors and H. pylori or endoscopic gastric mucosal atrophy, there are only two single-institution reports.11,12 Therefore, we conducted a multicenter, retrospective, case-control study involving 177 patients at seven Japanese institutions. In this study, conditional logistic regression analysis revealed that non-atrophic gastric mucosa was an independent factor associated with SNADETs; however, there was no association between H. pylori infection status and the prevalence of SNADET. To date, there have been conflicting data from two previous studies regarding whether duodenal tumors are caused by non-atrophic gastric mucosa or H. pylori positivity.11,12 In a letter, Kawai et al.,11 suggested a relationship between SNADETs and non-atrophic gastric mucosa, with 82% of endoscopic gastric mucosal atrophy classified as closed-type in 61 patients with SNADETs. In contrast, the rate of endoscopic gastric mucosal atrophy was 76% in this study, which was comparable to those reported in the studies mentioned above. Kakushima et al.12 reported that H. pylori positivity was a risk factor for NADAC in a retrospective, single-center, case-control study, and demographics and clinical findings of 156 patients with NADAC were compared with those of 468 age- and sex-matched controls selected from medical health check-up recipients. Multivariate analysis revealed that H. pylori positivity was a risk factor for NADAC, although gastric mucosal atrophy was not. Differences in the results reported by Kakushima et al.12 and those of the current study can be explained by the sample populations. The data generated by Kakushima et al.12 were collected only from patients with NADAC at a single tertiary cancer center. In contrast, our study examined SNADETs (40% of the cases were adenomas) and Kakushima et al.12 examined only cancers (37% of the cases were advanced). Therefore, the study by Kakushima et al.12 did not demonstrate a relationship between SNADETs and H. pylori infection. In addition, SNADETs can be classified into two main categories according to their phenotype: gastric or intestinal.23 Previous reports have indicated that the gastric type exhibits a higher biological malignancy than the intestinal type.23-25 No histopathological mucosal examinations of SNADETs were performed in the current study. However, because 40% of the subjects in this study had adenomas, it is expected that the proportion of intestinal type is higher than that in the study by Kakushima et al.12 The different results between the two studies may also be related to differences in the degree of malignancy and histopathological mucosal classification among the subjects. Furthermore, we speculate that patients with duodenal carcinoma at a cancer center may include those with a history of concomitant gastric cancer and severe atrophic gastritis; as such, this bias may reduce the association between less endoscopic gastric atrophy and SNADETs. It remains unclear why SNADETs occur more frequently in patients with non-gastric mucosal atrophy; however, we speculate that high acid secretion may be involved in this process. Previous studies have indicated that the mucosa and Brunner’s glands in the proximal duodenum can potentially transform into gastric foveolar-type epithelium by exposure to gastric acid,26,27 and SNADETs with a gastric mucin phenotype can arise from the gastric foveolar type of epithelium.23 Esophageal adenocarcinoma is associated with H. pylori negativity28 and a high-fat diet,29 the latter of which alters bile acid composition.30 Mukaisho et al.31 reported that an increase in taurine-conjugated bile acids in the refluxate under acidic conditions plays a role in Barrett’s carcinogenesis and cancer progression. Gastric juice flows directly into the oral side of the major duodenal papilla, while pancreatic juice and bile are expelled toward the anal side of the duodenum. Consequently, it has been suggested that the tumorigenesis of SNADETs may differ between the oral and anal sides of the major duodenal papilla.32 SNADETs can be classified into two main categories according to phenotype: gastric or intestinal.23 The gastric type reportedly occurs more frequently in the oral side of the major duodenal papilla, while the intestinal type is more prominent in the anal side of it.33 Furthermore, a previous study reported that, in patients with H. pylori infection, duodenal tumors were significantly more frequently located on the oral side of the major papilla compared with patients without H. pylori infection.34 On the other hand, that study reported that duodenal tumors located on the anal side of the major papilla were associated with bile acids.34 Accordingly, we investigated the association between H. pylori status and the degree of endoscopic gastric atrophy and location of SNADETs; however, a significant association was not found. Identifying the etiology of SNADETs is crucial for its early detection. Japan once had a high incidence of H. pylori infection; however, it is rapidly declining.35 This decreased incidence of H. pylori infection is particularly evident among younger individuals, which indicates the possibility of future increases in the number of SNADET cases associated with less gastric mucosal atrophy. Moreover, the number of SNADET cases is expected to increase due to the increasing number of esophagogastroduodenoscopies performed, the aging society, and the westernization of dietary habits. Increasingly, more SNADET cases are now being treated using endoscopy36; however, once surgical treatment cannot be avoided because there are no indications for endoscopic treatment, pancreaticoduodenectomy causes substantial patient morbidity. Therefore, the detection of duodenal tumors in SNADETs is critical for achieving a favorable outcome. In this regard, this age- and sex-matched case-control study highlights the importance of vigilant surveillance of SNADETs during esophagogastroduodenoscopy in patients with non-gastric mucosal atrophy. The present study had several limitations, the first of which was its retrospective design. Because the incidence of SNADETs is low, a prospective cohort study would require a large amount of effort and would be difficult to perform; therefore, results of this multicenter study are valuable. Second, histopathological mucosal examination of SNADETs was not performed. SNADETs can be classified into two main categories according to phenotype: gastric or intestinal.23 The gastric type reportedly occurs more frequently in the oral side of the major duodenal papilla, whereas the intestinal type is more prominent on the anal side.33 Future analyses of SNADETs and gastric mucosal atrophy or H. pylori infection should include histopathological mucosal classification. Third, the control subjects were patients who underwent endoscopic screening at only two general hospitals, which likely introduced selection bias. This was because the other five institutions were oncology hospitals, in which screening endoscopy was rarely performed. Furthermore, because the control group comprised subjects who were undergoing health checkups, these individuals may have been more interested in their own health than those in the general population. Furthermore, we did not routinely check the H. pylori status of all asymptomatic individuals who undergo screening endoscopy. For these reasons, selection bias may have occurred, and H. pylori infection status may have been slightly different from that of the general population. Fourth, the etiology of SNADETs has been suggested to be associated with colorectal polyps, high-fat diets, smoking, and alcohol consumption.12,20-22,37 However, the present study was not able to evaluate these relationships. Moreover, the intake of acid suppressants was not examined. Therefore, they were presumed to influence the present results as unmeasured confounders. These issues should be addressed in future studies.In conclusion, non-atrophic gastric mucosa, regardless of H. pylori infection status, may be an independent factor associated with SNADETs. In general, gastric cancer is less common in non-atrophic gastric mucosa. However, because SNADETs may be more common in non-atrophic gastric mucosa, close attention to the findings of non-atrophic gastric mucosa may lead to earlier detection of SNADETs.
