Pyoderma gangrenosum is an inflammatory skin disease, resulting from a process by which the body’s immune system malfunctions. It may progress aggressively unless early treatment is administered. However, early diagnosis is difficult because other diseases need to be ruled out. The incidence of the disease is in 3–10 patients per million population per year in Western countries. However, there have been lack of reports on Asian cases of pyoderma gangrenosum and limited data is available on treatment outcomes. In this observational study, the authors aimed to document clinical and microscopic features of all patients diagnosed with pyoderma gangrenosum between the years of 1999 to 2019. By reviewing medical records, 53 cases of this disease were detected, of which 37 were men and 16 were women. Their mean age was 43.3±18.5 years. The most frequently affected area was the lower legs (60.4%). Thirty patients had other diseases and the most common were cancers (24.5%). Nine (17.0%) patients experienced new occurrence of pyoderma gangrenosum after a mean period of 6.87±18.5 months. The type of microscopic features did not correlate with the clinical outcome. Patients with underlying haematologic disease (which primarily affect the blood and blood-forming organs) and pyoderma gangrenosum presenting as fluid-filled lesions showed earlier new occurrence of disease than others.
IntroductionPyoderma gangrenosum is a rare, non-infectious inflammatory neutrophilic dermatosis and often presents as painful ulcers.1 Ever since Brunsting et al. renamed this entity as “pyoderma gangrenosum,”2 many cases have been reported, warning about its rapid and aggressive course. However, even at present, new information on the disease is helping to update knowledge of this entity. The estimated incidence of pyoderma gangrenosum in western countries is 3–10 patients per million population per year.3 There are various clinical manifestations of pyoderma gangrenosum, with five types of pyoderma gangrenosum having been identified: classic (ulcerative), bullous, pustular, vegetative and post-operative.4 Nonetheless, few diagnostic pathologic features have been described in this entity. Due to its clinical variance and lack of reports, clinicians often face diagnostic difficulties with pyoderma gangrenosum because its diagnosis is based on the exclusion of other diseases.
Early immunosuppressant treatment can halt aggressive progression and thus, early diagnosis by awareness of pyoderma gangrenosum is essential. However, limited data is available on treatment outcomes and prognosis of pyoderma gangrenosum. There has been a paucity of reports on Asian cases of pyoderma gangrenosum as well, despite its possible ethnic differences.
In this study, we evaluated the clinical and pathologic characteristics of 53 patients with pyoderma gangrenosum. Treatment responses to various therapeutic agents were comprehensively reviewed. Clinical outcomes were also analysed to investigate the prognostic factors of pyoderma gangrenosum.
MethodsPatients were selected after retrospectively reviewing patient charts at Asan Medical Center (Seoul, South Korea) between March 1999 and December 2019. The institutional review board of the Asan Medical Center approved this study. Written informed consent was obtained from all patients. We aimed to investigate clinicopathologic and prognostic data, along with therapeutic response in patients with pyoderma gangrenosum. We included patients with a definitive diagnosis confirmed through clinicohistopathologic examination. Two different dermatopathologists (YJK and KHL) confirmed the exclusion of other histopathological diagnoses using uniform criteria. Since there are no specific diagnostic tests for pyoderma gangrenosum, proposed diagnostic criteria were used [Box 1]. Discrepancies were resolved by joint evaluation. For cases where information was not well described, three dermatologists (YJK, KHL and WJL) who participated in the study reviewed clinical data together and decided whether to include them, according to the majority opinion. From the medical records, we collected data on age, sex, onset, size and location of lesions, trauma history, symptoms and signs, clinical subtypes, underlying diseases, treatment and outcome. We defined delayed cases as all cases in which pyoderma gangrenosum was not the first top three diagnostic impressions in the clinicians’ initial examination. Multiple lesions were defined as those with two or more lesions rather than a single one. When accompanied by clear serous material, it was documented as oozing. Active bleeding or malodorous discharge was not considered oozing. With regard to histologic analysis, we defined a granuloma as the proliferation and focal aggregation of histiocytic cells. Neutrophilic infiltration was classified into absent, mild, moderate and severe.5
Box 1: Inclusion diagnostic criteria for pyoderma gangrenosum in this study
Major criteria*
Sudden onset of a painful lesion with the characteristic pyoderma gangrenosum morphology (ulcerative, bullous, pustular, or vegetative) in a patient who does not have fever, significant toxaemia, or relevant drug intake
Histopathologic exclusion of malignancy and infective organisms by special histologic studies and negative tissue cultures. Significant vascular stasis and occlusion also excluded.
Minor criteria
Occurrence in an individual with systemic diseases, such as inflammatory bowel disease (IBD), monoclonal gammopathy, haematologic disease, inflammatory arthritis, malignancy, hidradenitis suppurativa
Classic histologic pyoderma gangrenosum findings according to each subtype**: Abscess formation with intense dermal neutrophilic infiltration in ulcerative pyoderma gangrenosum. Epidermal bulla and/or epidermal necrosis and marked upper dermal oedema with prominent neutrophils in bullous pyoderma gangrenosum. Dermal neutrophilic infiltration with subepidermal oedema and epidermal neutrophil infiltration in pustular pyoderma gangrenosum. The presence of pseudoepitheliomatous hyperplasia, sinus tract formation and the presence of palisading granulomas with focal dermal neutrophilic abscesses in vegetative pyoderma gangrenosum.
