Figure 1. SARS-CoV-2 infection in the upper and lower part of the lungs (A); virus-infected host cell and virus propagation cycle (B). The SARS-CoV-2 virus consists of membrane proteins (M) in the lipid membrane along with envelope proteins (E) and spike glycoproteins linked to the envelope. RNA bound to the nucleocapsid protein exists inside the virus. External binding protein S can enter the lungs by binding to angiotensin-converting enzyme-2 (ACE-2) receptors present in human lung cells. In this case, the virus releases RNA together with genetic information and performs RNA replication to synthesize all components of the virus. Synthetic components are assembled and released out of lung cells.
Figure 1. SARS-CoV-2 infection in the upper and lower part of the lungs (A); virus-infected host cell and virus propagation cycle (B). The SARS-CoV-2 virus consists of membrane proteins (M) in the lipid membrane along with envelope proteins (E) and spike glycoproteins linked to the envelope. RNA bound to the nucleocapsid protein exists inside the virus. External binding protein S can enter the lungs by binding to angiotensin-converting enzyme-2 (ACE-2) receptors present in human lung cells. In this case, the virus releases RNA together with genetic information and performs RNA replication to synthesize all components of the virus. Synthetic components are assembled and released out of lung cells.
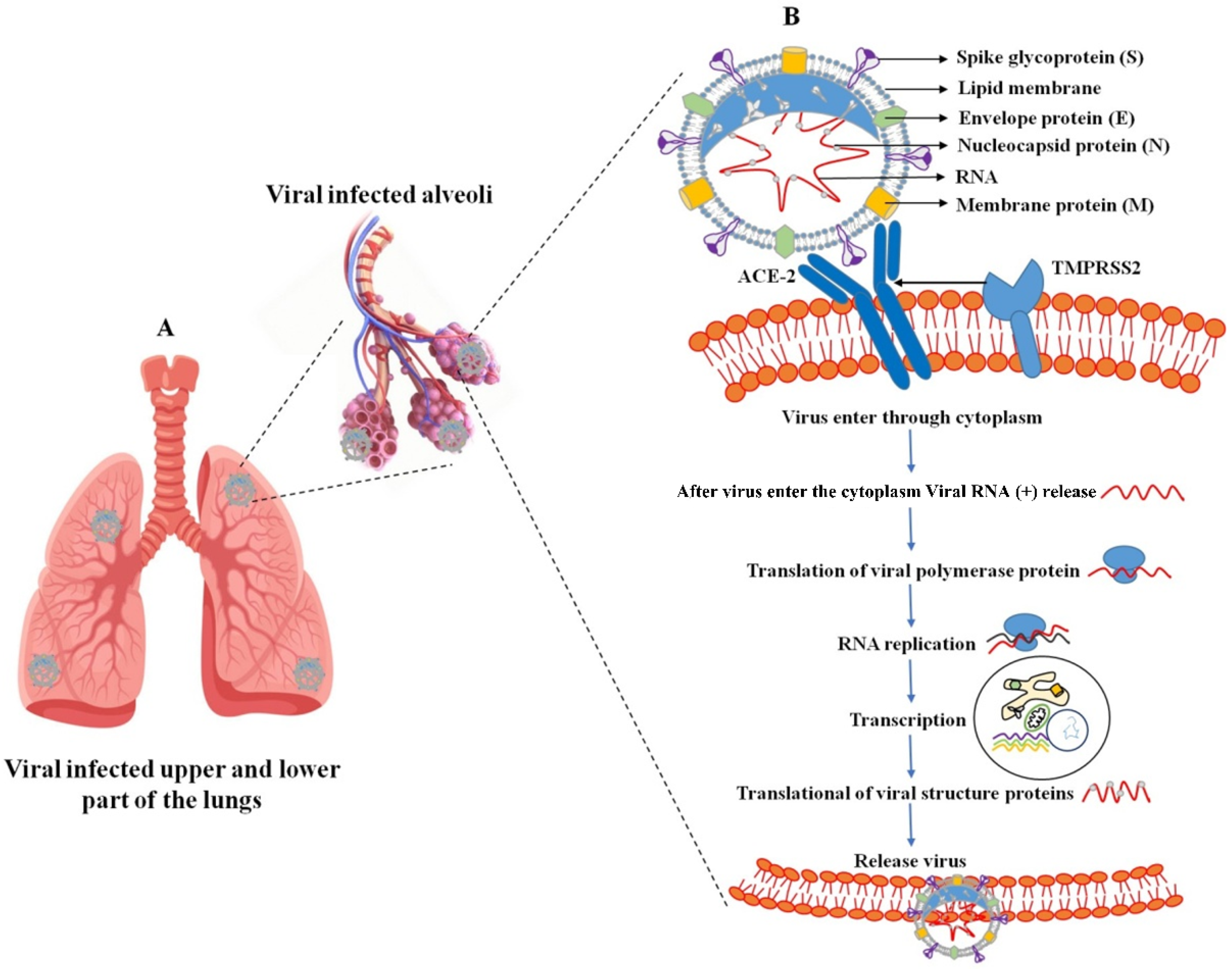
Figure 2. Coronavirus infections and immune approach. During coronavirus infections, the lower part of the lungs collects fluid in the bronchioles and disrupts surface coating types I and II pneumocytes. Additionally, they disrupt the immune response and increase cytokine release, which leads to the accumulation of reactive oxygen species. Various cytokines are secreted by CD163 macrophages activated by a viral infection in infected endothelial cells, and immune responses are caused by IL1, IL6, TNF-α, IFN, etc. Chemical catalysts by this cytokine attract various immune cells, such as neutrophils, lymphocytes, and T cells, through blood vessels. (The specific cells are indicated by dotted lines, and the direction of cell movement is indicated by an arrow).
Figure 2. Coronavirus infections and immune approach. During coronavirus infections, the lower part of the lungs collects fluid in the bronchioles and disrupts surface coating types I and II pneumocytes. Additionally, they disrupt the immune response and increase cytokine release, which leads to the accumulation of reactive oxygen species. Various cytokines are secreted by CD163 macrophages activated by a viral infection in infected endothelial cells, and immune responses are caused by IL1, IL6, TNF-α, IFN, etc. Chemical catalysts by this cytokine attract various immune cells, such as neutrophils, lymphocytes, and T cells, through blood vessels. (The specific cells are indicated by dotted lines, and the direction of cell movement is indicated by an arrow).
