Endoscopic ultrasound (EUS) has become an essential diagnostic and therapeutic tool. EUS was introduced in 2013 in Indonesia and is considered relatively new. This study aimed to describe the current role of interventional EUS at our hospital as a part of the Indonesian tertiary health center experience.
MethodsThis retrospective study included all patients who underwent interventional EUS (n=94) at our center between January 2015 and December 2020. Patient characteristics, technical success, clinical success, and adverse events associated with each type of interventional EUS procedure were evaluated.
ResultsAltogether, 94 interventional EUS procedures were performed at our center between 2015 and 2020 including 75 cases of EUS-guided biliary drainage (EUS-BD), 14 cases of EUS-guided pancreatic fluid drainage, and 5 cases of EUS-guided celiac plexus neurolysis. The technical and clinical success rates of EUS-BD were 98.6% and 52%, respectively. The technical success rate was 100% for both EUS-guided pancreatic fluid drainage and EUS-guided celiac plexus neurolysis. The adverse event rates were 10.6% and 7.1% for EUS-BD and EUS-guided pancreatic fluid drainage, respectively.
ConclusionsEUS is an effective and safe tool for the treatment of gastrointestinal and biliary diseases. It has a low rate of adverse events, even in developing countries.
Keywords: Drainage; Endoscopic ultrasound-guided fine needle aspiration; Endosonography; Gastrointestinal endosocopy
INTRODUCTION Endoscopic ultrasound (EUS) has emerged as an essential tool for diagnostic and therapeutic procedures. EUS was first developed in the 1980s and is predominantly used as a guide for fine-needle aspiration. EUS allows real-time visualization of many organs and lesions adjacent to the gastrointestinal tract, making it possible to target them while avoiding vascular and other structures in their proximity. This ability paves the way for EUS in the treatment of various gastrointestinal diseases.1 Currently, EUS plays a role in the management of biliary drainage, pancreatic fluid drainage, celiac plexus neurolysis, and targeted chemotherapy and/or radiotherapy. EUS-guided biliary drainage (EUS-BD) has been used in patients with failed cannulation, inaccessible papilla due to obstruction of the stomach or the duodenum, or surgically altered anatomy. EUS is also used in patients with pancreatic pseudocysts (PPCs) and walled-off necrosis (WON) to guide fluid collection. In malignant diseases, EUS plays a role in the delivery of antitumor agents, brachytherapy, tumor ablation, placement of fiducial markers, or laser therapy. EUS-guided celiac plexus neurolysis (EUS-CPN) or celiac plexus blockage is used for pain management in pancreatic cancer and chronic pancreatitis.2 Recent studies have shown that EUS-guided interventions are safe and effective, have fewer complications than percutaneous radiological interventions, and are less invasive than surgical procedures. Currently, EUS-guided drainage of PPCs or WON has a technical success rate above 95%, which is higher than that of conventional transmural drainage (technical success rate of 72%). However, EUS-guided interventions have a higher risk of complications when compared with conventional endoscopy. Depending on the type of intervention, these complications include bleeding, infection, perforation, and other complications.3 EUS is relatively new in Indonesia. It was first introduced in 2013 at Cipto Mangunkusumo General Hospital, which is a tertiary health center and teaching hospital. EUS-BD was first introduced in 2015. Since its introduction, it has been the treatment of choice for biliary drainage in case of failure of endoscopic retrograde cholangiopancreatography (ERCP). Makmun et al.4 demonstrated a technical success rate of 100% for EUS-BD procedures in patients with malignant biliary obstruction and clinical success rates of 78.2% and 100% for EUS-BD via the choledochoduodenostomy route (EUS-CDS) and EUS-BD via the hepaticogastrotomy route (EUS-HGS), respectively. Since then, the role of interventional EUS has expanded in Indonesia. This study aimed to describe the current role of interventional EUS at our hospital as a part of the Indonesian tertiary health center experience. METHODS Study designWe performed a retrospective study at Cipto Mangunkusumo National General Hospital, a tertiary health center and a major referral hospital in Indonesia. All adult patients who underwent interventional EUS at the hospital between January 2015 and December 2020 were included in this study. The profiles of all the patients and the results of these procedures were reviewed. Data were obtained from electronic medical records. The obtained information included patient demographics such as age and sex, diagnosis, type of procedure, laboratory data, technical and clinical success of the procedure, and complications or adverse events. The decision to perform interventional EUS was made based on the judgment of experienced gastroenterologists. The interventional EUS procedures were performed by four experienced endoscopists who performed more than 150 ERCP procedures and 75 EUS-FNA procedures annually.