*Both major criteria and at least one minor criterion should be fulfilled to diagnose as pyoderma gangrenosum.
**The histopathologic findings are not diagnostic but can be supportive of the diagnosis of pyoderma gangrenosum to rule out alternative diagnoses.
Response to treatment was evaluated one month after initial treatment, as either a complete remission with extinction of lesions, partial remission with improvement, stable disease without improvement or aggravation and progression of disease state with aggravation and/or change to second line treatment because of insufficient response to first line treatment. Progression-free period was measured from the date of diagnosis until the date of local or regional aggravation or recurrence on last follow-up. Recurrence was defined as occurrence of lesions two weeks or more after being confirmed as complete remission. The Kaplan–Meier method was used to plot survival curves and a Cox proportional hazard model was used to identify the factors associated with progression-free period. Pearson’s Chi-square test and Fisher’s exact test was used to compare the categorical variables. One-way analysis of variance and Kruskal-Wallis test with post hoc was performed to compare the continuous variables among different subtypes. All data were statistically analysed using SPSS version 18.0 (SPSS Inc., Chicago, IL); P < 0.05 was considered to be statistically significant.
Results Clinicopathological characteristics of pyoderma gangrenosumA total of 76,080 skin biopsy reports were reviewed and 53 (0.07%) cases of pyoderma gangrenosum were found between March 1999 and December 2019, of which 37 were men and 16 were women [Table 1]. Patient ages at initial diagnosis ranged from 12 to 81 years (mean ± standard deviation: 43.3 ± 18.5 years). The period of onset to diagnosis ranged from three days to 60 months (mean ± SD 6.91 ± 14.5 months). The mean size of skin lesion was 5.09 ± 4.13 cm. There were 25 cases (47.2%) of the ulcerative type, 