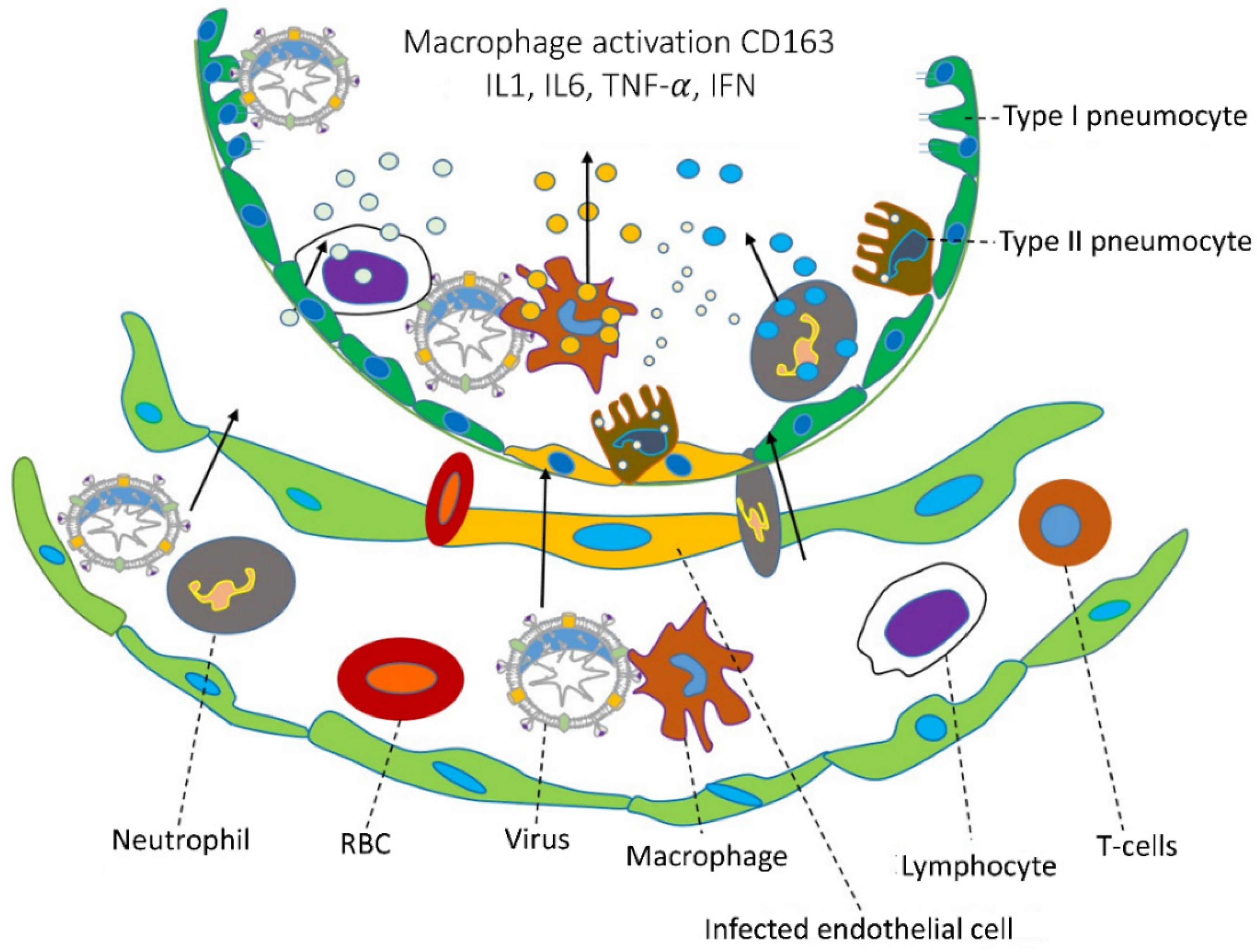 Figure 3.
(a) SAMDI-MS spectra of duplexed reactions before and after 3CLpro and HRV3C activity (top and bottom). (b–d) Structural analysis of protease inhibitor binding sites. Rupintrivir binding mode in HRV3C protease (b,c). (d) Superimposition of previously reported SARS-CoV-2 3CL-pro structures with bound GC376. Reprinted with permission from [60]. Copyright 2021, Elsevier. (e) The most powerful and effective inhibitor, YH-53, can effectively prevent SARS-CoV-2 replication. Reprinted with permission from [59]. Copyright 2022, American Chemical Society.
Figure 3.
(a) SAMDI-MS spectra of duplexed reactions before and after 3CLpro and HRV3C activity (top and bottom). (b–d) Structural analysis of protease inhibitor binding sites. Rupintrivir binding mode in HRV3C protease (b,c). (d) Superimposition of previously reported SARS-CoV-2 3CL-pro structures with bound GC376. Reprinted with permission from [60]. Copyright 2021, Elsevier. (e) The most powerful and effective inhibitor, YH-53, can effectively prevent SARS-CoV-2 replication. Reprinted with permission from [59]. Copyright 2022, American Chemical Society.
Figure 3.
(a) SAMDI-MS spectra of duplexed reactions before and after 3CLpro and HRV3C activity (top and bottom). (b–d) Structural analysis of protease inhibitor binding sites. Rupintrivir binding mode in HRV3C protease (b,c). (d) Superimposition of previously reported SARS-CoV-2 3CL-pro structures with bound GC376. Reprinted with permission from [60]. Copyright 2021, Elsevier. (e) The most powerful and effective inhibitor, YH-53, can effectively prevent SARS-CoV-2 replication. Reprinted with permission from [59]. Copyright 2022, American Chemical Society.
Figure 3.
(a) SAMDI-MS spectra of duplexed reactions before and after 3CLpro and HRV3C activity (top and bottom). (b–d) Structural analysis of protease inhibitor binding sites. Rupintrivir binding mode in HRV3C protease (b,c). (d) Superimposition of previously reported SARS-CoV-2 3CL-pro structures with bound GC376. Reprinted with permission from [60]. Copyright 2021, Elsevier. (e) The most powerful and effective inhibitor, YH-53, can effectively prevent SARS-CoV-2 replication. Reprinted with permission from [59]. Copyright 2022, American Chemical Society.