EUS was performed using an Olympus EU-ME2 ultrasound processor with a curvilinear array ultrasound gastrovideoscope (GF-UCT180; Aizu Olympus Co., Ltd., Tokyo, Japan). For the puncture, we used the Boston Scientific Expect Slimline EUS aspiration needle (Boston Scientific Ltd., Spencer, IN, USA). We also used cystotomes for EUS-BD.
The primary outcomes of this study were the results of these procedures. The results of the procedures were assessed based on their technical and clinical success. Technical success in EUS-BD and EUS-guided pancreatic fluid drainage was defined as successful stent deployment at the end of the procedure. Technical success in other procedures was defined as successful execution of the procedures. Clinical success in EUS-BD was defined as a 50% reduction in total serum bilirubin at 1 week after the procedure. Clinical success in other procedures was defined as a reduction in the symptoms after the procedure. The secondary outcomes were the complications and adverse events associated with the procedures.
The collected data were analyzed using descriptive statistics. Numerical data were presented as mean and standard deviation or median and interquartile range. Categorical data were presented as counts and percentages. All analyses were performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA).
Ethical statementsThis study was approved by the ethical committee of Faculty of Medicine University of Indonesia, Cipto Mangunkusumo National General Hospital (IRB No: 21-05-5019).
RESULTS Patient characteristics Altogether, 94 patients who underwent interventional EUS procedures at our center between January 2015 and December 2020 were included. Among these, 47.9% were male and 52.1% were female. The mean age of the patients was 57±14.2 years. The characteristics of the patients and the types of interventional EUS procedures performed at our center are presented in Table 1. EUS-BD EUS-BD was the most frequent EUS-guided intervention (74 patients, 78.7%). Characteristics of patients who underwent EUS-BD are presented in Table 2. At our center, most of the patients underwent EUS-CDS rather than EUS-HGS. The EUS-CDS procedure for pancreatic head cancer is shown in Figure 1, while the EUS-HGS procedure in a patient with advanced ampullary adenocarcinoma is shown in Figure 2. Plastic stents were used more frequently than metal stents at our center in patients who underwent EUS-BD (Table 1). The overall technical success rate of EUS-BD at our center was 98.6%. The overall clinical success rate was 52%, with no follow-up data available for two patients. The complication rate for EUS-BD was 10.6% (eight adverse events). The adverse events included perforation, pneumoperitoneum, and bile peritonitis (Table 1).No significant differences were observed between EUS-CDS and EUS-HGS in terms of technical success, clinical success, and complication rates. The technical success rate for EUS-CDS was 98.5%, while that for EUS-HGS was 100%. EUS-CDS achieved a clinical success rate of 50%, while EUS-HGS achieved a clinical success rate of 66.7%. All complications occurred during EUS-CDS, with a complication rate of 11.3%. No complications occurred during EUS-BD-HGS.
Indications for EUS-CDS were difficult cannulation and unidentifiable ampulla in 55.9% and 44.1% of the cases, respectively. Pancreatic head cancer was the most common diagnosis (50.0%) among patients who underwent EUS-CDS, followed by ampullary and periampullary tumors (16.2%). The indications for EUS-HGS were difficult cannulation and unidentifiable ampulla in 66.7% and 33.3% of the cases, respectively. Pancreatic mass (33.3%) was the most common diagnosis among patients who underwent EUS-HGS.