12 cases (22.6%) of the bullous type, 11 cases (20.7%) of the vegetative type and two cases (3.8%) of the pustular type [Figure 1]. Three cases (5.7%) were of the post-operative type, all occurring after total colectomy with ileostomy, resection of small intestine and right lower lobe wedge resection.
Table 1:: Clinical features of pyoderma gangrenosum
Characteristics Pyoderma gangrenosum Total patients, n 53 Age, year (mean±standard 43.3±18.5 deviation) Sex ratio (male: female) 37 (69.8%):16 (30.2%) Onset to diagnosis (month) 6.91±14.5 Delayed diagnosis 14 (26.4%) Mean size (cm) 5.09 Location Lower extremities 32 (60.4%)
Figure 1a:: Ulcerative type, which is the most common form of pyoderma gangrenosum, showing a rapidly enlarged deep ulceration with erythematous borders
Export to PPT
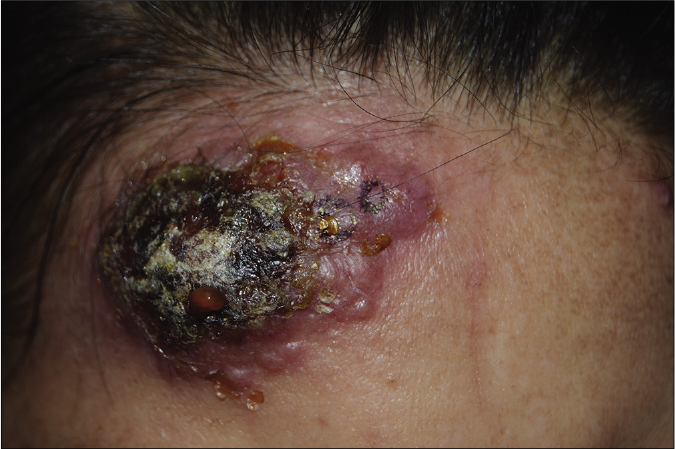
Figure 1b:: Bullous pyoderma gangrenosum, a rapidly developed bulla with crusts and central necrosis
Export to PPT
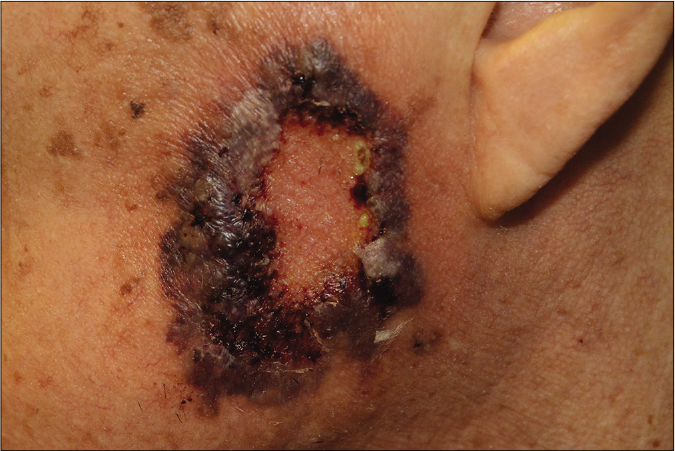
Figure 1c:: Pustular pyoderma gangrenosum, a rare type of pyoderma gangrenosum presenting with grouped pustular eruption
Export to PPT
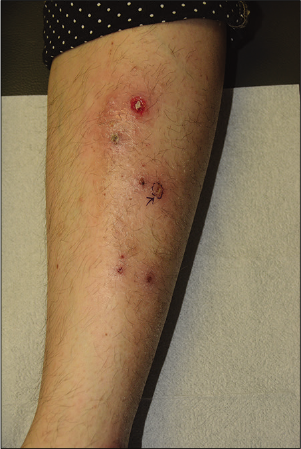
Figure 1d:: Vegetative pyoderma gangrenosum, a superficial and non-aggressive type of pyoderma gangrenosum, showing shallow erosion
Export to PPT
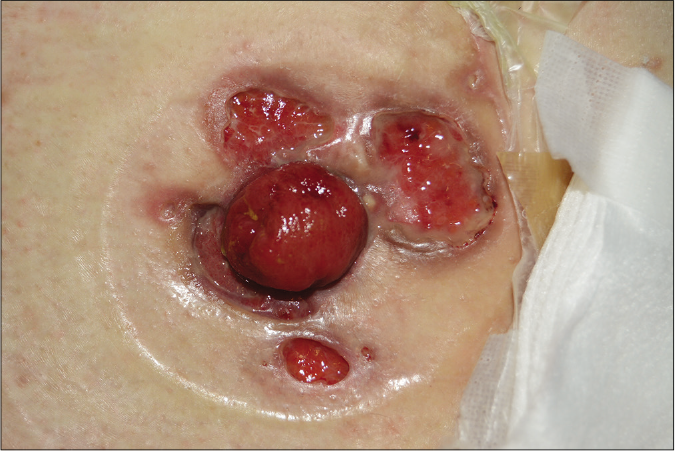
Figure 1e:: Post-operative pyoderma gangrenosum, an atypical type of pyoderma gangrenosum located on the fistulous tracts and the surrounding areas
Export to PPT
Lower extremities were the most affected site (n = 32, 60.4%). Pain/tenderness (n = 42, 79.2%) was the most common initial symptom/sign of skin lesion. Twenty cases (37.7%) had an antecedent history of trauma at the site of the skin lesion. Eight cases (15.1%) had underlying haematologic disorders. Five cases (9.4%) had fever at the time of initial diagnosis. Ulcerative colitis was observed in four cases (7.5%) and Crohn’s disease in six cases (11.3%). Associated malignancies were found in five cases (9.4%), most commonly in early gastric cancer (n = 4, 7.5%) followed by ovarian cancer (n = 1, 1.9%).
