The use of medicinal plants in plaque psoriasis has not been systematically studied. In this meta-analysis, some medicinal plants demonstrated significant therapeutic effects on reducing psoriatic plaques and can be recommended by physicians for use to improve the quality of life.
IntroductionPsoriasis is a chronic inflammatory disease of the skin affecting about 2-3% of the population.1,2 It sometimes affects the joints. The disease commonly presents as itchy, thick, dry, erythematous and scaly plaques that negatively affect the quality of life.3
Psoriasis in not curable and its treatment is individualised. In milder cases with limited skin involvement, topical therapy with corticosteroids, vitamin D3-derived drugs, dithranol (anthralin), retinoids (tazarotene) and tacrolimus, among others, are appropriate. Long-term adverse effects include local irritation, skin atrophy, telangiectasia and striae. However, in more extensive psoriasis, systemic treatments such as PUVA (psoralen plus ultraviolet-A radiation), cyclosporine, methotrexate and various biologics are often used.5 Systemic treatments may be contraindicated in patients with premalignant (e.g., xeroderma pigmentosum, albinism) and photosensitive dermatoses, patients with a history of melanoma or multiple skin cancers, in pregnant and lactating patients, in liver or kidney failure and in patients with immunodeficiency or blood dyscrasias.5,6
Medicinal plants contain organic substances that have pharmacological actions (e.g., anti-inflammatory effects). This vast field needs further development and there is an urgent need for clinical studies.
For any plant-derived products to be introduced for the treatment of disease, it is necessary to have extensive knowledge of the plant, its cultivation and harvest characteristics, its composition (active substances), pharmacological effects, dose and forms of use. Safety and efficacy studies both in animals and humans also need to be performed. Medicinal plant products can induce adverse events such as intoxication, acute nephritis, liver injury and abortion.7
The aim of this study was to carry out a systematic review of the literature on clinical trials of medicinal plants or substances derived from them in plaque psoriasis published from 2016 to 2020.
Methods Search strategyA systematic review was performed in accordance with the Cochrane guidelines. The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO; Regn No. CRD42021269042). The search for relevant randomized controlled trials published between 2016 and 2020 was performed in the MEDLINE, Embase, Lilacs and in the Cochrane library databanks. References found in the potentially relevant publications were screened for additional records. The search was conducted in 2021.
Selection criteriaStudies were selected by two blinded reviewers (LW, GG). Discrepancies were settled by consensus. The PICOT (Population, Intervention, Comparison(s), Outcome and Time/study period and date started) strategy was applied to originate the guiding question for the performance of the study. This resulted in the research question: “Do patients with psoriasis exhibit improvement when using products or substances derived from medicinal plants?”
Sub-questions were asked to obtain detailed specifications such as:
Can patients with psoriasis show improvement in itching, redness and scaling of the skin with the use of medicinal plant-based products?
Can patients with psoriasis improve their skin disease if they use herbal products?
Can psoriasis patients use herbal medicinal products as a combined therapeutic treatment?
Can patients with persistent psoriatic plaques improve with the use of herbal medicinal products?
The search strategy was carried out using the Cochrane Library and PubMed platforms to select the MeSH terms. These were organised into five categories of interest guiding the review and later combined with each other for the search using Boolean operators AND, OR for a match between search terms. The resulting categories were:
Category A - for terms specific to medicinal plants (healing plants; herbs; medicinal; medicinal herbs; medicinal plants; pharmaceutical plants; phytochemicals and flavonoids)
Category B - for generic search for randomized clinical trials and controlled clinical trials (randomized controlled trial; controlled clinical trial; comparative study; clinical trial; randomized; placebo; drug therapy; randomly; trial; groups)
Category C - specific for psoriasis (psoriasis; published from 2016 to 2020; arthritis, psoriatic)
Category D - for results (inflammatory activity; inflammatory; activity; follow-up; diagnose; diagnoses; diagnosis; diagnostic; treatment; treatments; therapy; therapies; therapeutic)
Category E - for types of psoriasis considering timeline (early; very early; immediate; immediate early; subclinical; late; late diagnoses).
A Prisma (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flowchart was used (http://prisma-statement.org/prismastatement/flowdiagram.aspx) to describe the information in the different phases of the review, mapping the number of identified entries, included and excluded, for the studies to be eligible for consideration.
Eligibility criteriaArticles published between 2016 and 2020 were considered eligible for study. The inclusion criteria adopted were in accordance with the PROSPERO protocol:
Population: individuals diagnosed with mild to moderate to severe psoriasis;
Intervention: use of medicinal plants (as an aid and/or therapeutic treatment or association with other psoriasis drugs);
Outcome: especially considering the outcomes that indicated an improvement in psoriatic lesions and/or an improvement in the quality of life of psoriasis patients, although other outcomes were also included;
Type of studies: randomized clinical trials to assess efficacy;
Time: period of treatment or intervention.
