Cutaneous abnormalities were among the first clinical findings reported in patients infected with SARS-CoV-2 at the onset of the COVID-19 pandemic, but the significance was initially unclear. Correlations have since been drawn between many of these cutaneous eruptions and their diagnostic or prognostic value. Additionally, COVID-19 vaccines have generated acute and delayed cutaneous reactions with which clinicians should be familiar. In this narrative review, we update the cutaneous abnormalities associated with COVID-19 infection for pediatric and non-White populations, and common cutaneous reactions to COVID-19 vaccines.
KEY POINTSThere has been an increase in cutaneous manifestations together with and following COVID-19 infection and messenger RNA vaccination, respectively.
Unique manifestations have been described in children, including erythema multiforme, acute hemorrhagic edema of infancy, papular acrodermatitis of childhood, and various skin changes in multisystem inflammatory syndrome in children.
Adenoviral vector vaccine appears to result in vaccine-induced thrombotic thrombocytopenia in some patients.
As the impact of the covid-19 pandemic has extended across the globe, cutaneous presentations deserve specific attention in both adult and pediatric patient populations.1–8 The development and dissemination of COVID-19 vaccines has also generated notable cutaneous findings,9–16 and studies have noted skin phenotype as a factor in cutaneous COVID-19 manifestations.17–21 Recognition of these reactions and their implications is beneficial to clinicians in shaping patient counseling and anticipatory guidance (Table 1).9,12,17–32
TABLE 1Cutaneous reactions to COVID-19 vaccines in pediatric patients and non-White patients
PEDIATRIC OVERVIEWThe reported prevalence of cutaneous manifestations ranges between 0.25% and 8.1% in pediatric COVID-19 cases,1,2 with studies suggesting that the face is the most commonly affected site.3 Review articles have detailed similarities in cutaneous findings and their implications between adult and pediatric patients.3–6 However, unique manifestations have been described in children, including erythema multiforme, acute hemorrhagic edema of infancy, papular acrodermatitis of childhood, and various skin changes in multisystem inflammatory syndrome in children (MIS-C) that we will detail in this article.4–6,33,34
An early review article found that cutaneous involvement was described prior to other systemic symptoms in 77.9% of pediatric COVID-19 cases and simultaneously in 13.2% of cases.3 Additionally, a cohort study of more than 12,000 children noted that the prevalence of fever in conjunction with cutaneous lesions was lower in adolescents when compared with younger children and infants.2 Their analyses also suggested that hospitalized COVID-19 pediatric patients more frequently had rash, urticaria, and conjunctivitis at the time of presentation compared with nonhospitalized patients, although specific incidence rates and comparative statistics were not reported.2
MULTISYSTEM INFLAMMATORY SYNDROME IN CHILDRENAlthough there is overlap in the cutaneous manifestations between adult and pediatric populations,7 the most notable cutaneous abnormalities in pediatric COVID-19 patients relate to MIS-C. Reported findings in this syndrome include a nonexudative conjunctivitis, polymorphic rash, oral mucositis, hand and foot anomalies, and perineal and facial desquamation (Figures 1 and 2).35–37 These manifestations suggest that MIS-C shares many similarities with Kawasaki disease. However, children with MIS-C tend to be older, with higher rates of gastrointestinal symptoms, myocarditis, and shock than in classic Kawasaki disease.8,22,37,38 Mucocutaneous manifestations are important clues to the diagnosis of MIS-C,22 although not significantly associated with overall disease severity,36,37 and in some studies have been associated with lower rates of intensive care unit admission, shock, and requirement for invasive mechanical ventilation.37,38

 Figure 1
Figure 1 (A) A previously healthy 11-year-old girl with known COVID-19 exposure was hospitalized after 5 days of fever along with bilateral neck erythema and swelling. Workup revealed mildly reduced left ventricular ejection fraction of 47%, elevated erythrocyte sedimentation rate of 60 mm/hr, and a highly elevated C-reactive protein of 15.3 mg/dL, resulting in a diagnosis of multisystem inflammatory syndrome in children. The patient was treated with intravenous immunoglobulin G, steroids, and antithrombotics, with subsequent improvement in left ventricular ejection fraction and rash. She was discharged home with close clinical follow-up. (B) A previously healthy 10-year-old boy was admitted to the pediatric intensive care unit with 5 days of fever, nausea, vomiting, and erythematous, blanchable patches on the back and extremities that began 1 month after confirmed COVID-19 infection. His condition stabilized after treatment with intravenous steroids and immunoglobulin G, and broad-spectrum antibiotics, and he was ultimately discharged home on oral steroids and aspirin, with resolution of the rash confirmed at outpatient follow-up 3 days later.