When we analysed histopathologic characteristics, all cases revealed neutrophil infiltrations at various levels [Figure 2]. However, the degree of neutrophilic infiltration was not significantly different in the subtypes of pyoderma gangrenosum (P = 0.277). Epidermal ulceration (n = 33, 62.3%) was the second most common feature of pyoderma gangrenosum. However, these features were not significantly correlating with clinical outcomes.
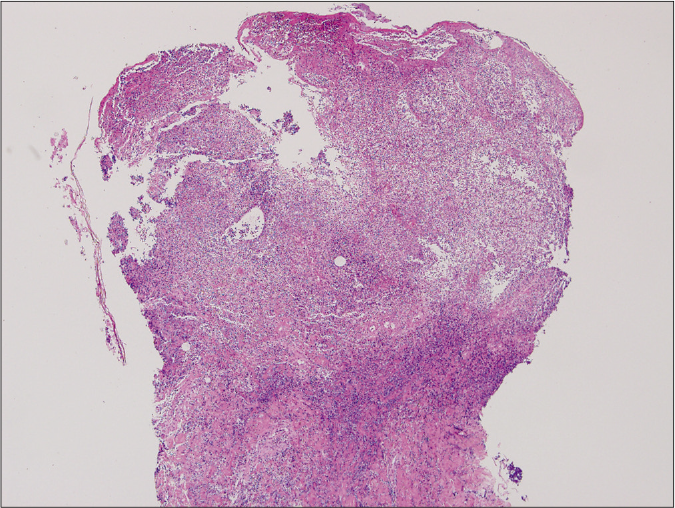
Figure 2a:: Ulcerative type revealed diffuse epidermal necrotic ulcer with neutrophilic infiltrations and dermal granulation tissue (haematoxylin and eosin stain, ×125)
Export to PPT
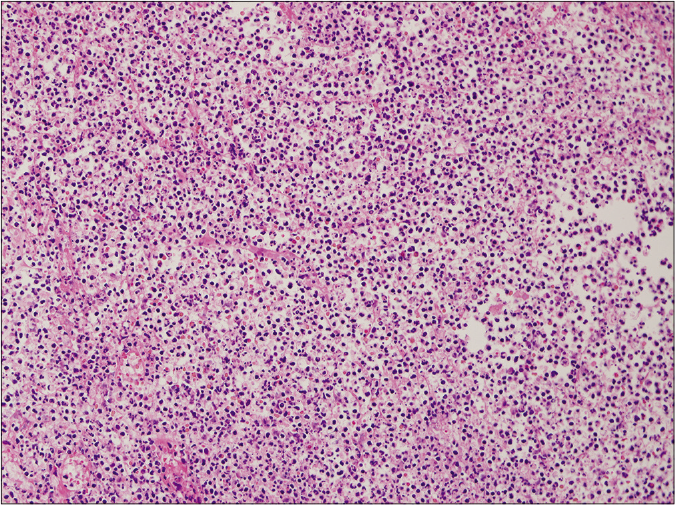
Figure 2b:: Ulcerative type, on high power (haematoxylin and eosin stain, ×2000)
Export to PPT
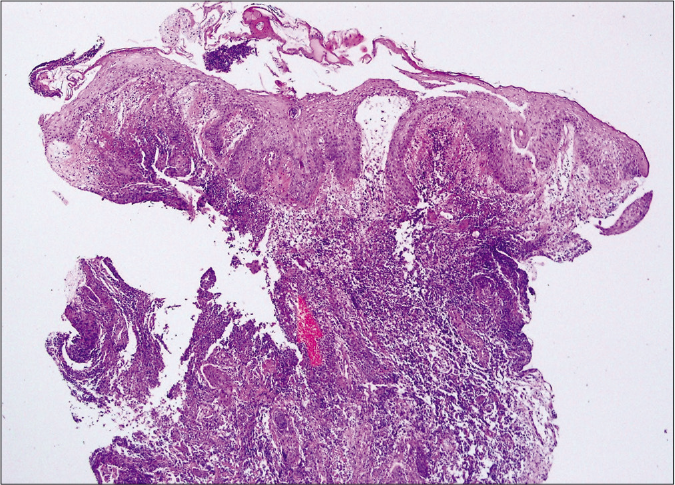
Figure 2c:: Bullous type showed epidermal ulcers with subepidermal bulla and upper dermal oedema. Diffuse dermal massive neutrophilic and eosinophilic infiltrations were also noted. (haematoxylin and eosin stain, ×400)
Export to PPT
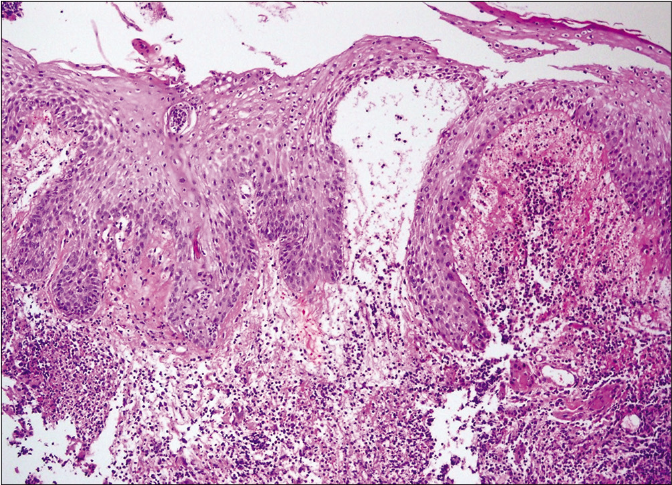
Figure 2d:: Bullous type on higher magnification (haematoxylin and eosin stain, ×1000)
Export to PPT
Comparative clinicopathologic subgroup analysisOf the 53 patients diagnosed with pyoderma gangrenosum, comparative subgroup analysis was performed. The presence of trauma history was significantly different among subtypes (P = 0.023), being highest in the ulcerative variety, whereas other clinical variables were not different among subtypes [Table 2]. Histopathologic analysis showed that the presence of granulation tissue and granuloma was significantly different among subtypes (P = 0.012) with 7 of 12 patients (58.3%) with bullous type exhibiting these [Table 2]. There was no statistical difference among subgroups regarding other histopathologic features.