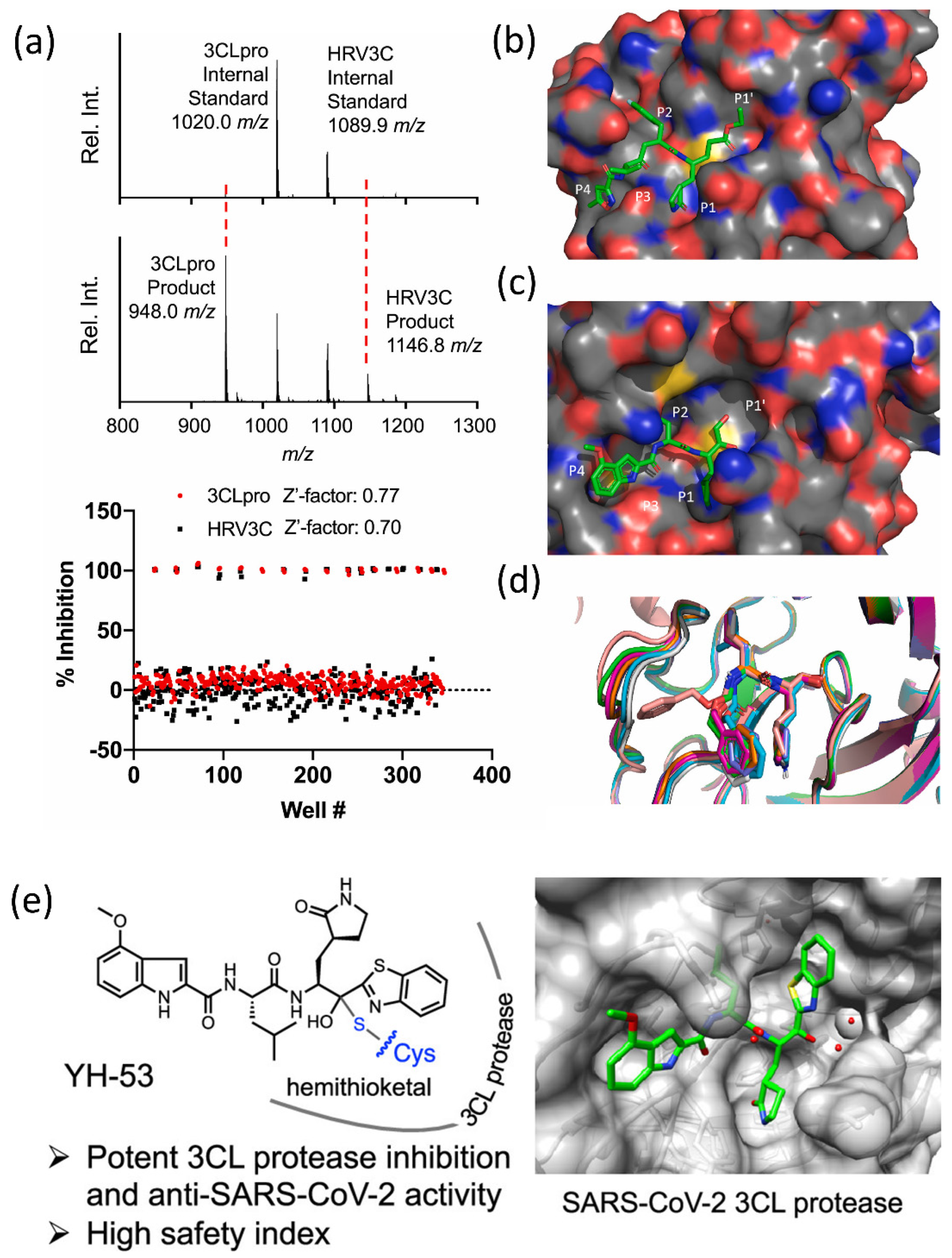
Figure 4. (A) The major protease of the SARS-CoV-2 dimer’s structural characteristics. The primary protease of the SARS-CoV-2 virus. (B) In the active site groove, a sphere represents the primary protease monomer.
Figure 4. (A) The major protease of the SARS-CoV-2 dimer’s structural characteristics. The primary protease of the SARS-CoV-2 virus. (B) In the active site groove, a sphere represents the primary protease monomer.
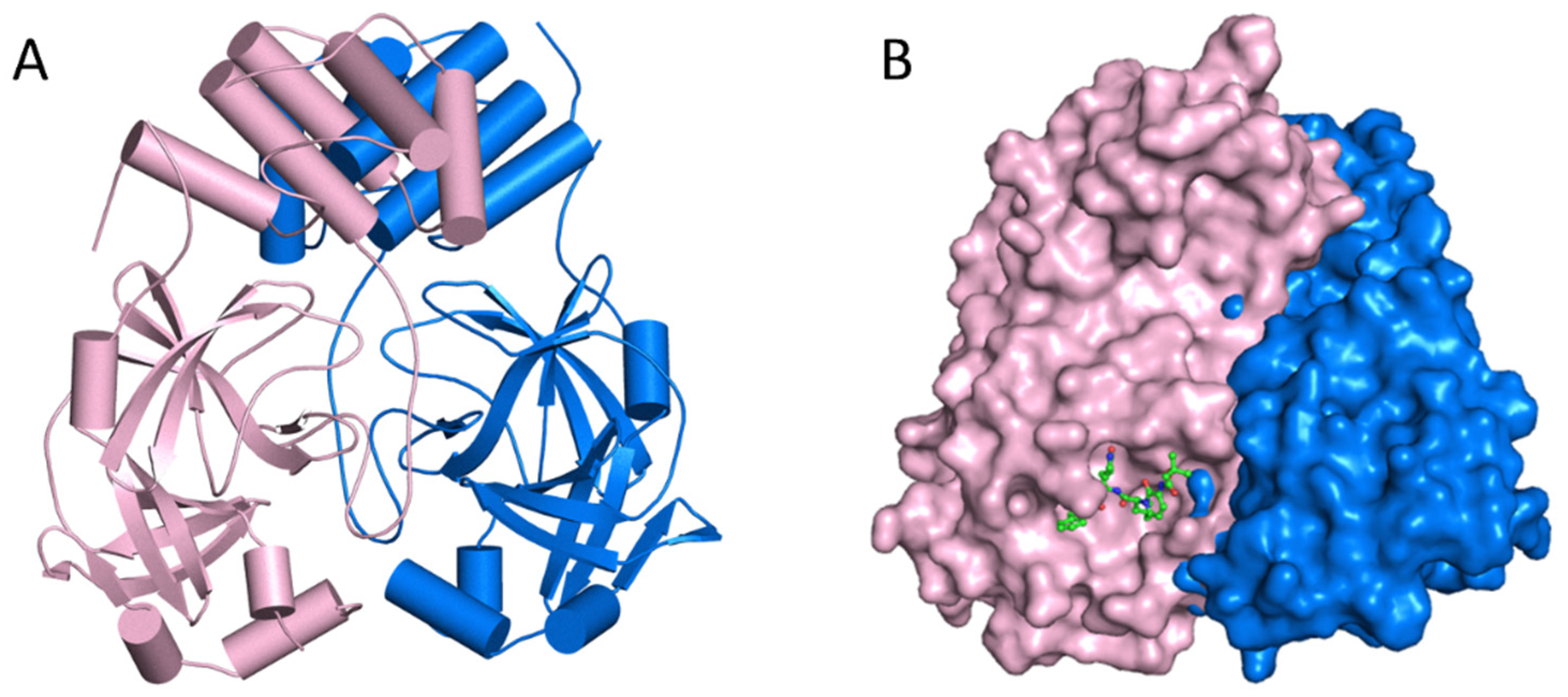
Figure 5. (A) The major protease of the SARS-CoV-2 monomer has three domains and is structurally unique. A linker connects Domains II and III, which is necessary for protein dimerization. (B) Subsite groups in the substrate-binding subsites of SARS-CoV-2 MPro: S1′, S1, S2, S3, and S4.
Figure 5. (A) The major protease of the SARS-CoV-2 monomer has three domains and is structurally unique. A linker connects Domains II and III, which is necessary for protein dimerization. (B) Subsite groups in the substrate-binding subsites of SARS-CoV-2 MPro: S1′, S1, S2, S3, and S4.
