Most cytologic preparations and CNB samples were adequately subclassified into WHO lymphoma categories
B-cell lymphomas, specifically diffuse large B-cell lymphoma, were most common
Most cases had IHC; few had flow cytometry or cytogenetic or molecular studies
INTRODUCTIONThe use of fine-needle aspiration biopsy (FNAB) and/or core needle biopsy (CNB) for primary diagnosis and subclassification of lymphomas has been controversial, especially when the diagnosis is based on cytomorphologic features alone for directing disease management.[1-3] Some hematopathologists are skeptical about whether FNAB can be used to accurately diagnose and classify lymphoma, even if combined with flow cytometry (FC).[4] Nonetheless, its usefulness has been recognized for verifying residual or recurrent lymphoma and for triaging patients with benign or malignant lymphadenopathy for further work-up.[5,6] Improvement in image guidance and ancillary studies, including immunohistochemical staining (IHC), FC, and molecular and cytogenetic testing, has facilitated the diagnoses, despite excisional biopsy still being the diagnostic standard.[7-9] The trade-off of using these additional studies, however, is that the process is now more time consuming and technically challenging than just using FNAB cytologic analysis alone.[10] Caraway[10] also stated that “ancillary studies that include immunophenotyping are mandatory to successfully distinguish between” certain neoplastic lymphocytic entities (P = 0.433). A recent report by Jin and Wakely[11] compares ancillary techniques, advantages, and disadvantages, FNAB versus core biopsies, recommended IHC lymphoma panels, molecular testing options, and some of the corresponding results for the most common lymphoma subtypes, along with providing several useful charts and an overall review.
This paper describes a single-institution’s multiparameter approach to diagnosing lymphoproliferative disorders as per the 2017 World Health Organization (WHO) classification terminology of “Tumors of the Hematopoietic and Lymphoid Tissues.”[12] By this classification, conditions “are diagnosed not only based on their morphologic features, but also based on their immunophenotypic, cytogenetic, and molecular profiles;” therefore, it can be used with limited cytologic preparations (fluids, cytologic smears, and cell block) and/ or CNB samples with less emphasis on the histopathologic pattern.[1,6] The aim of this study was to evaluate the general reliability of combined cytologic preparations (including fluid cytology, FNAB, and cell block) and CNB for diagnosing lymphoproliferative disorders with appropriate ancillary studies. Our main objective was to determine the diagnostic utility of combined CNB and cytologic preparations for triaging and appropriately classifying lymphoproliferative disorders using limited sampling.
MATERIAL AND METHODSThe Mayo Clinic Institutional Review Board approved this study. We used SNOMED codes to search the electronic laboratory information system of Mayo Clinic, Jacksonville, Florida, for the records of all patients with cytology specimens collected between January 1, 2012, and August 1, 2017, and who were given a final diagnosis of “atypical lymphoid cells,” “suspicious for lymphoma,” or “definitive lymphoma.” No other inclusion or exclusion criteria were applied. For all identified patients, we collected demographic information: age, sex, date of last follow-up, and current status. Specimen-specific data included specimen sources, subclassification of the lymphoma, IHC, FC, and molecular testing results, if available. A total of 389 cases were collected and analyzed.
Sampling and slide preparationsOut of 389 cases, 46 (11.8%) cases were body fluid specimens, including 19 cerebrospinal fluid (CSF), 15 pleural fluid, 10 peritoneal fluid, and two other body fluids (pericardial and nasopharyngeal). For CSF, three cytospin slides were prepared; two slides were for Papanicolaou staining; and one for Giemsa-Wright staining. For non-CSF body fluid, two cytospin slides were prepared for Papanicolaou staining; and the remaining of the cell button (sediment after centrifuge) was prepared for cell block processing.