EUS-guided pancreatic fluid drainage EUS also plays a role in pancreatic fluid drainage from PPCs and WON. Fourteen patients underwent EUS-guided pancreatic fluid drainage at our center from 2015 to 2020. The characteristics of the patients who underwent EUS-guided pancreatic fluid drainage are presented in Table 1. The technical success rate was 100%. Plastic stents were used in 50% of the patients, metal stents were used in 42.9% of the patients, and no stents were used in 7.1% of the patients. EUS-guided PPC drainage using a lumen-apposing metal stent (LAMS) is shown in Figure 3. Among the 14 patients who underwent EUS-guided pancreatic fluid drainage, stent migration was observed in one patient. EUS-CPN At our center, five EUS-CPN procedures were performed in four patients with pancreatic malignancy and in one patient with chronic pancreatitis. The technical success rate was 100%. Ethanol and Bupivacaine were used as neurolytic agents. Pain relief was observed in all patients. No complications occurred during the procedures. The list of patients who underwent EUS-CPN is presented in Table 2. The degree of pain relief was measured using the visual analog scale score. The median change in the visual analog scale score was 6 points (range, 4–8 points). The EUS-CPN procedure is shown in Figure 4. DISCUSSION At our center, EUS has played various roles in the management of gastrointestinal and biliary diseases. EUS-BD was the most common interventional EUS procedure performed at our center as CDS was the most common access route. The overall technical success rate of EUS-BD at our center was 98.6%. This result is consistent with those from previous studies that reported a technical success rate of approximately 90% for EUS-BD. However, the overall clinical success rate at our center was only 52%. In a previous study at our center, we reported a clinical success rate of 78.3% in achieving biliary drainage.4 This percentage is lower than the clinical success rates reported in other studies. Kanno et al.5 reported a technical success rate of 98% and a clinical success rate of 93% in patients with unresectable malignant biliary obstruction who underwent EUS-BD. Paik et al.6 also reported a technical success rate of 93.8% and a clinical success rate of 90% in a similar patient population. The low clinical success rate at our center might be due to the more frequent use of plastic stents compared to metal stents. The choice between plastic and metal stents usually depends on the etiology of the disease. Plastic stents are used for benign obstructions such as biliary strictures, while metal stents are more commonly used for malignant obstruction.7 Theoretically, metal stents such as self expandable metallic stent, should be more advantageous than plastic stents, since they have a larger diameter that can provide better biliary drainage and better prevention of bile leakage. However, metal stents are more expensive and have a higher risk of stent migration.8Another possible cause of the low clinical success rate at our center is the advanced stage of the disease. Due to the referral system in Indonesia, most of the patients visiting our center have advanced stages of cancer, severe cholangitis, and sepsis. Moreover, we defined clinical success of EUS-BD as a 50% reduction in total serum bilirubin at 1 week after the procedure, while other studies defined clinical success as a 50% reduction in total serum bilirubin at 2–4 weeks after the procedure.