Table 2:: Clinical and histopathological features of pyoderma gangrenosum according to subtypes
Clinical features Ulcerative (n=25) Bullous (n=12) Vegetative (n=11) Pustular (n=2) Post-operative (n=3) P-value Age, year (mean±SD) 44.2±17.7 50.8±16.5 34.5±19.2 54.5±34.6 30.0±8.67 0.145 (Age≥60: Age<60) (6:19) (4:8) (2:9) (1:1) (0:3) 0.659 Sex ratio (male: female) 16:9 (64%:36%) 9:3 (75%:25%) 8:3 (73%:27%) 2:0 (100%:0%) 2:1 (67%:33%) 0.911 Mean size of initial lesion (cm) 5.32±4.36 5.89±5.24 3.91±2.42 4.50±0.70 4.67±4.61 0.829 Duration of skin lesion (months) 10.70±20.93 3.25±5.66 9.54±21.03 1.62±1.94 1.16±0.28 0.701 Time to diagnosis (months) 9.74±18.40 2.92±4.76 7.36±13.95 1.62±1.94 1.16±0.28 0.639 Delayed diagnosis (%) 9 (36.0) 2 (16.7) 3 (27.3) 0 0 0.588 Location (%) Lower extremities 19 (76.0) 8 (66.7) 4 (36.4) 1 (50.0) 0 0.120 Head and neck 3 (12.0) 1 (8.3) 4 (36.4) 1 (50.0) 0 0.127 Trunk 0 2 (16.7) 3 (27.3) 0 3 (100.0) 0.063 Upper extremities 3 (12.0) 1 (8.3) 0 0 0 0.817 Multiple lesions (%) 13 (52.0) 8 (66.7) 4 (36.4) 0 2 (66.7) 0.393 Trauma history (%) 10 (40.0) 1 (8.3) 5 (45.5) 1 (50.0) 3 (100.0) 0.023* Past medical history (%) Haematologic malignancy 2 (8.0) 4 (33.3) 1 (9.1) 0 1 (33.3) 0.181 Other solid organ cancers 2 (8.0) 2 (16.7) 0 1 (50.0) 0 0.329 Inflammatory bowel disease 6 (24.0) 2 (16.7) 1 (9.1) 0 1 (33.3) 0.754 Aortic aneurysm or dissection 2 (8.0) 1 (8.3) 0 0 0 0.923 Behcet’s disease 2 (8.0) 0 0 0 0 0.782 Rheumatoid arthritis 0 1 (8.3) 1 (9.1) 0 0 0.317 Autoimmune pancreatitis 1 (4.0) 0 0 0 0 0.764 Primary sclerosing cholangitis 0 1 (8.3) 0 0 0 0.415 Spondyloarthropathy 1 (4.0) 0 0 0 0 0.764 Histopathologic features (%) Epidermal ulceration 14 (56.0) 9 (75.0) 6 (54.5) 1 (50.0) 3 (100) 0.514 Perivascular infiltration 8 (32.0) 7 (58.3) 2 (18.2) 0 0 0.177 Vasculitis 4 (16.0) 1 (8.3) 3 (27.2) 0 0 0.768 Granulation tissue 4 (16.0) 7 (58.3) 1 (9.1) 0 2 (66.7) 0.012* Granuloma 2 (8.0) 7 (58.3) 2 (18.2) 0 0 0.012* Haemorrhage 4 (16.0) 3 (25.0) 1 (9.1) 0 0 0.865 Pseudoepitheliomatous hyperplasia 4 (16.0) 4 (33.3) 1 (9.1) 0 0 0.539 Neutrophil infiltration 0.277 Minimal 5 (20.0) 1 (8.3) 4 (36.4 1 (50.0) 1 (33.3) Mild 10 (40.0) 9 (75.0) 2 (18.2) 1 (50.0) 1 (33.3) Moderate 6 (24.0) 0 3 (27.2) 0 0 Severe 4 (16.0) 2 (16.7) 2 (18.2) 0 1 (33.3) Lymphocyte infiltration 4 (16.0) 1 (8.3) 3 (27.2) 0 0 0.686 Plasma cell infiltration 1 (4.0) 1 (8.3) 2 (18.2) 0 0 0.498 Eosinophil infiltration 1 (4.0) 4 (33.3) 0 0 0 0.079 Mixed cell inflammation 7 (28.0) 5 (41.6) 4 (36.4) 1 (50.0) 0 0.678The subgroup aged <60 years presented with multiple lesions more commonly as compared to others [P = 0.004, Table 2]. Underlying malignancy was also more frequent in this subgroup (P = 0.005). Other clinical variables including progression-free period were not significantly different. When we further subdivided the patients into smaller age groups of 20-year units for further analysis, involvement of the upper extremities (P = 0.007), multiple skin lesions (P = 0.002) and underlying malignancy (P = 0.008) was significantly different. Since 37 out of 53 patients (69.8%) were male, a subgroup analysis was also performed between men and women; however, no statistically significant difference in clinical features was observed.
Treatment outcome to variable therapeutic agentsOf the 53 patients diagnosed with pyoderma gangrenosum, 22 cases (41.5%) received steroids as initial treatment and 15 of those cases (68.2%) showed at least a partial response. Treatment response and related side effects to each of the main treatment modalities are summarised in Table 1.