The remaining 343 cases were CNBs obtained with an 18–20-gauge core needle under ultrasound, interventional radiology, or computed tomographic guidance by an interventional radiologist. A cytotechnologist was available on-site to evaluate tissue adequacy during these procedures (rapid on-site evaluation). For any cases of suspected lymphoma either with history or clinical presentation or initial morphologic evaluation, two cores were obtained initially. Touch prep or roll prep slides were made from each core and stained with modified Giemsa stain (Diff-Quick stain). The cytotechnologist evaluated the slides for adequacy, sometimes further reviewed by a hematopathologist or a cytopathologist to decide whether FC analysis was necessary or not. If more tissue was needed (either inadequate or FC is deemed necessary), and the patient can tolerate additional passes, the cytotechnologists requested additional material, and interventional radiologists performed several more passes with caution.
Additional touch prep or roll prep smears were prepared from each core. In some cases (possible carcinoma or lymphoma), some slides were immediately fixed in 95% ethyl alcohol to preserve the cellular morphology followed by Papanicolaou staining. While one core was placed in the RPMI media for FC analysis, other cores were stored in a container with 10% formalin. Upon return to the cytology laboratory, we processed the cores with the rule of “one core in each cassette/paraffin block,” followed by routine tissue processing in the histology laboratory for hematoxylin and eosin (H&E) sections. All cases were accessioned under the cytopathology laboratory. Therefore, for cerebrospinal specimens, each specimen included three cytospin slides (one Giemsa stain and two Papanicolaou stains); for other body fluid specimens, each specimen included two Papanicolaou stains and two H&E slides from the cell block. For CNB cases, each case included multiple touch/roll prep smears and H&E slides (one slide of each block). All the slides were initially reviewed by a cytopathologist, then consulted by a hematopathologist based on the consultation policy in our department. The lymphoma cases usually were signed out by cytopathologists after consultation with our hematopathologists’ colleagues.
FC analysisThe core tissue was transferred from RPMI media in a Petri dish and grounded through the wire mesh. The grounded specimen goes through lysing and wash steps to eliminate red cells, then mixed with a panel of fluorescein-coated antibodies. A specifically designed tissue panel including CD45, CD19, CD20, CD5, CD10, CD3, CD7, CD23, kappa, lambda, and 7-AAD was used as an initial panel; if abnormal blasts or T cells were suspected, an additional T-cell panel or acute leukemia panel was applied. In the case of body fluid specimens or effusions, the sample was submitted fresh within 48 h for flow cytometric analysis. Body fluid specimens were centrifuged; then, the supernatant was lysed and washed following further processing with a triage panel including CD45, CD19, CD10, CD34, CD3, CD16, kappa, and lambda.
The FC analysis is performed on BD FACS Canto II, and the data analysis was performed by Kaluza software. For the tissue specimens, the analysis algorithm started with the viability tested by 7-aminoactinomycin D. The data from the specimen with an acceptable rate >80% or suboptimal 50–80% were analyzed. If the specimen’s viability was unacceptable (<50%), it was rejected and designated as “insufficient for analysis.” The minimum volume requirement for FC analysis for a core tissue was 5 mm3 or larger biopsy; for body fluids was 20 mL; and for spinal fluid was 1.0– 1.5 mL.
IHCIHC was performed with the avidin-biotin-peroxidase complex technique using the fully automated Ventana BenchMark ULTRA staining system (Roche) with standard procedures. Appropriate positive and negative controls were used throughout these procedures. A wide panel of markers (commercially available antibodies, Dako) was used as necessary for exact subtyping. Antibody panels were chosen on the basis of sample morphologic features and patient clinical history. Immunohistochemical stains were performed on all cell blocks and CNB only. Ki-67 was used to distinguish between low- and high-grade lymphomas in almost all cases where there were no obvious morphologic features of a high-grade process.
Fluorescence in situ hybridization (FISH)FISH was used to examine genetic aberrations and translocations. FISH was performed on either formalin-fixed and paraffin-embedded cell blocks or CNB. For visualization, hybridized probes were further conjugated with fluorescein isothiocyanate-labeled avidin and rhodamine-labeled anti-digoxigenin. The analysis of fluorescent signals was performed by counting of 50–100 nuclei. Probes used in our analysis were targeted for c-MYC, BCL-2, BCL-6, IgH, and IgL.