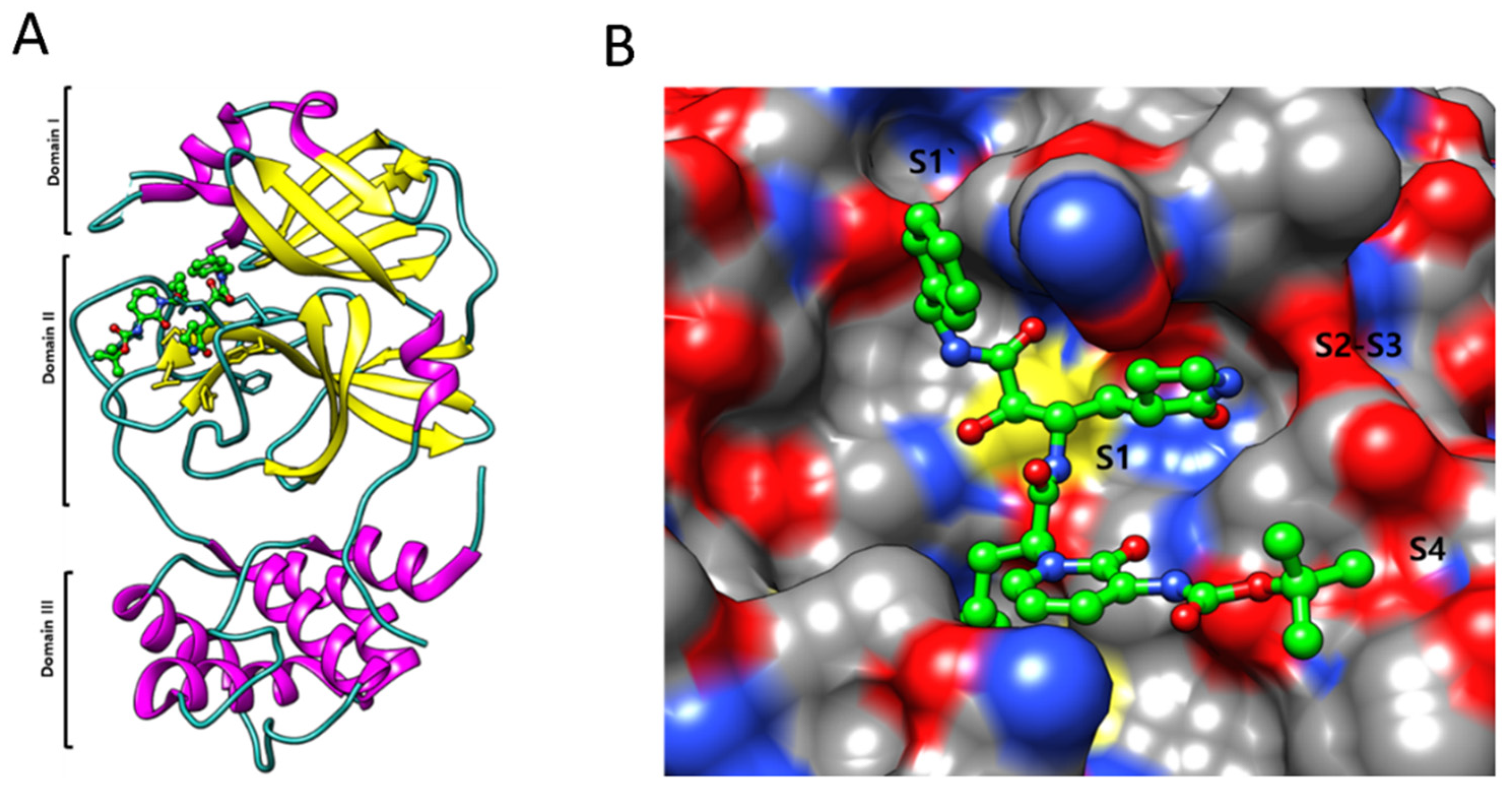
Figure 6. Illustration of the direct and indirect impacts of several types of radiation on microorganisms. Radiation can cause direct DNA and protein damage which leads to virus inactivation. On the other hand, radiation can also cause the generation of ROS in response to oxidative stress, which also causes to damage the DNA and proteins of microorganisms and lead to inactivation.
Figure 6. Illustration of the direct and indirect impacts of several types of radiation on microorganisms. Radiation can cause direct DNA and protein damage which leads to virus inactivation. On the other hand, radiation can also cause the generation of ROS in response to oxidative stress, which also causes to damage the DNA and proteins of microorganisms and lead to inactivation.
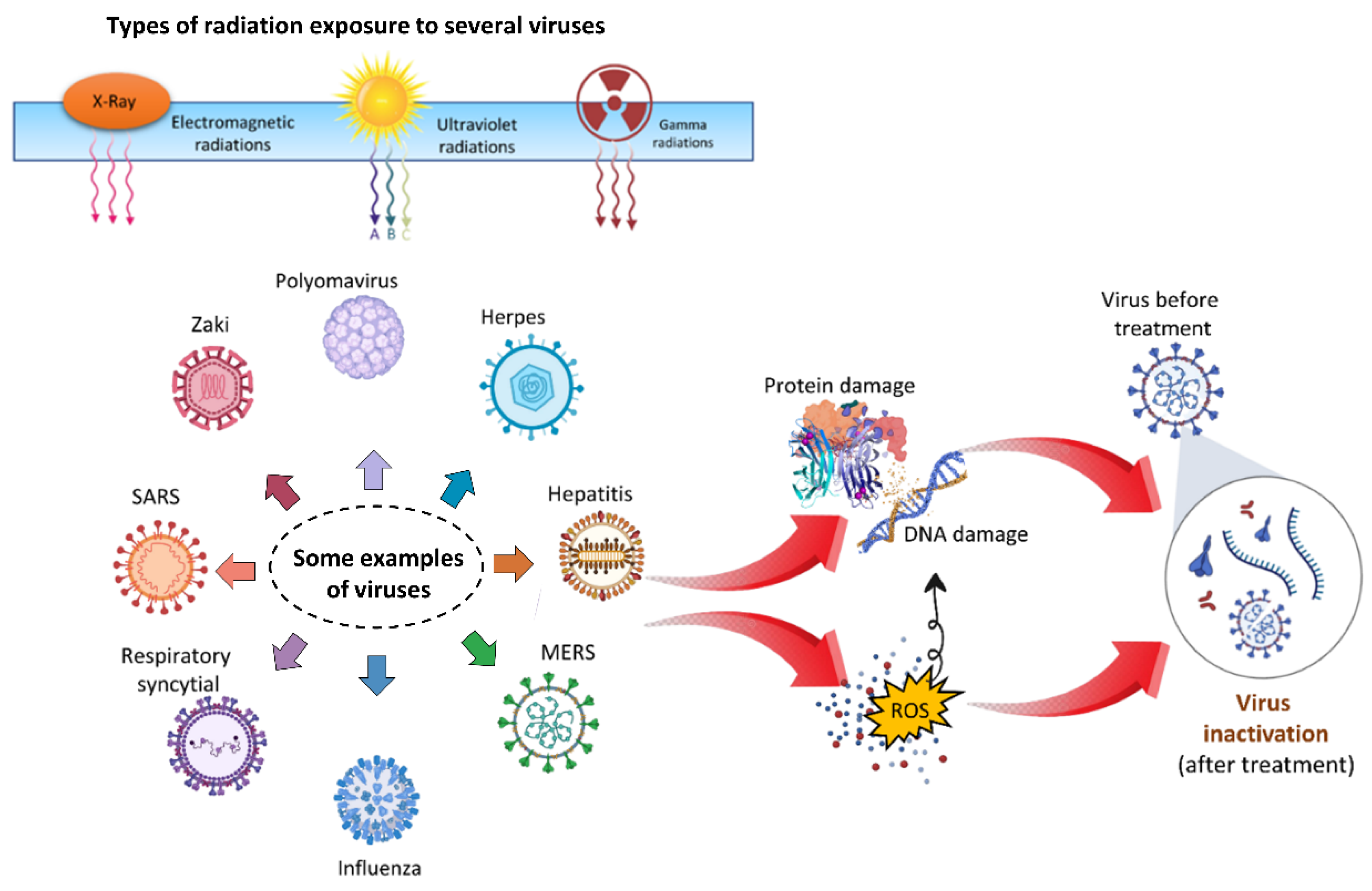 Figure 7.