 Figure 2
Figure 2 A previously healthy 2-year-old girl was admitted with concern for multisystem inflammatory syndrome in children after 5 days of fever, and vomiting, as well as palmar erythema, (seen in photo) a blanchable erythematous rash, conjunctival injection, periorbital edema, and lip erythema with desquamation and fissuring. Results of laboratory testing were notable for an elevated erythrocyte sedimentation rate of 36 mm/hr, a highly elevated C-reactive protein of 26.9 mg/dL, and COVID-19 immunoglobulin G antibody positivity. Because of cardiopulmonary deterioration during intravenous immunoglobulin G infusion, she was transferred to the intensive care unit and started on steroids, diuretics, antithrombotics, and anakinra, with improvement and eventual discharge after a 13-day hospitalization.
NON-WHITE POPULATIONSAs of September 2021, the US Centers for Disease Control and Prevention (CDC) reported that rates of COVID-19 infections were 1.1 and 1.5 times higher in Black and Hispanic populations, respectively, compared with White peers.39 Notably, these disparities increased when rates of hospitalization and death were considered.39 Despite this, there has been a relative dearth of published information describing these findings.40 Interestingly, 3 studies based on race and ethnicity found that COVID-19–specific cutaneous manifestations, including chilblain-like lesions, were uncommon in patients with darker skin phenotypes.23,24,41 As these studies were relatively limited in size, it is not yet clear whether these observations reflect the subtleties of appreciating inflammation in darker skin tones or true variations in presentation. Additionally, multiple small retrospective studies found disproportionate rates of telogen effluvium in patients with darker skin tones during the COVID-19 pandemic, and the presence of medical comorbidities has been described as a risk factor.17–19 However, larger prospective studies are needed to clarify this association. Furthermore, self-reported rates of scalp erythema and scaling were significantly higher in non-White Brazilian patients with confirmed COVID-19 infection.20 Our institutional experience suggests cutaneous abnormalities seen in patients with darker phenotypes may be somewhat more variable compared with those described and seen in White patients (Figures 3 and 4).
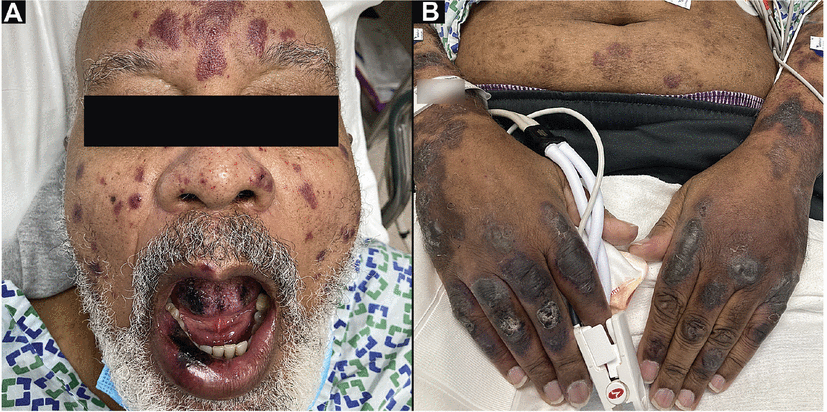
 Figure 3
Figure 3 A 74-year-old man, fully vaccinated against COVID-19 and with a remote history of cutaneous leukocytoclastic vasculitis, was seen in the emergency room after developing new purpuric patches, plaques, and bullae (A) on the face, oral mucosa, trunk, and (B) extremities. Testing confirmed acute breakthrough COVID-19 infection, and skin biopsy results were consistent with immunoglobulin A vasculitis. Hospitalization for intravenous steroids and supportive care was complicated by methicillin-resistant Staphylococcus aureus bacteremia and poor food and fluid intake due to oral pain. He was discharged in stable condition after a 22-day hospital stay.
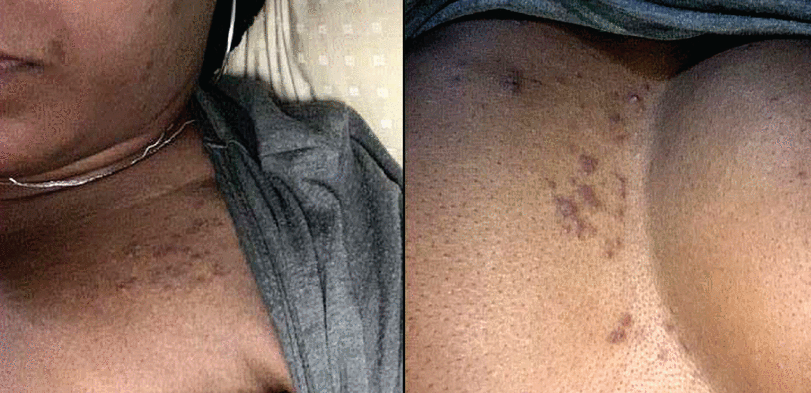
 Figure 4
Figure 4 A 40-year-old woman with no known dermatologic history developed a pruritic maculopapular rash on the trunk following hospitalization for COVID-19 infection. Her COVID-19 course was complicated by bilateral pneumonia requiring supplemental oxygen and treatment with remdesivir and dexamethasone. Her rash was managed with oral antihistamines.