Molecular studies by BIOMED-2 multiplex polymerase chain reaction (PCR) (Invivoscribe Technologies) were also performed for some of the cases, with appropriate primer sets for T-cell receptor and B-cell gene (IgH and IgK) rearrangement. FISH and molecular studies were only performed on either cell block preparations or CNB specimens.
RESULTSAmong 389 patients identified and included in the analysis, the mean age was 66.0 years (range, 21.0–98.0 years), and 218 of 388 patients (56.2%) were men.
The diagnosis was atypical lymphoid cells in 17 cases (4.4%) and suspicious for lymphoma in 31 cases (8.0%), for an overall rate of indeterminate findings of 12.3%. Of the 17 atypical lymphoid cases, 8 (47.1%) had follow-up excisional biopsies (three Hodgkin lymphoma [HL], one high-grade B-cell lymphoma, one low-grade B-cell lymphoma, one diffuse large B-cell lymphoma, one T-cell prolymphocytic leukemia, and one benign process with chronic inflammation). Of the 31 suspicious cases, 23 (74.2%) had follow-up excisional biopsies (five diffuse large B-cell lymphoma, four HL, three large B-cell lymphoma, three low-grade B-cell lymphoma, three follicular lymphoma, two high-grade B-cell lymphoma, one chronic lymphocytic leukemia/small lymphocytic lymphoma, one sclerosing T-cell rich B-cell lymphoma, and one with no evidence of lymphoproliferative disorder). The remaining 17 (nine atypical and eight suspicious) cases were lost to follow-up. The other 341 malignant cases (87.7%) were reclassified using the current 2017 WHO lymphoid tissue classification system as follows: 284 mature B-cell neoplasms (73.0%), 32 HL (8.2%), 14 mature T-cell and natural killer cell neoplasms (3.6%), 9 precursor lymphoid neoplasms (2.3%), 1 blastic plasmacytoid dendritic cell neoplasm (0.3%), and 1 post-transplant lymphoproliferative disorder (0.3%) [Table 1]. Among the B-cell lymphomas, six low-grade and 26 high-grade neoplasms were not further subclassified (“not otherwise specified”) because of limited tissue or otherwise unclear disease features.
Table 1:: All study cases diagnosed per the 2017 WHO classification including atypical and suspicious cases (48 cases).
Diagnostic categories per 2017 WHO classification including atypical and suspicious cases (48) No. (%) of cases (n=389) Blastic plasmacytoid dendritic cell neoplasm 1 (0.3) Precursor lymphoid neoplasms 9 (2.3) B-cell lymphoblastic leukemia/lymphoma 5 (1.3) T-cell lymphoblastic leukemia/lymphoma 4 (1) Mature B-cell neoplasms 284 (73.0) Low grade 167 (42.9) Follicular lymphoma, any grade 68 (17.5) Chronic lymphocytic leukemia/small lymphocytic lymphoma 43 (11.1) Marginal zone lymphoma 17 (4.4) Mantle cell lymphoma, including blastoid variant 16 (4.1) Lymphoplasmacytic lymphoma 9 (2.3) Plasmacytoma 7 (1.8) Low-grade B-cell lymphoma, NOS 6 (1.5) Extranodal marginal zone lymphoma 1 (0.3) High grade 117 (30.1) Diffuse large B-cell lymphoma, all variants 84 (21.6) High-grade B-cell lymphoma, NOS 26 (6.7) Plasmablastic lymphoma 3 (0.8) Burkitt lymphoma 2 (0.5) Primary mediastinal large B-cell lymphoma 1 (0.3) Primary effusion lymphoma 1 (0.3) Mature T-cell and NK-cell neoplasms 14 (3.6) T-cell prolymphocytic leukemia 3 (0.8) Peripheral T-cell lymphoma, NOS 3 (0.8) Angioimmunoblastic T-cell lymphoma 3 (0.8) Anaplastic large cell lymphoma 3 (0.8) Mycosis fungoides 1 (0.3) Sézary syndrome 1 (0.3) Hodgkin lymphoma 32 (8.2) Classic Hodgkin lymphoma 17 (4.4) Hodgkin lymphoma, NOS 8 (2.1) Nodular sclerosis classic Hodgkin lymphoma 5 (1.3) Lymphocyte-rich classic Hodgkin lymphoma 1 (0.3) Mixed cellularity classic Hodgkin lymphoma 1 (0.3) Post-transplant lymphoproliferative disorder 1 (0.3) Suspicious for lymphoma 31 (8) Various atypical lymphoid cells 17 (4.4)Among the B-cell lymphomas, which were the most common overall, the largest subcategories were diffuse large B-cell lymphomas (21.6%), followed by follicular lymphoma (17.5%), and chronic lymphocytic leukemia/small lymphocytic lymphoma (11.1%).