(a) Typical cold atmospheric plasma configurations for biomedical applications, such as plasma jet, dielectric barrier discharges (DBD), and spark; (b) plasma environment, which includes plasma generating reactive species, electrons, other ions, emissions, waves, and physical forces; (c) plasma delivery strategies, which include direct application, plasma-activated media, device-assisted delivery, and a combination of these. Reproduced with permission from [116]. Copyright 2022, Elsevier.
Figure 7.
(a) Typical cold atmospheric plasma configurations for biomedical applications, such as plasma jet, dielectric barrier discharges (DBD), and spark; (b) plasma environment, which includes plasma generating reactive species, electrons, other ions, emissions, waves, and physical forces; (c) plasma delivery strategies, which include direct application, plasma-activated media, device-assisted delivery, and a combination of these. Reproduced with permission from [116]. Copyright 2022, Elsevier.
Figure 7.
(a) Typical cold atmospheric plasma configurations for biomedical applications, such as plasma jet, dielectric barrier discharges (DBD), and spark; (b) plasma environment, which includes plasma generating reactive species, electrons, other ions, emissions, waves, and physical forces; (c) plasma delivery strategies, which include direct application, plasma-activated media, device-assisted delivery, and a combination of these. Reproduced with permission from [116]. Copyright 2022, Elsevier.
Figure 7.
(a) Typical cold atmospheric plasma configurations for biomedical applications, such as plasma jet, dielectric barrier discharges (DBD), and spark; (b) plasma environment, which includes plasma generating reactive species, electrons, other ions, emissions, waves, and physical forces; (c) plasma delivery strategies, which include direct application, plasma-activated media, device-assisted delivery, and a combination of these. Reproduced with permission from [116]. Copyright 2022, Elsevier.
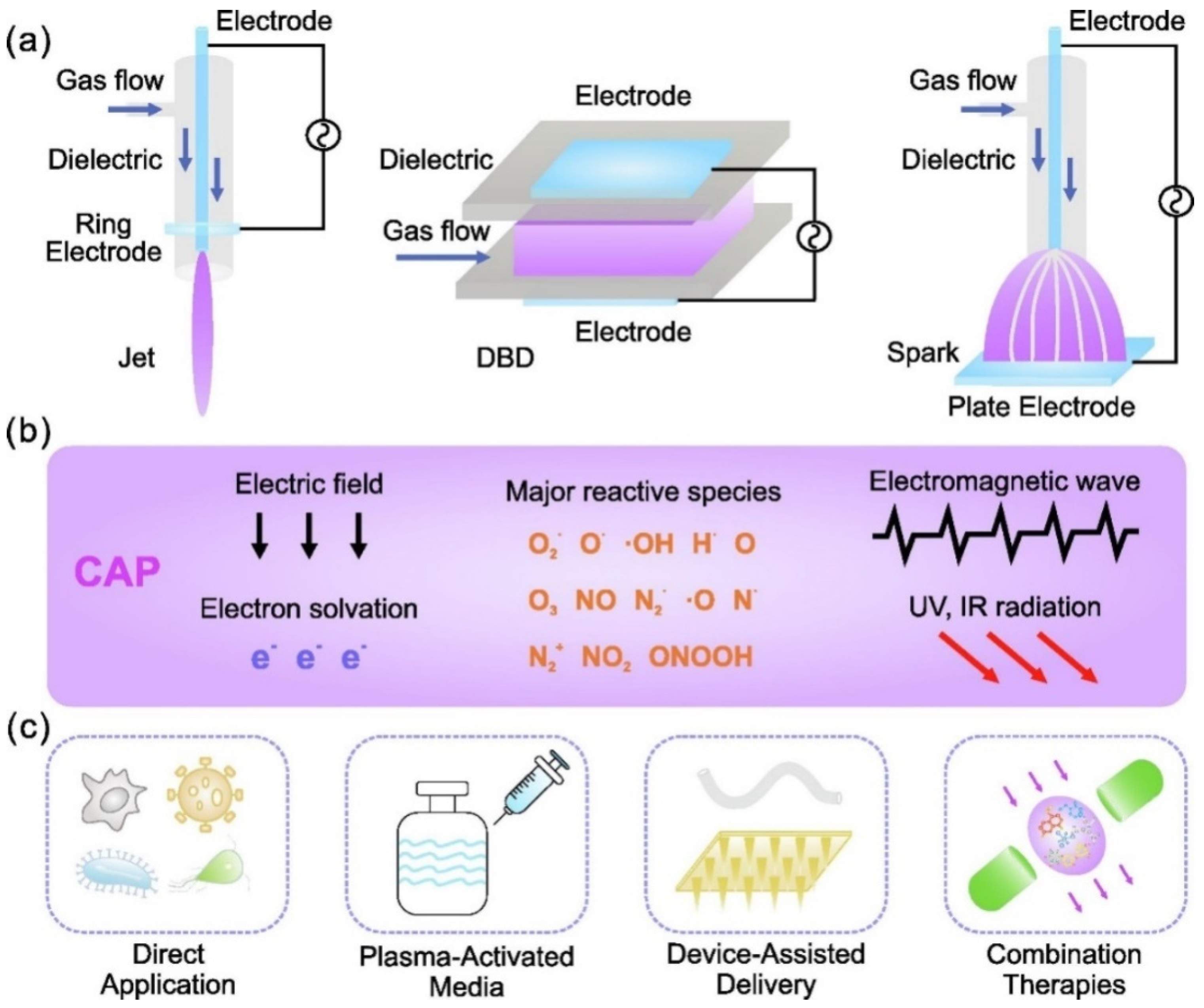 Figure 8.