Fig. 1.Endoscopic image of superficial non-ampullary duodenal epithelial tumors using white light imaging. (A) A whitish, slightly elevated lesion (10 mm in diameter) was observed in the descending part of the duodenum (oral side of the major duodenal papilla). The final histopathological diagnosis was low-grade adenoma. (B) A whitish, slightly depressed lesion (8 mm in diameter) was observed in the descending part of the duodenum (anal side of the major duodenal papilla). The final histopathological diagnosis was a well-differentiated intramucosal adenocarcinoma.
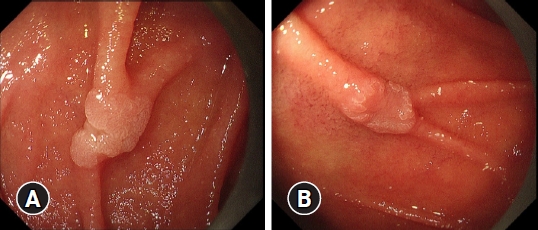 Table 1.
Demographic
SNADET (n=177)
Age (yr)
65 (25–88)
Sex
Male
133 (75.1)
Female
44 (24.9)
Tumor size (mm)
12 (2–50)
Tumor location
Bulb
30 (16.9)
Descending part, oral side of papilla
63 (35.6)
Descending part, anal side of papilla
79 (44.6)
Horizontal part
5 (2.8)
Macroscopic type of tumor
0–I
32 (18.1)
0–IIa
120 (67.8)
0–IIc
25 (14.1)
Histopathology
Category 3
71 (40.1)
Category 4
106 (59.9)
Category 5
0 (0)
Table 2.
Table 1.
Demographic
SNADET (n=177)
Age (yr)
65 (25–88)
Sex
Male
133 (75.1)
Female
44 (24.9)
Tumor size (mm)
12 (2–50)
Tumor location
Bulb
30 (16.9)
Descending part, oral side of papilla
63 (35.6)
Descending part, anal side of papilla
79 (44.6)
Horizontal part
5 (2.8)
Macroscopic type of tumor
0–I
32 (18.1)
0–IIa
120 (67.8)
0–IIc
25 (14.1)
Histopathology
Category 3
71 (40.1)
Category 4
106 (59.9)
Category 5
0 (0)
Table 2.
Prevalence of Helicobacter pylori infection status and endoscopic gastric atrophy in the SNADET and control groups
Characteristic SNADET (n=177) Control (n=531) p-value Median age (yr) 65 (25–88) 65 (25–88) Sex Male 133 (75.1) 399 (75.1) Female 44 (24.9) 132 (24.9) H. pylori infection status <0.001 Non-infection 85 (48.0) 112 (21.1) Current infection 37 (20.9) 155 (29.2) Past infection 55 (31.1) 264 (49.7) Endoscopic gastric atrophy <0.001 C0 83 (46.9) 50 (9.4) C1 13 (7.3) 62 (11.7) C2 26 (14.7) 53 (10.0) C3 12 (6.8) 109 (20.5) O1 21 (11.9) 125 (23.6) O2 11 (6.2) 93 (17.5) O3 11 (6.2) 39 (7.3) Table 3.Multivariable analysis of risk factors for superficial non-ampullary duodenal epithelial tumor
Characteristic Adjusted odds ratio (95% confidence interval) p-value Helicobacter pylori infection status 0.15 Non-infection 1.10 (0.63–1.84) Current/past infection 1 Endoscopic gastric atrophy <0.001 None 5.10 (2.44–8.40) Mild and severe 1 Table 4.Comparison of superficial non-ampullary duodenal epithelial tumor location with respect to major duodenal papilla in gastric mucosal atrophy and Helicobacter pylori infection status
Characteristic Tumor location to major duodenal papilla p-value Oral side (n=93) Anal side (n=84) H. pylori infection status 0.30 Non-infection 43 46 Current/past infection 50 38 Endoscopic gastric atrophy 0.23 None 46 50 Mild and severe 47 34 REFERENCES 1. Hoffman BP, GrayzeL DM. Benign tumors of the duodenum. Am J Surg 1945;70:394–400.
留言 (0)