Most of the cases (319, 82.0%) had a baseline IHC panel performed using a combination of 28 hematologic-specific stains [Table 2]. The remaining cases (70, 18%) in which IHC was not performed were diagnosed and classified by FC and/or molecular testing since they were recurrent cases. A total of 140 cases (36.0%) had FC performed, but 30 of these specimens (21.4%) were insufficient to yield any results [Table 3]. Only a few cases showed typical FC immunophenotypic results for acute leukemia (1, 0.7%), T-cell leukemia (1, 0.7%), and polytypic B cells (4, 2.9%). Thus, the majority of cases that were sufficient for testing showed a typical B-cell immunophenotypic profile (104, 74.3%).
Table 2:: IHC used by neoplastic lymphoma groupings.
Marker tested by IHC Mature B-cell neoplasms Mature T-cell neoplasms High-grade B-cell neoplasms Low-grade B-cell neoplasms Hodgkin lymphoma None* 26 1 13 30 CD20 233 3 90 118 6 Ki-67 117 1 52 59 Bcl-6 110 1 55 69 1 Bcl-2 82 1 43 44 CD10 80 2 26 44 PAX-5 58 1 25 47 27 CD23 39 2 32 CD79a 36 12 19 CD5 31 6 28 MUM1 29 30 3 CD3 25 10 4 5 4 CD21 23 2 4 24 CD138 17 1 11 Cyclin D1 16 12 CD45 14 10 1 CD30 5 2 13 28 CD15 24 CD4 12 LCA 2 2 1 Kappa 3 1 Lambda 3 1 CD279 1 2 1 TdT 3 TCR 2 CD8 2 CXCL13 2 TCL1 1 CD2 1Table 3:: Summary of cases with ancillary FC testing.
FC diagnostic categories Cases/total % Cases submitted for FC 140/389 36 Cases insufficient for FC 30/140 21.4 Cases sufficient for FC 110/140 78.6 B-cell lymphoma 104/140 74.3 Polytypic B cells 4/140 2.9 Acute leukemia 1/140 0.7 T-cell leukemia 1/140 0.7Cytogenetic and molecular studies, FISH (c-MYC, BCL-2, and BCL-6), and testing for immunoglobulin gene and T-cell receptor gene rearrangements were performed for 78 cases (20.0%) [Table 4]. Of the 14 mature T-cell neoplasms, seven were tested with PCR and found to have clonal T-cell receptor gene rearrangements. Of 284 mature B-cell neoplasms, 58 were tested with PCR and 51 of which had clonal gene rearrangements. Among atypical and suspicious cases with PCR testing, one atypical and four suspicious had clonal B-cell gene rearrangements, and two atypical and two suspicious had clonal T-cell receptor gene rearrangements.
Table 4:: Summary of lymphomas diagnosed by PCR.