The mechanism of viral inactivation by plasma. (a) Morphological differences between viruses exposed to plasma. (b) A magnified view of the plasma properties responsible for viral inactivation. (c) These virus particles are partially or completely destroyed after being exposed to plasma, resulting in non-infective components. Reprinted with permission from [127]. Copyright 2020, Elsevier. (d) TEM images of GX_P2V before and after 3 min of CAP exposure. (e) Disinfection of virus with plasma exposure. (f) The mechanism through which RONS is produced in Ar-based NTAP damaged S protein. Plasma jet-generated reactive species dissolve into liquid and cross-react in the liquid phase. RONS ONOO− and O2− oxidized tyrosine, tryptophan, and histidine are predominate at RBD and NTD, thereby impairing RBD’s capacity to bind to cell receptor ACE2 and NTD’s function. After 3 min of cold atmospheric plasma (CAP) or NTAP treatment, the viral genome remained intact. Reprinted with permission from [146]. Copyright 2022, Elsevier.
Figure 8.
The mechanism of viral inactivation by plasma. (a) Morphological differences between viruses exposed to plasma. (b) A magnified view of the plasma properties responsible for viral inactivation. (c) These virus particles are partially or completely destroyed after being exposed to plasma, resulting in non-infective components. Reprinted with permission from [127]. Copyright 2020, Elsevier. (d) TEM images of GX_P2V before and after 3 min of CAP exposure. (e) Disinfection of virus with plasma exposure. (f) The mechanism through which RONS is produced in Ar-based NTAP damaged S protein. Plasma jet-generated reactive species dissolve into liquid and cross-react in the liquid phase. RONS ONOO− and O2− oxidized tyrosine, tryptophan, and histidine are predominate at RBD and NTD, thereby impairing RBD’s capacity to bind to cell receptor ACE2 and NTD’s function. After 3 min of cold atmospheric plasma (CAP) or NTAP treatment, the viral genome remained intact. Reprinted with permission from [146]. Copyright 2022, Elsevier.
Figure 8.
The mechanism of viral inactivation by plasma. (a) Morphological differences between viruses exposed to plasma. (b) A magnified view of the plasma properties responsible for viral inactivation. (c) These virus particles are partially or completely destroyed after being exposed to plasma, resulting in non-infective components. Reprinted with permission from [127]. Copyright 2020, Elsevier. (d) TEM images of GX_P2V before and after 3 min of CAP exposure. (e) Disinfection of virus with plasma exposure. (f) The mechanism through which RONS is produced in Ar-based NTAP damaged S protein. Plasma jet-generated reactive species dissolve into liquid and cross-react in the liquid phase. RONS ONOO− and O2− oxidized tyrosine, tryptophan, and histidine are predominate at RBD and NTD, thereby impairing RBD’s capacity to bind to cell receptor ACE2 and NTD’s function. After 3 min of cold atmospheric plasma (CAP) or NTAP treatment, the viral genome remained intact. Reprinted with permission from [146]. Copyright 2022, Elsevier.
Figure 8.
The mechanism of viral inactivation by plasma. (a) Morphological differences between viruses exposed to plasma. (b) A magnified view of the plasma properties responsible for viral inactivation. (c) These virus particles are partially or completely destroyed after being exposed to plasma, resulting in non-infective components. Reprinted with permission from [127]. Copyright 2020, Elsevier. (d) TEM images of GX_P2V before and after 3 min of CAP exposure. (e) Disinfection of virus with plasma exposure. (f) The mechanism through which RONS is produced in Ar-based NTAP damaged S protein. Plasma jet-generated reactive species dissolve into liquid and cross-react in the liquid phase. RONS ONOO− and O2− oxidized tyrosine, tryptophan, and histidine are predominate at RBD and NTD, thereby impairing RBD’s capacity to bind to cell receptor ACE2 and NTD’s function. After 3 min of cold atmospheric plasma (CAP) or NTAP treatment, the viral genome remained intact. Reprinted with permission from [146]. Copyright 2022, Elsevier.
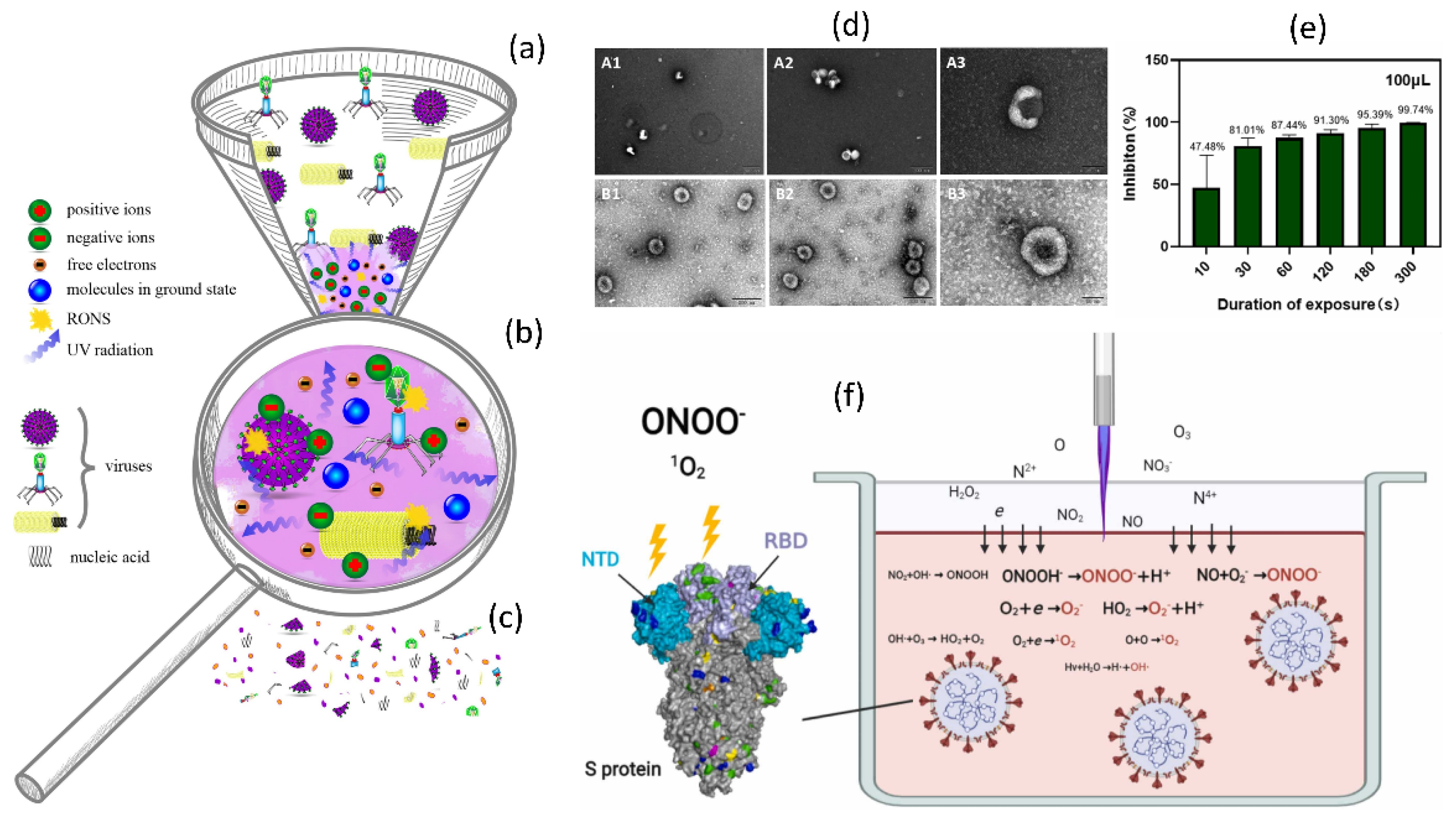 Figure 9.