Articles in all languages were included. Studies with interventions or investigations performed in animals were excluded. Also excluded were studies that did not provide information on the use of medicinal plants as a therapeutic resource in patients with psoriasis and those that involved only conventional pharmacological treatments.
Study sourcesSearches were conducted electronically through the Cochrane Collaboration online library and LILACS, MEDLINE via PubMed and Embase platforms. Relevant studies were detected by crossing key words.
Data extractionTitles and abstracts were independently reviewed by two reviewers. Disagreements were settled by consensus. In cases of unsettled disagreement, the decision was to be taken by a third reviewer specialised in the subject; however, there were no unsettled disagreements and this was not necessary.
Data extraction from the articles was performed and recorded in a spreadsheet indicating the type of study, number of participants, comparator (if any), medicinal plant used, dosage, time of product use, main outcome, type of study and level of evidence. To ensure a structured review a narrative synthesis of studies meeting the inclusion criteria was conducted. The studies were screened to identify, select and evaluate their eligibility and adherence to the PRISMA 2020 recommendations. For transparency and completeness, a checklist of 27 items was applied to the review.8
Bias riskAll studies included were randomized and the risk of bias was jointly assessed by three reviewers, using the ROB2.0 as an instrument in which the risk in each bias domain was graded as either “high,” “unclear” or “low.”9
Evidence levelThe certainty of evidence was assessed using the Cochrane GRADE criteria.
Results Study selectionThe initial search retrieved a total of 2,268 studies. After applying the filters and crosswords only seven randomized clinical studies remained that constituted the final sample. Figure 1 shows the flowchart (PRISMA) of the bibliographic searches and demonstrates the results obtained initially and after application of the filters “in vivo,” “clinical study” and “period from 2016 to 2020”, indicating the scarcity of clinical trials in humans using active medicinal ingredients (both oral and topical) for the treatment of plaque psoriasis according to the criteria defined in this research.
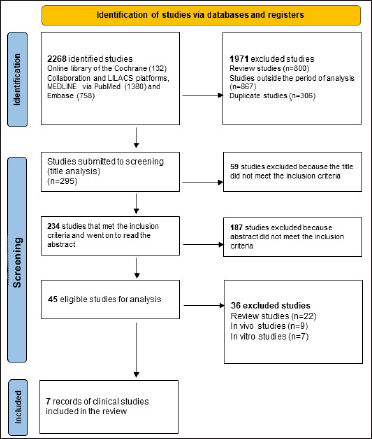
Figure 1:: Preferred reporting items for systematic reviews and meta-analysis flow diagram showing the methodology in selecting articles for final analyses10
Export to PPT
Table 1 details the characteristics of the publications included. All studies included in the review used PASI. The number of participants in each study ranged from 24 to 100 and a total of 389 patients with psoriasis completed the studies till the end. The studies ranged from 4 to 12 weeks. In 3 of the 7 studies an ointment formulation was used, while cream, lotion and oral formulations were used in 1 study each. The mode of usage was not specified in one study.
Table 1:: Characteristics of the randomized clinical trials included
Authors, year Number of participants included in the study Number of participants completed the study Intervention Form of product presentation Dosage/ dosage of extract Route of administration Use prescription Comparator Outcome Adverse events Study time Shatalebi et al. 202015 30 30 Glacilariaalgae Cream 3% Dermatological route Once a day Cream containing 0.05% clobetasol Psoriatic plaque reduction Mild itching 8 weeks Bahraini et al. 201814 73 73 Curcuma longa Hair tonic Not submitted by the authors Dermatological route Not submitted by the authors Placebo Decrease psoriasis nuisances Dryness of psoriatic lesions (only in two patients in the placebo group) 9 weeks Zorko et al. 201810 61 56 Abies alba Ointment 2% Dermatological route Two applications a day Placebo Improvement of psoriatic plaques, according to PASI None declared 12 weeks Lin et al. 201813 100 98 Indigo naturalis Ointment 200, 100, 50 or 10 µg/g of indirubin Dermatological route Twice a day Same vehicle in four different concentrations Regression of psoriatic plaques Eczema and contact dermatitis 12 weeks Cheng et al. 201711 24 23 Indigo naturalis Ointment 1:10 of Indigo naturalis powder Dermatological route Twice a day Placebo Significant improvement of psoriatic plaques Itching, rash and nasopharyngitis 8 weeks Yu et al. 201716 100 84 Peonia+ acitretin − 0,6 g Orally Twice a day Placebo + acitretin Improvement of psoriatic plaques None declared 12 weeks Rerknimitr et al. 201612 25 Gynura pseudochina Ointment Extract and vehicle (1:10) Dermatological route Twice a day Cream with 0.1% triamcinolone Non-significant statistical analysisFive of the 7 studies were clearly randomized [Figure 2]. In the remaining two studies, one10 did not mention the form of randomization and although the other11 stated that the study was conducted in a randomized manner but did not mention the type of randomization. All studies were blinded, but the form of blinding was unclear in the study by Rerknimitr et al.12 The risk of bias analysis was performed in 6 domains (Fig.2), with about 30% of high risk in domain 1 (randomization) and medium risk (some concerns) in about 20% in the other domains.9
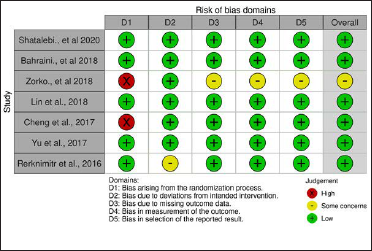
Figure 2:: Risk of bias summary; review of authors’ judgement about each risk of bias item for each study included
Export to PPT
The results were presented differently in the studies. Six studies clearly and objectively outlined the selection, measurement and description of the results, but Zorko et al.10 failed to describe how they carried out the measurement of the results, as well as the selection of results in relation to the effect measures.