Diagnosis Cases tested/total (%) Clonal B-cell gene rearrangement by PCR (%) Clonal T-cell gene rearrangement by PCR (%) Mature B-cell neoplasms 58/284 (20.4) 51 (87.9) 0 Mature T-cell neoplasms 7/14 (50.0) 0 7 (100.0) Suspicious for lymphoma 6/31 (19.4) 4 (66.7) 2 (33.3) Atypical for lymphoma cells 3/17 1 (33.3) 2 (66.7)Some of the high-grade B-cell neoplasms (n = 23) had subsequent reflex c-Myc FISH testing, which, if amplified, then were subsequently reflexed to Bcl-2 and Bcl-6. Among these 23 cases with c-Myc amplification, one had a diagnosis of Burkitt lymphoma confirmed. The remaining cases included 14 diffuse large B-cell lymphomas (c-MYC rearrangement only), six double-hit, high-grade, B-cell lymphoma (c-MYC plus BCL-2, or BCL-6 rearrangement), and two triple-hit, high-grade, and B-cell lymphoma (c-MYC plus BCL-2 and BCL-6 rearrangement). [Table 5] lists the numerous different specimen types that were collected.
Table 5:: Target organs (lymph nodes or others) sampled by centesis (spinal tap, thoracocentesis, paracentesis, and pericardiocentesis) and core biopsy for cytologic analysis.
Site No. of cases (n=389) Body fluid 46 Cerebrospinal fluid (CSF) 19 Pleural fluid 15 Peritoneal fluid 10 Other body fluids (pericardial and nasopharyngeal) 2 Lymph node 213 Neck includes paratracheal, submandibular, and submental 27 Inguinal/femoral 28 Supraclavicular 22 Axillary 25 Retroperitoneal/mesenteric/abdominal 66 Hilar includes subcarinal 15 Gastrointestinal: Celiac, perigastric, and periportal 8 Mediastinal/pericardial 16 Specific site not listed 2 Iliac 1 Paraspinal 1 Peripancreatic mass/lymph node 1 Subpectoral 1 Other body sites 130 Bone: Humerus, iliac, ischium, sacrum, scapula, tibia, rib, elbow, femur, sternum, maxilla, and vertebrae 32 Soft tissue: Various, includes muscle 25 Neck includes thyroid and salivary glands 23 Lung 13 Liver 12 Kidney includes perirenal 9 Specific site not stated 5 Adrenal gland 2 Pancreas 3 Spleen 3 Gastric 2 Small bowel 1 DISCUSSIONFNAB and CNB are generally fast, cost-effective, and relatively safe procedures to collect tissue for microscopic evaluation and are better tolerated and less invasive than excisional biopsies. They are also amenable to performing real-time adequacy checks to ensure sufficient cells for all tests. They can also be used in conjunction with imaging modalities for accessing and sampling deep-seated lesions. Collected cells or tissues can be placed in various media, such as RPMI, for ancillary testing (e.g., FC) as needed. Houcine et al.[5] asserted that one of the most significant advantages of FNAB is its ability “to separate the lymphomas from the carcinomas before more invasive surgery” (P = 0.1,119). The shortcomings of FNAB and CNB, however, should not be ignored, such as suboptimal results because of too few passes, too small needle gauge, CNB done before FNAB, physician inexperience with the procedure, size of the lesion, amount of sclerosis and necrosis, or blood contamination present.[1,13] Other drawbacks include lack of histologic architecture, stripped nuclei, possible neoplastic cell dilution by nonneoplastic cells, distinguishing reactive from neoplastic cells, grading in certain conditions such as follicular lymphoma, and limited tissue for cancer biobanking.[1,2,13,14] The predominant disadvantage of combined FNAB and CNB is the lack of reliable subtyping, which is critical for disease management, such as between the different low-grade subtypes (P = 0.1,119).[5] In some situations, however, grading is possible and can be clinically significant, although the National Comprehensive Cancer Network guidelines state not to grade follicular lymphomas by FNAB.[1,8,11] Jin and Wakely[11] agreed with this point by stating that FNAB does not allow for all lymphoma subtypes to be equally precisely diagnosed; their review of deep-seated lymphoma studies reported a specificity of only 38–88.8%. They describe several noteworthy reasons for this, not including the previously described inherent FNAB problems, but also the “expertise, interest, and philosophy of the individual pathologist” and the continually evolving WHO subclassification (P = 0. 622).