Clinical course of pyoderma gangrenosum according to their clinicopathologic characteristicsA total of 53 patients were included, while 17 patients (32.1%) were lost to follow-up. Forty-five patients survived at the last follow-up. Among the eight patients who died, two patients died due to myelodysplastic syndrome, two patients due to leukaemia and two patients due to septic shock. Cerebral infarction and aplastic anaemia were found as the cause of death in the other two patients, respectively. Nine (17.0%) patients had recurrence and three (33.3%) of them eventually died. The time interval from diagnosis to recurrence was between two weeks to 105 months (6.87 ± 18.50 months). Of note, ten cases (18.9%) underwent debridement and three of those cases (30.0%) showed prompt recurrence within one month. Only three patients (5.7%) had complete remission. A comparison between the groups which showed complete remission plus those with partial remission and the group of stable disease plus progressive disease showed that underlying malignancy was more frequently associated in the group of stable disease and progression disease [P = 0.058, Table 3].
Table 3:: Clinical response to initial treatment of pyoderma gangrenosum according to the clinical features
Clinical response to initial treatment, n (%) P-value‡ Complete remission (n = 3) Partial remission (n = 17) Stable disease (n = 24) Progression disease (n = 9) Progression free period (median, interquartile range) CR: 6.00, 4.0–35.0 PR: 2.0, 1.0–5.0 SD: 1.0, 0.5–1.0 PD: 6.0, 3.0–9.0 CR + PR: 2.5, 1–6 SD + PD: 1.0, 0.5–3.0 Age 0.471 Aged ≥ 60 1 (33.3%) 5 (29.4%) 5 (20.8%) 2 (22.2%) Aged < 60 2 (66.7%) 12 (70.6%) 19 (79.2%) 7 (77.8%) Sex 0.208 Male 1 (33.3%) 15 (88.2%) 14 (58.3%) 7 (77.8%) Female 2 (66.7%) 2 (11.8%) 10 (41.7%) 2 (22.2%) Mean size of initial lesion (cm) 2.00 ± 1.00 6.21 ± 5.06 4.20 ± 2.26 6.33 ± 5.85 0.501 Duration of skin lesion (month) 2.00 ± 2.59 13.81 ± 24.54 6.89 ± 15.07 1.33 ± 0.79 0.402 Time to diagnosis (month) 2.00 ± 2.59 12.40 ± 21.48 5.72 ± 10.56 1.33 ± 0.79 0.310 Delayed diagnosis 1 (33.3%) 4 (23.5%) 6 (25.0%) 3 (33.3%) 0.871 Subtype Ulcerative 1 (33.3%) 10 (58.8%) 13 (54.2%) 1 (11.1%) 0.332 Bullous 1 (33.3%) 4 (23.5%) 2 (8.3%) 5 (55.6%) 0.623 Vegetative 1 (33.3%) 2 (11.8%) 7 (29.2%) 1 (11.1%) 0.399 Pustular 0 0 1 (4.2%) 1 (11.1%) 0.328 Post-operative 0 1 (5.9%) 1 (4.2%) 1 (11.1%) 0.774 Anatomical location Head and neck 1 (33.3%) 3 (17.6%) 4 (16.7%) 1 (11.1%) 0.585 Upper extremities 1 (33.3%) 1 (5.9%) 2 (8.3%) 0 0.456 Trunk 0 1 (5.9%) 3 (12.5%) 4 (44.4%) 0.185 Lower extremities 1 (33.3%) 12 (70.6%) 15 (62.5%) 4 (44.4%) 0.772 Multiple lesions 2 (66.7%) 6 (35.3%) 13 (54.2%) 6 (66.7%) 0.211 Trauma history 2 (66.7%) 4 (23.5%) 10 (41.7%) 4 (44.4%) 0.397 Underlying malignancy 0 5 (29.4%) 3 (12.5%) 5 (55.6%) 0.058†When prognosis was correlated with progression-free period, it was significantly shorter in the pyoderma gangrenosum with haematologic disorder group (P = 0.002) and the bullous subtype (P = 0.014) [Figures 3a and 3b]. The pyoderma gangrenosum group in which the size of the initial skin lesion was >4 cm also showed a shorter progression-free period which was however of a borderline significance (P = 0.050) [Figure 3c]. Other clinical variables and histopathologic features did not affect progression-free period [Table 2].