(a) A graphical representation of NTAP water treatment to prepare PAW and its application to COVID-19. (b) PAW inactivation of pseudovirus. Infection of COS-7 and HEK-293T cells with control and PAW-treated pseudovirus. (c) TEM analysis of PAW-treated pseudoviruses. The pseudoviruses treated with PAW-5 min and PAW-10 min, as well as the untreated pseudovirus, were negative stained and TEM examined. (d) The effects of PAW inactivation after storage. (e) Storage of long-lived species. The concentrations of H2O2 and NO2/NO3 in PAW-10 min stored for the indicated times were measured. (f) A comparison of the inactivation effects of PAW and a mixture of H2O2, NO2, and NO3. (g) Short-lived species in PAW and the H2O2, NO2, and NO3 mixed solution. Reused with permission from [147]. Copyright 2021, Elsevier.
Figure 9.
(a) A graphical representation of NTAP water treatment to prepare PAW and its application to COVID-19. (b) PAW inactivation of pseudovirus. Infection of COS-7 and HEK-293T cells with control and PAW-treated pseudovirus. (c) TEM analysis of PAW-treated pseudoviruses. The pseudoviruses treated with PAW-5 min and PAW-10 min, as well as the untreated pseudovirus, were negative stained and TEM examined. (d) The effects of PAW inactivation after storage. (e) Storage of long-lived species. The concentrations of H2O2 and NO2/NO3 in PAW-10 min stored for the indicated times were measured. (f) A comparison of the inactivation effects of PAW and a mixture of H2O2, NO2, and NO3. (g) Short-lived species in PAW and the H2O2, NO2, and NO3 mixed solution. Reused with permission from [147]. Copyright 2021, Elsevier.
Figure 9.
(a) A graphical representation of NTAP water treatment to prepare PAW and its application to COVID-19. (b) PAW inactivation of pseudovirus. Infection of COS-7 and HEK-293T cells with control and PAW-treated pseudovirus. (c) TEM analysis of PAW-treated pseudoviruses. The pseudoviruses treated with PAW-5 min and PAW-10 min, as well as the untreated pseudovirus, were negative stained and TEM examined. (d) The effects of PAW inactivation after storage. (e) Storage of long-lived species. The concentrations of H2O2 and NO2/NO3 in PAW-10 min stored for the indicated times were measured. (f) A comparison of the inactivation effects of PAW and a mixture of H2O2, NO2, and NO3. (g) Short-lived species in PAW and the H2O2, NO2, and NO3 mixed solution. Reused with permission from [147]. Copyright 2021, Elsevier.
Figure 9.
(a) A graphical representation of NTAP water treatment to prepare PAW and its application to COVID-19. (b) PAW inactivation of pseudovirus. Infection of COS-7 and HEK-293T cells with control and PAW-treated pseudovirus. (c) TEM analysis of PAW-treated pseudoviruses. The pseudoviruses treated with PAW-5 min and PAW-10 min, as well as the untreated pseudovirus, were negative stained and TEM examined. (d) The effects of PAW inactivation after storage. (e) Storage of long-lived species. The concentrations of H2O2 and NO2/NO3 in PAW-10 min stored for the indicated times were measured. (f) A comparison of the inactivation effects of PAW and a mixture of H2O2, NO2, and NO3. (g) Short-lived species in PAW and the H2O2, NO2, and NO3 mixed solution. Reused with permission from [147]. Copyright 2021, Elsevier.
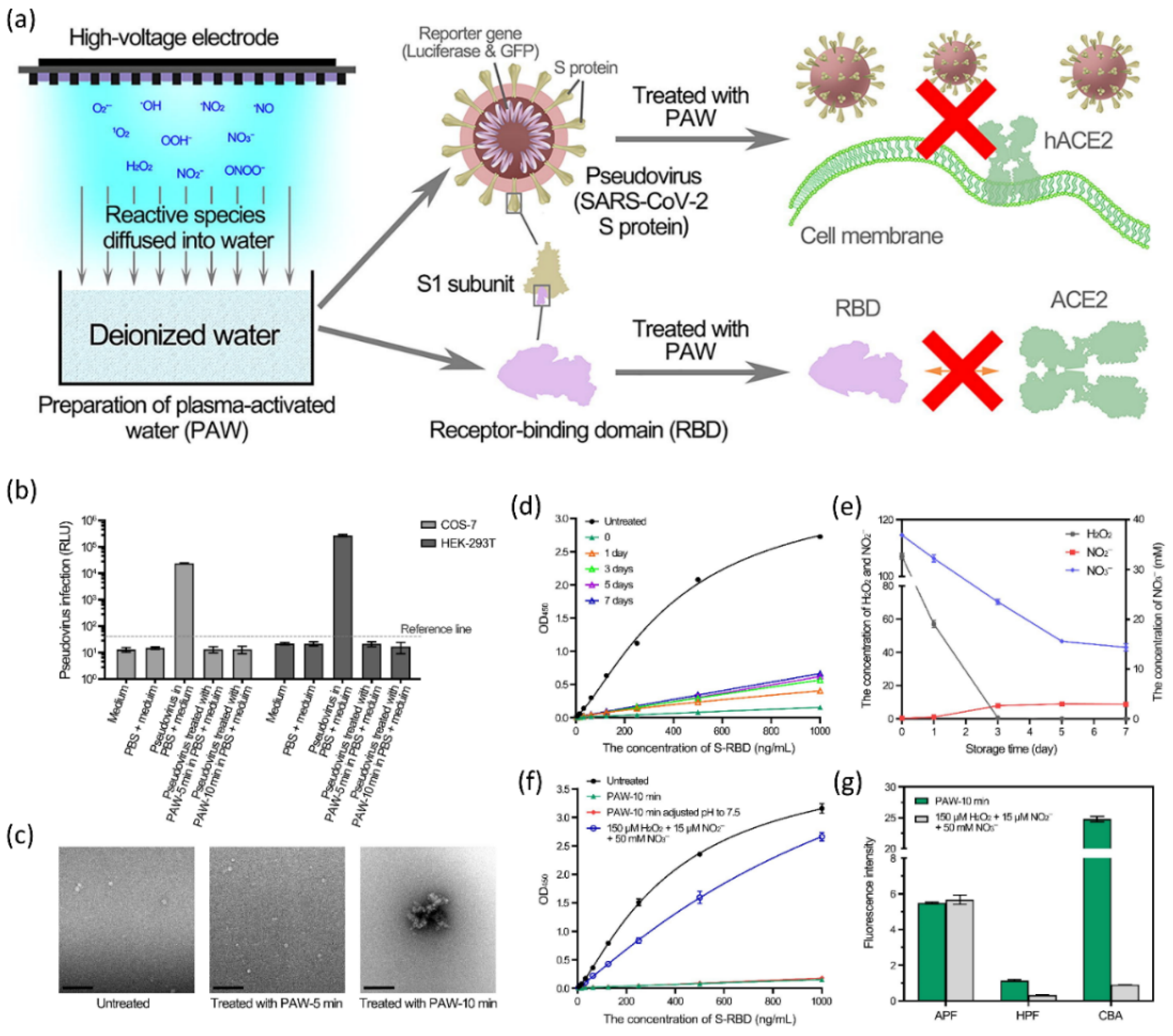
Figure 10. The mechanisms of NTAP in microorganisms in response to biological challenges. The NTAP is a cocktail of reactive species, UV, and RONS that is beneficial in iniactivating COVID-19 through DNA damage, protein denaturation, membrane damage, and oxidative stress, which ultimately results in the virus being rendered inactive or restricted to replication.