Lymph node diagnosis by cytology is a relatively straightforward algorithm starting with determining whether the sample is benign or malignant. If it is malignant, the next step is determining whether it is a B- or T-cell lymphoma or acute leukemia. If it is a B-cell lymphoma, a low or high grade is next ascertained; if it is a T-cell lymphoma, then determining whether it is an anaplastic large cell lymphoma. The reporting pathologist has the option to request additional tissue or rebiopsy or recommend an excisional biopsy for a more definitive diagnosis if one cannot be rendered with the tissue already submitted. [Figures 1 and 2] show recommendations for additional testing needed in instances of adequate and inadequate tissue sampling, respectively.
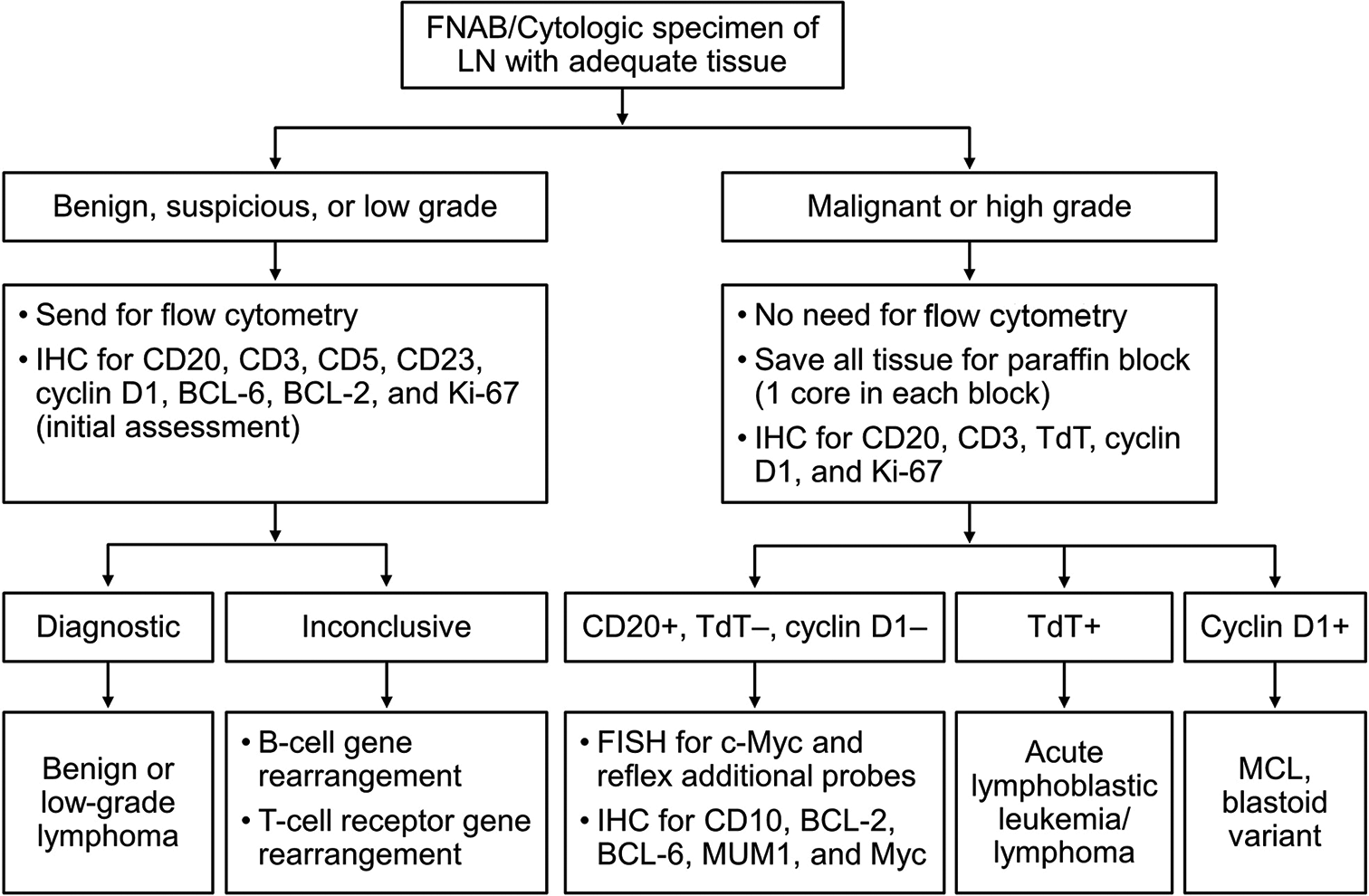
Figure 1:: Algorithm for cytologic specimen/core needle biopsy with adequate tissue. FISH: Fluorescence in situ hybridization, IHC: Immunohistochemical staining, LN: Lymph node, MCL: Mantle cell lymphoma.