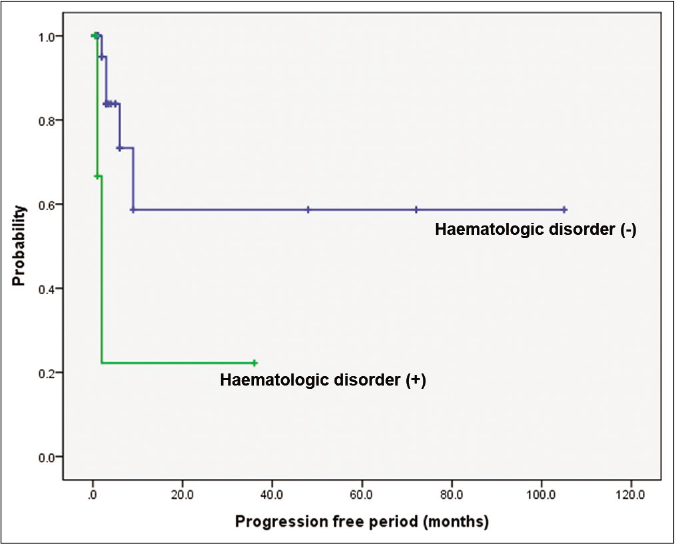
Figure 3a:: Comparison of survival according to underlying haematologic disorders and bullous type. Difference in progression-free period depending on underlying haematologic disorders. Worse progression-free period in patients with haematologic disorders (P = 0.002)
Export to PPT
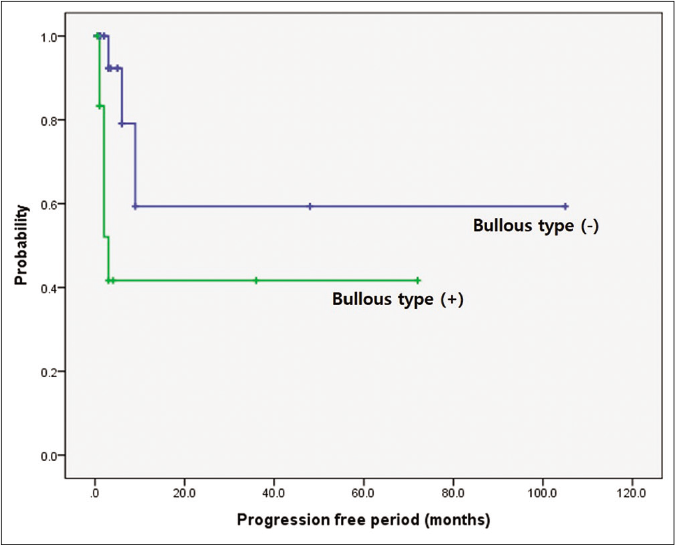
Figure 3b:: Comparison of survival according to underlying haematologic disorders and bullous type. Difference in progression-free period depending on bullous subtype. Worse progression-free period in patients with bullous type (P = 0.014)
Export to PPT
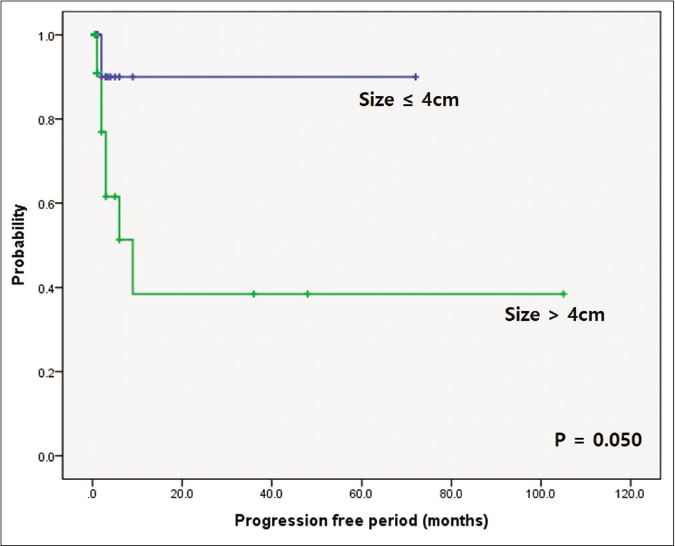
Figure 3c:: Comparison of survival according to underlying haematologic disorders and bullous type. Difference in progression-free period depending on initial skin lesion. Worse progression-free period in patients with initial skin lesion over 4 cm (P = 0.050)
Export to PPT
DiscussionIn our Korean cases, male predominance (M : F ratio = 37/16 vs. 9/27) and younger mean age (43.3 ± 18.5 years vs. 50.9 ± 19.0) were noted as opposed to the Western and Japanese reports.6-8 With regard to pyoderma gangrenosum subtypes, ulcerative type was the most common, which was consistent with previous reports.9 However, ulcerative type (49.1% vs. 78.9%) was less frequent and bullous type (24.5% vs. 1.9%) was more frequent in comparison to the observations in other case series.8,9 Ten cases (18.9%) were associated with inflammatory bowel diseases (Ulcerative colitis and Crohn’s disease), which was less frequent than the incidence rate reported from other countries.10 Brodell et al. found that pyoderma gangrenosum patients of the African– American race had a higher likelihood of development of inflammatory bowel diseases, implying ethnic differences in the clinical manifestations of pyoderma gangrenosum.10
Age-focused workup of patients with pyoderma gangrenosum was suggested by Ashchyan et al.11 as a higher prevalence of solid organ and haematologic malignant disorders was noted in patients aged ≥65 years. They also found a higher prevalence of inflammatory bowel diseases in patients aged <65 years.
However, only five Asians were included among 356 cases in Aschyan’s study. In our study of the Korean population, seven cases aged ≥65 years were observed, and their prognosis was not significantly different than that of others. Patients aged ≥65 years showed no significant association with malignancy (P = 0.343), inflammatory bowel diseases (P = 0.469) or rheumatoid arthritis (P = 0.117) in contrast to Aschyan’s study. Therefore, we concluded that different Asian groups may have ethnical differences and older age does not seem to be a critical prognostic factor for Korean cases at least.