Figure 10. The mechanisms of NTAP in microorganisms in response to biological challenges. The NTAP is a cocktail of reactive species, UV, and RONS that is beneficial in iniactivating COVID-19 through DNA damage, protein denaturation, membrane damage, and oxidative stress, which ultimately results in the virus being rendered inactive or restricted to replication.
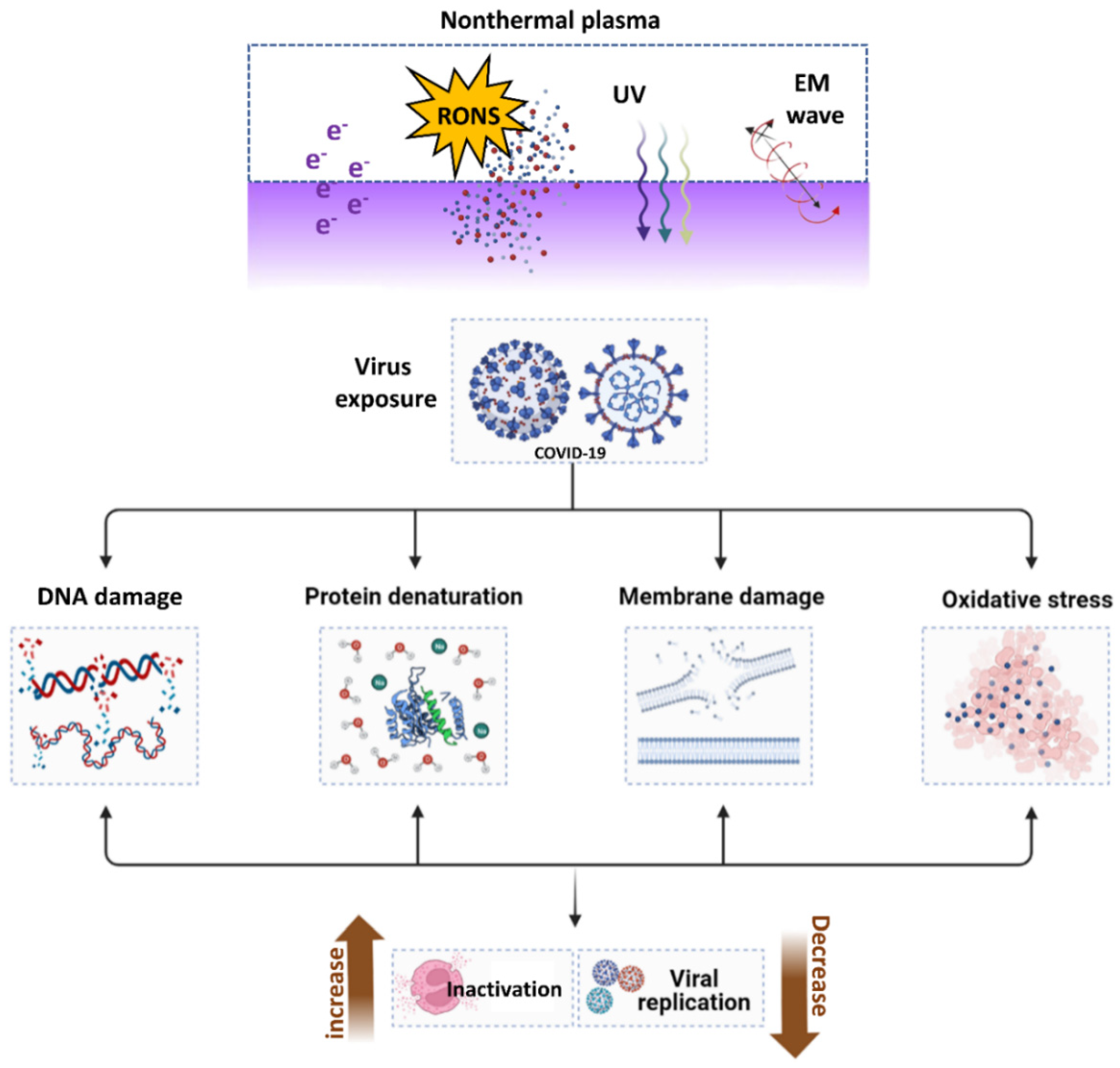
Table 1. The summary vaccines and its types.
Table 1. The summary vaccines and its types.
Vaccine PlatformTotal VaccineVaccine TypeProtein subunit (PS)55Maximum spike protein-based and less combined with nanoparticle (4 no)Viral vector non-replicating (VVnr)23Adenovirus, lentiviral and Sendai viral vector-basedDNA16DNA plasmid encoding RDB, S and othersInactivated virus (IV)22Inactivated whole virus or CpG1018RNA40mRNAViral vector replicating (VVr)4Based on the influenza A virusVirus-like particle (VLP)6expressing S, E, M, and NVVr + antigen-presenting cell (VVr + APC)2-Live attenuated virus (LAV)2Codon deoptimized live attenuated virusVVnr + antigen-presentingcell (VVnr + APC)1Mesenchymal stem cell expressing with S and NBacterial antigen-spore expression vector (BacAg-SpV)1Oral salmonella enteritidis (3934 vac)
留言 (0)