Export to PPT

Figure 2:: Algorithm for cytologic specimen/core needle biopsy with inadequate or scanty tissue. FISH: Fluorescence in situ hybridization, IHC: Immunohistochemical staining.
Export to PPT
Caraway[1] stated that T-cell lymphomas, as compared with B-cell lymphomas, are “more difficult to diagnose and subclassify with” FNAB and “often require tissue biopsy;” the same applies to HLs because of the paucity of cellularity and diagnostic Reed-Sternberg cells and neoplastic cell mimickers (P = 0. 390). In one study of 74 cases of CNBs and FNABs, FNAB had fewer diagnostic cases of diffuse large B-cell lymphoma (25%) than CNB (37%), but FNABs had more diagnostic cases of small B-cell non-Hodgkin lymphomas (NHLs) than CNB (15% vs. 8%).[14]
In possibly the first investigational paper to question the validity of using FNAB in diagnosing lymphoma, Hehn et al.[2] reported a 12% sensitivity rate. Among 93 FNABs studied for initial disease evaluation, 67 had a subsequent excisional biopsy to compare FNAB results, only eight of which showed concordance. They stated that the low concordance rate is most likely due to the inherent problems with FNAB, as previously mentioned, but the specimens that needed supplementary testing had a better correlation with biopsy results.[2]
One study of 24 patients showed a diagnostic accuracy rate of 79% by endobronchial ultrasound-guided FNAB or use of the Tru-Cut Biopsy Device (Merit Medical) combined with FC.[4] Their subclassification rate was 67% (n = 16), which consisted of seven of nine large B-cell lymphomas and nine of 15 non-large B-cell lymphomas.[4] In addition, of 23 cases that had FNAB, 13 had a diagnosis of definite lymphoma or suspicious for lymphoma and none were able to be subclassified unless combined with FC (only 19 of 23 cases), in which 18 of the 19 had a lymphoma diagnosis, and 14 of 18 were able to be subclassified.[4] In contrast, a study by Yasuda et al.[15] reported a 98% accuracy in lymphoma diagnosis and an 88% subclassification rate when combining large gauge needle aspiration biopsy and IHC. This was concordant with a study by Houcine et al.[5] of 65 lymphomas from cervical lymph node FNABs, which showed a sensitivity of 95.5% and a specificity of 98.7% when comparing cytologic findings with the histologic standard diagnoses.