Although more studies are needed to better characterize the skin manifestations of COVID-19 in patients with darker skin and to investigate potential prognostic implications, a recent review highlighted clinical clues for identifying predominant cutaneous findings in patients with darker skin tones and emphasized the importance of palpation when considering diagnoses of urticaria, morbilliform eruptions, or even chilblain-like lesions, as the associated erythema may be more difficult to appreciate.21 Additionally hyperpigmentation was noted to provide insight into previous skin inflammation and may be concerning to affected patients.21
VACCINE REACTIONSMessenger RNA vaccinesVast clinical trial data42–44 noted that self-limited nonspecific, acute, local reactions were the most commonly described cutaneous findings following vaccination with a messenger RNA (mRNA) vaccine. However, a study of 414 cutaneous reactions to mRNA COVID-19 vaccines noted the most frequently reported reactions were delayed large, confluent, local reactions involving the lateral upper arm and deltoid at the injection site (Figure 5),9 often referred to as “COVID arm.”10,12 International registry data suggest delayed cutaneous reactions are more commonly seen with the Moderna mRNA vaccine9 and in female patients,9,10 presented on average 1 week after the first vaccine dose, with no reports of severe adverse events in patients who went on to receive a second dose.9 The reaction is often associated with mild tenderness and pruritus, and less commonly with concomitant fever and malaise, resolving within 1 to 2 weeks without treatment.11

 Figure 5
Figure 5 Eight days after her first dose of the Moderna messenger RNA vaccine, a 39-year-old woman developed a warm, confluent, (A) erythematous rash on her arm, characterized by a burning sensation. She also developed a pruritic, erythematous papular eruption on her chest, neck, and upper back 3 weeks after vaccination. Her primary care physician had treated her for presumed cellulitis with clindamycin without improvement. (B) The rash and associated symptoms began to improve by 23 days after vaccination. (C) Biopsy study of the lesions on her chest revealed a mild perivascular inflammatory infiltrate (arrows) consistent with a dermal hypersensitivity reaction. The patient deferred her second dose of Moderna vaccine due to concern for reaction recurrence. She eventually received the Johnson & Johnson COVID-19 vaccine at 10 months after the initial Moderna vaccine dose.
In our experience, nonconfluent rashes, rashes involving the lateral arm, or rashes involving other anatomic locations close to the injection site may also occur (Figure 6). Notably, when looking at all cutaneous reactions, in the above-mentioned study, 43% of patients who initially had a cutaneous reaction developed another cutaneous reaction after the second dose.9

 Figure 6
Figure 6 Uncharacteristic COVID-19 vaccine reactions near the injection site. (A) A 28-year-old woman developed a rash localized to the arm after her receiving her second dose of the Moderna vaccine. She developed multiple, ill-defined, erythematous macules and thin papules extending from the vaccination site in the deltoid area distally to the elbow 16 days after vaccination. Treatment with antihistamines, topical steroids, and a methylprednisolone dose pack brought resolution within 1 week. (B) A 51-year-old man developed confluent erythema with mild axillary tenderness and lymphadenopathy 48 hours after a third dose (ie, booster dose) of the Pfizer-BioNTech vaccine. He had experienced no cutaneous reactions after his first or second vaccine doses. His symptoms resolved within 2 days without treatment.
Other commonly reported cutaneous manifestations include findings associated with COVID-19 infection, such as functional angiopathies, urticarial eruptions, and morbilliform rashes.12 Another important adverse event to be aware of is herpes reactivation, which was reported in 13.8% of cutaneous reactions from a cohort of 405 cases.13 Reassuringly, a review of 40 cases of herpes reactivation found that none of the patients had a repeat viral flare after the second vaccination dose.14
The connection between reactogenicity and immunogenicity of the mRNA vaccines continues to be explored. Recent studies have suggested that systemic adverse effects are correlated with increased antibody production.15,16,45 However, others have found that the presence and severity of local and systemic adverse reactions are not reliable indicators of a humoral response,25–27 specifically when adjustments are made for age and sex that have been independently associated with increased antibody production.28 As the number of vaccinated patients continues to climb and increasingly available metrics of immune response are developed, the relationship between cutaneous reactions and immunogenicity may become clearer.