The complication rate of EUS-BD was 10.6% at our center. This is relatively low compared to the complication rates reported in previous studies. In previous studies, the complication rate of EUS-BD varied from as low as 6.3% to as high as 35%.2,6,9 Possible complications of EUS-BD include perforation, bile leakage, bleeding, and stent migration or dysfunction. At our center, the most common adverse event was pneumoperitoneum. However, this adverse event was self-limiting and did not require surgical treatment. Risk factors for complications in EUS-BD include the use of plastic stents, track dilatation, and the use of gases other than carbon dioxide. The safety of the procedure also depends on the experience of the operator and the assistant. Bile leakage, which can lead to bile peritonitis, occurs more frequently in patients with plastic stents than in those with metal stents. Kawakubo et al.10 reported that the percentage of bile leakage was 11% in patients with plastic stents and 4% in those with metal stents. EUS is also used for pancreatic fluid drainage of PPCs and WON at our center. The technical success rate of EUS-guided drainage at our center was 100%. The success rates in other studies varied from 70% to 100%. Kumta et al.11 reported a technical success rate of 98.4% and a clinical success rate of 92.6% in patients with pancreatic fluid collections (PFCs) who underwent EUS-guided drainage using LAMS. EUS-guided drainage is comparable to surgical drainage12 and has a higher success rate than percutaneous drainage.13 Keane et al.14 reported no significant difference in the success rates of EUS-guided drainage between patients with PPCs and those with WON (p=0.77). Adverse event rates in other studies varied from 1.5% to 14.3%.12,15 Adverse events associated with EUS-guided pancreatic drainage include stent migration, pneumoperitoneum, perforation, gastrointestinal bleeding, pneumothorax, and aspiration pneumonia.14 In a study by Xie et al.,13 EUS-guided drainage was associated with a significantly lower number of reinterventions (p=0.047) and a significantly shorter length of hospital stay (pp=0.035 and p=0.017, respectively).12 Only one patient experienced an adverse event (stent migration) at our center. However, no data are available regarding PFC recurrences and reinterventions after EUS-guided pancreatic fluid drainage at our center. The technical success rate for EUS-CPN was 100% at our center and pain relief was achieved in patients with pancreatic cancer and chronic pancreatitis. Puli et al.16 performed a meta-analysis including eight studies on pain due to pancreatic cancer and nine studies on pain due to chronic pancreatitis. They reported that the pooled proportion of patients with pain relief after the procedure was as high as 80.12% (95% confidence interval [CI], 74.47–85.22) among patients with pancreatic cancer and 59.45% (95% CI, 54.51–64.30) among those with chronic pancreatitis. Kaufman et al.17 also reported that EUS-CPN was effective in managing pain associated with pancreatic cancer in 72.45% of the patients.In conclusion, EUS plays various roles in the treatment of gastrointestinal and biliary diseases. As a tertiary center in Indonesia, our hospital has implemented interventional EUS as a therapeutic approach in many cases. EUS-BD is the treatment of choice for biliary drainage in case of failure of cannulation during ERCP. EUS-guided drainage is also considered a safe and effective approach for PFC from PPCs and WON. EUS also has an extensive role in the management of malignant diseases, which includes reducing cancer-associated pain using EUS-CPN.
Fig. 1.Endoscopic ultrasound biliary drainage via the choledochoduodenostomy route in advanced pancreatic head cancer. (A) Puncture of the dilated common bile duct with a 19-gauge needle. (B) Cholangiography and wire placement. (C) Fistulization using a cystotome. (D) Placement of a plastic stent.
 Fig. 2.
Fig. 2.
Endoscopic ultrasound biliary drainage via the hepaticogastrotomy route (patient with advanced ampullary adenocarcinoma. (A) Puncture of the dilated intra-hepatic bile duct with a 19-gauge needle. (B) Cholangiography, wire placement, and fistulization. (C, D) Deployment of a fully covered self-expandable metal stent.
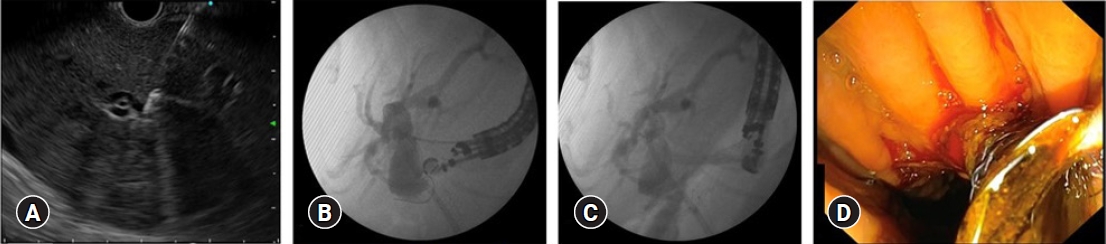 Fig. 3.
Fig. 3.
Endoscopic ultrasound-guided pseudocyst drainage using a lumen-apposing metal stent. (A) Puncture of the lesion under endoscopic ultrasound guidance with a 19-gauge needle. (B) Insertion of a guidewire into the cyst. (C) Deployment of a lumen-apposing metal stent.
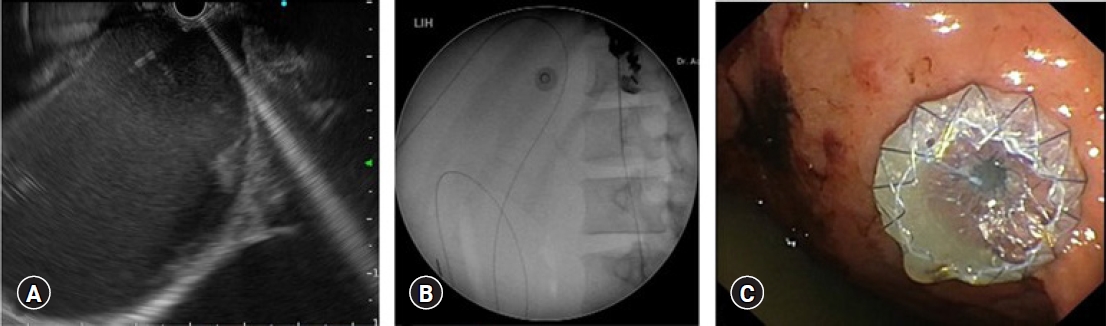 Fig. 4.