Notably, early gastric cancers were detected in four (7.5%) patients with pyoderma gangrenosum in this study. Gastric cancer is one of the most frequent cancers in the Korean population; however, considering that the incidence of gastric cancer in the general population is only 35.4 cases/100000 persons in the year of 2016,12,13 the higher frequency of gastric cancer in pyoderma gangrenosum is worth remarking upon. Supporting this theory of an increased predisposition to gastric cancer in pyoderma gangrenosum is that it has been reported as the first and isolated clinical manifestation of advanced gastric cancer.14 With regard to pathophysiological considerations, the level of pro-inflammatory cytokines, including tumour necrosis factor α, interleukin-1, interleukin-6 and interleukin-8, were elevated in pyoderma gangrenosum patients,15 and tumour necrosis factor-α inhibitor has been an effective treatment option for pyoderma gangrenosum.16 As the overexpression of tumour necrosis factor-α may lead to an increased gastric cancer risk,17 pyoderma gangrenosum and gastric cancer may be associated through a tumour necrosis factor-α related inflammatory cascade. Conventionally, studies with pyoderma gangrenosum reported other solid tumours, mainly breast cancer, renal caner and colorectal cancer.18-20 A relatively higher incidence of gastric cancer in Korean pyoderma gangrenosum cases is an interesting finding although further investigation is warranted.
A relatively higher frequency of bullous pyoderma gangrenosum in our study is also notable. Bullous pyoderma gangrenosum was found in 13 (24.5%) patients. Only nine cases with bullous pyoderma gangrenosum among 473 cases with pyoderma gangrenosum (1.9%) were observed in a Japanese epidemiologic study.8 Another Japanese regional long-term study also revealed a lower frequency of bullous pyoderma gangrenosum at 6.5%.7 Because up to 70% of bullous pyoderma gangrenosum cases are associated with underlying haematologic disorders,21 clinicians should be cautious when they encounter bullous-type pyoderma gangrenosum. Duguid et al. reported that 75% pyoderma gangrenosum and acute myeloid leukaemia patients died within one year after the manifestation of skin lesions.22 Five cases (38.5%) had haematologic disorders in our study and the presence of haematologic disorders was associated with a frequent recurrence and poor survival rate, consistent with the findings of previous studies.21-24 Due to the association between bullous pyoderma gangrenosum and haematologic disorders, patients with bullous pyoderma gangrenosum should be followed up closely.
Three of 53 cases showed a history of abdominal aortic aneurysm characterised by atherosclerotic changes with chronic inflammation of aortic walls. Two cases with Behcet’s diseases were also observed. The activation of matrix metalloproteinases (MMPs), particularly matrix metalloproteinase-2 and matrix metalloproteinase-9, was the main pathogenesis of both diseases.25 Marzano et al. found that immunoreactivities of matrix metalloproteinase-2 and matrix metalloproteinase-9 were significantly high in pyoderma gangrenosum.26 In light of these findings, although rare, clinicians should be aware of the possibility of underlying fatal aneurysmal diseases, particularly in pyoderma gangrenosum cases.
The histopathologic findings in pyoderma gangrenosum are not specific. Nevertheless, a skin biopsy is recommended in most cases to exclude the other diseases, such as infections and malignancy.3,7,15,21 We initially aimed to find a correlation between the clinical subtype and histopathologic features. Histologic features with granulation tissue were frequently found in post-operative and bullous types. In addition, bullous type pyoderma gangrenosum frequently exhibited granulomas. For these features, the response to initial treatment and the prognosis related to recurrence were analysed for any correlation but no significant difference was found. Since pyoderma gangrenosum has been considered as one of neutrophilic dermatosis,3,27 we also initially thought that the level of neutrophil infiltration may have prognostic significance. However, no significant difference was found between the level of neutrophil infiltration and clinical outcome. Although there are only a few studies on this aspect, we considered it difficult to predict the prognosis of pyoderma gangrenosum based on pathologic features.
The first line of the treatment of pyoderma gangrenosum is an empirical systemic steroid administration because of the lack of controlled studies to date.3,7,15,21 However, delayed diagnosis interferes with the prompt use of systemic steroids. In this study, the use of systemic glucocorticoid or oral immunosuppressant had been chosen for more severe or extensive pyoderma gangrenosum cases, whereas topical agents such as steroid and calcineurin inhibitors had been considered for mild cases with shallow ulcers. In this study, 22 (41.5%) patients with pyoderma gangrenosum were initially treated with a systemic corticosteroid and 15 (68.1%) patients showed partial-to-complete remission. Most of systemic steroid treatment initially taken as prednisolone 1 mg/kg/day and slowly tapered off within two weeks if the treatment response was noticed. Of note, first-line treatment with dapsone showed a remarkable response, as validated in other studies.3,27 In our study, nine (17.0%) of 53 cases experienced recurrence; however, this frequency was lower than the recurrence rates of 24–46% reported in a previous study.
留言 (0)