Owing to more recent approval of the Pfizer-BioNTech COVID-19 vaccine for pediatric patients, data regarding cutaneous reactions in this population are limited. However, clinical study data noted that rash was seen in 0.3% of children ages 5 to 11 years and redness and swelling at the injection site reported in less than 20% of patients.46 As increased numbers of pediatric patients undergo vaccination, clinicians should be familiar with commonly seen reactions as well as the potential for more severe presentations (Figure 7), which may relate to a more intense immune response in younger individuals. Additionally, a recent nationwide analysis of French adolescents ages 12 to 18 suggests that COVID-19 mRNA vaccination could be associated with a lower incidence of MIS-C, although data regarding younger patients are not yet available.47
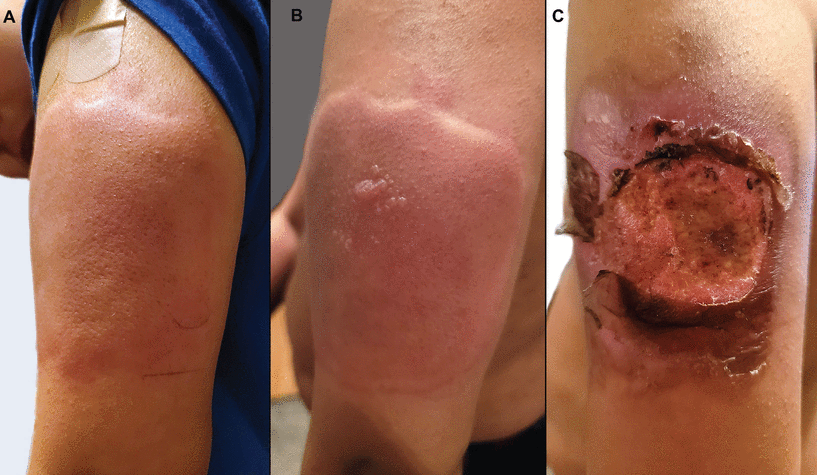
 Figure 7
Figure 7 At a well visit with his pediatrician, an 11-year-old boy received 4 age-appropriate vaccines. In the right arm, he received the tetanus, diphtheria, and acellular pertussis vaccine and the meningitis vaccine. In the left arm, he received the Pfizer-BioNTech COVID-19 vaccine (12-year-old dose) and the human papillomavirus vaccine. Within minutes of receiving the vaccines, he developed a large, pruritic, erythematous and edematous plaque on his left arm (A), with subsequent vesiculation (B). Over the next 1 to 2 days, this progressed to a large, painful ulceration (C). He had no systemic symptoms with this reaction, and the lesions eventually healed.
Adenoviral vector vaccineAlthough the Johnson & Johnson adenoviral vector vaccine appears to have relatively few dermatologic side effects, with clinical trial data and safety analyses reporting only local adverse reactions and urticaria,29 rare reports of vaccine-induced immune thrombotic thrombocytopenia have garnered significant attention.30,31,48 Patients have demonstrated concomitant petechiae, suggesting their presence may be a clue to this reaction.30,31 Although only a few cases describing vaccine-related cutaneous manifestations of immune thrombotic thrombocytopenia have been reported to date, affected patients are often critically ill, and subsequent deaths have been reported.49 Thus, any cutaneous manifestation that provides insight to this diagnosis could be valuable. Other cutaneous reactions have also been reported in association with the Johnson & Johnson COVID-19 adenoviral vector vaccine.32
TAKE-HOME MESSAGESIn summary, cutaneous findings of COVID-19 infection in pediatric patients appear to overlap those in adult patients. Although the constellation of cutaneous findings in MIS-C can aid in diagnosis, mucocutaneous involvement is not correlated with more severe disease. While there are reports of fewer cutaneous findings in COVID-19 infection in non-White patients, palpation may be helpful in appreciating subtle inflammation not readily apparent on visual examination. Fortunately, the vast majority of cutaneous mRNA vaccine reactions are short-lived, associated with only minimal or mild symptoms, and in some cases represent molecular mimicry causing rashes similar to those seen with COVID-19 infection. Given the limited number of available studies and variable strength of current data, future research is warranted to more definitively characterize cutaneous manifestations of COVID-19 in pediatric and non-White patients, in addition to cutaneous manifestation following COVID-19 vaccines.
DISCLOSURESDr. Fernandez discloses consulting for Abbvie Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Mallinckrodt, Novartis, and UCB; research or independent contracting for Abbvie Pharmaceuticals, Mallinckrodt, Novartis, and Pfizer; being an advisor or research panel participant for Abbvie Pharmaceuticals; and teaching and speaking for Abbvie Pharmaceuticals, Kyowa Kirin, Mallinckrodt, and Novartis. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
AcknowledgmentThe authors would like to acknowledge Janine Sot, MBA, for her help and expertise in preparing the figures displayed in this manuscript.
Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.
留言 (0)