Another study of 25 follow-up biopsy-confirmed lymphoma cases showed that 22 (88.0%) were correctly diagnosed and subclassified on FNAB.[16] Raddaoui et al.’s[9] study demonstrated a combined diagnostic sensitivity for NHL and HL of 83%, specificity of 100% for NHL, and an NHL and HL accuracy rate of 87% when using FNAB combined with FC. Another study of FNAB with FC by Ensani et al.[17] agreed with these rates: Sensitivity of 75% and specificity of 93.8% in correctly diagnosing NHL only. Two other studies reported a sensitivity of 90% and specificity of 88% using IHC and concurrent biopsy comparison in 48 of 53 lymphoma cases (90.6%),[6] and a specificity of 85% using cytomorphology and histology comparisons.[18]
Numerous other studies, especially from the late 1990s to mid-2000s, reported diagnostic rates using FNAB combined with FC ranging from 66.0% to 95.6%.[13,19-26] Frederiksen et al.[8] performed a comprehensive literature review spanning 1989–2012 and 42 studies of FNAB and/or CNB with ancillary testing in which diagnoses of lymphoma subtypes, inadequate/ inconclusive, or lymphoma not otherwise specified were given. That study reported a median rate of subtype-specific lymphoma diagnosis of 74% (3791/5707) and also showed a lack of improvement of subtyping with the advancement of adjuvant testing over the 25-year period. They suggested that additional testing “only occasionally compensates for the loss of diagnostic specificity inherent in limited sampling” (P = 0.250).[8]
Our study substantiates the benefit of having ancillary testing in combination with cytomorphology from cytologic preparations and CNBs that other studies have demonstrated as well. Only 40 (10.3%; all not otherwise specified) of our 389 cases (six low-grade B-cell lymphoma-NOS, 26 high-grade B-cell lymphoma-NOS, and eight HL-NOS; [Table 1]) were unable to be given a more specific subclassification, despite further testing in some, not including the atypical and suspicious cases (48, 12.3%). This left 301 cases that were adequately classified given the 2017 WHO subtyping based on morphology, IHC, and molecular findings. As in other papers, a percentage of samples (21.4%) were unable to yield FC results, and few underwent molecular testing (20.0%). Our study even included the indeterminate rate of 12.3% (atypical and suspicious), whereas other investigations usually have not.
Of interest, 70 cases in our study had no IHC performed, but these were recurrent cases that were exclusively diagnosed with the use of FC and/or molecular testing only. In terms of mature B-cell neoplasms, the most commonly ordered IHC stains were CD20, Ki-67, Bcl-6, Bcl-2, CD10, and PAX-5, whereas the most frequently ordered stains for T-cell neoplasms were CD4, CD3, CD5, TdT, and CD20 [Table 2]. Among the high-grade B-cell neoplasms, the most commonly performed IHC was for CD20, Bcl-6, Ki-67, Bcl-2, and CD5, and staining for low-grade B-cell neoplasm consisted mostly of CD20, Ki-67, Bcl-6, CD10, and Bcl-2. The HL panel usually comprised CD15, CD30, PAX-5, CD20, and CD3.
In our study, half of the mature T-cell neoplasms tested with PCR (7 of 14, 50%) had clonal T-cell receptor gene rearrangements. Most of the mature B-cell neoplasms tested with PCR had clonal gene rearrangements (51 of 58, 87.9%). The high-grade B-cell neoplasms tested with reflex c-MYC FISH testing had various results, such as Burkitt lymphoma, diffuse large B-cell lymphoma, double-hit, high-grade, B-cell lymphoma, and triple-hit, high-grade, and B-cell lymphoma.
This study has several limitations. It is a single-center study and, therefore, is limited to only a few pathologists’ diagnostic experiences. We also did not review patient histories to distinguish primary from recurrent lymphoma except for the few cases that were documented on the pathology report (70 cases total). We did not analyze whether a superficial versus deep-seated tumor influenced the diagnosis. It also would have been advantageous to molecularly test and conduct FC on all specimens.
Recently, two new classifications of lymphoma (the 5th edition of WHO classification and international consensus classification) were published by the WHO working group and the clinical advisory committee, respectively.[27,28] We acknowledge that the updated definitive and classification of some lymphomas slightly differ from the 2017 WHO classification, which we used for this study. Nevertheless, the principle, the ancillary tests, and the algorithms which not only did we utilize to render the diagnosis in the clinical practice, but are also summarized in this article are minimal, if not at all, affected by the update of classification.
CONCLUSIONOur study confirms the findings of the previous studies that cytologic preparations coupled with CNB, especially in conjunction with other ancillary testing such as IHC, FC, and molecular testing, can be used to determine a specific and definitive diagnosis of lymphoma. This supports the statement by Jin and Wakely[11] that “it has been well accepted that the addition of ancillary techniques is mandatory for a confident cytologic diagnosis of NHL and HL” (P = 0. 619). The combination of cytologic preparations and CNB can be viewed as an attractive first sampling procedure in the diagnostic process for this disease group.
Although the definitive diagnosis was possible only on IHC studies, molecular profiling is mandatory for further evaluation.
留言 (0)