Fig. 4.
Endoscopic ultrasound-guided celiac plexus neurolysis in patient with pancreatic head cancer. (A) Identification of the celiac artery. (B) Puncture with a 19-gauge needle with initial injection of 2 mL bupivacaine followed by injection of absolute alcohol solution around the celiac trunk.
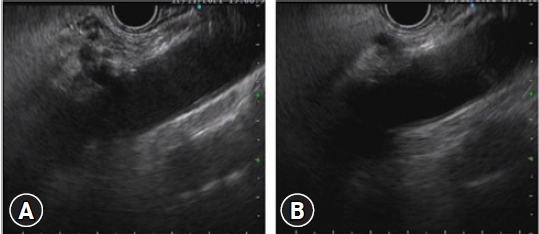 Table 1.
Table 1.
Characteristics of patients who underwent interventional EUS procedures
Characteristic Total (n=94) EUS-BD (n=75) EUS-guided pancreatic drainage (n=14) Sex Male 45 (47.9) 35 (46.7) 8 (57.1) Female 49 (52.1) 40 (53.3) 6 (42.9) Age (yr) <60 53 (56.4) 36 (48.0) 13 (92.9) ≥60 41 (43.6) 39 (52.0) 1 (7.1) Primary disease Tumor of the head of pancreas 38 (40.4) 35 (46.7) - Ampulla/periampullary tumor 12 (12.8) 12 (16.0) - Pancreatic pseudocyst 9 (9.6) - 9 (64.3) Pancreatic mass 6 (6.4) 6 (8.0) - Duodenal mass 6 (6.4) 6 (8.0) - Obstruction or stenosis of CBD 5 (5.3) 5 (6.7) - Metastasis from other organs 4 (4.3) 4 (5.3) - Walled-off necrosis 2 (2.1) - 2 (14.3) Klatskin tumor 2 (2.1) 2 (2.7) - CBD stone 2 (2.1) 2 (2.7) - Pancreatic cyst 2 (2.1) - 1 (7.1) Chronic pancreatitis 2 (2.1) - 1 (7.1) Cholangiocarcinoma 2 (2.1) 2 (2.7) - Pyogenic abscess of the pancreas 1 (1.1) - 1 (7.1) Biliary sepsis 1 (1.1) 1 (1.3) - Stents Plastic stent 62 (66.0) 55 (73.3) 7 (50.0) Metal stent 26 (27.6) 20 (26.7) 6 (42.9) No stent 6 (6.4) - 1 (7.1) Technique NA NA Choledochoduodenostomy 68 (90.7) Hepaticogastrotomy 6 (8.0) Rendezvous 1 (1.3) Indication NA NA Difficult cannulation 43 (57.3) Unidentifiable ampulla 32 (42.7) Complications Overall 9 (9.6) 8 (10.6) 1 (7.1) Pneumoperitoneum 6 (6.4) 6 (8.0) - Bile peritonitis 1 (1.1) 1 (1.3) - Perforation 1 (1.1) 1 (1.3) - Stent migration 1 (1.1) - 1 (7.1) Table 2.List of patients who underwent endoscopic ultrasound-guided celiac plexus neurolysis
Patient Sex Age (yr) Diagnosis Technical success Clinical success 1 Female 41 Cancer of the head of pancreas Yes Yes 2 Male 48 Cancer of the head of pancreas Yes Yes 3 Female 47 Chronic pancreatitis Yes Yes 4 Male 66 Cancer of the head of pancreas Yes Yes 5 Female 36 Serous cystic neoplasm Yes Yes REFERENCES 1. Friedberg SR, Lachter J. Endoscopic ultrasound: current roles and future directions. World J Gastrointest Endosc 2017;9:499–505.






留言 (0)