Trend in favour of herbal treatmentsAfter reviewing and analysing the included studies, a spreadsheet populated with the information collected was developed followed by a statistical analysis of the data. The Forest Plot Graphic [Figure 3] shows considerable confidence intervals between the studies, I2 = 91%, 2.16 (0.97, 4.78) 95% confidence interval, (P < 0.00001) demonstrating no important inconsistency even with a high heterogeneity rate. The Funnel Plot Graphic [Figure 4] however, demonstrates that although there are less than 10 studies to carry out the meta-analysis, there was considerable homogeneity in their distribution with a trend favouring the herbal treatment.
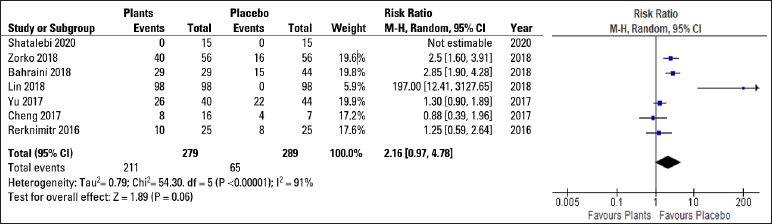
Figure 3:: Forest plot graphic
Export to PPT
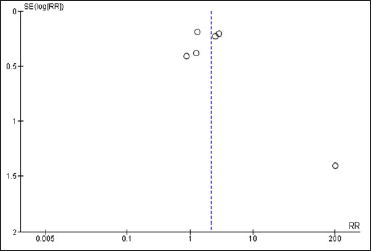
Figure 4:: Funnel plot graphic
Export to PPT
The certainty of evidence was assessed using the GRADE framework [Figure 5] which demonstrated a high level of evidence in the conduct of clinical trials that exhibited favourable action of herbal products for psoriasis.
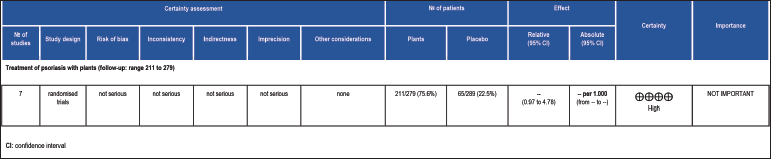
Figure 5:: Grading of recommendations assessment, development and evaluation
Export to PPT
DiscussionSince ancient times, medicinal plants have been used to treat various diseases. Most of the medicinal plants in the trials included in this review contain substances with an anti-inflammatory action. Although Cheng et al.11 and Lin et al.13 found that Indigo naturalis was effective in the treatment of psoriasis, it has not found widespread acceptance in the long-term treatment of this condition.
Bahraini et al.14 treated scalp psoriasis with Curcuma longa hair tonic and noted significant improvement in the quality of life. Improvements in the PASI score were assessed by Zorko et al.,10 who studied Abies alba and Rerknimitr et al.,12 who studied Gynura pseudochina. They did not find statistically relevant outcomes but they observed significant improvement in the clinical assessments using the PASI. They also noted improvement in the quality of life of patients with psoriasis after treatment.
With conventional dermatological treatments validated for psoriasis, the use of medicinal plants can be an alternative and auxiliary option. Shatalebi et al.15 studied the Gracilaria algae in comparison with clobetasol, while Yu et al.16 used Paeonia officinalis in combination with acitretin-the outcome in both studies were statistically significant, showing the beneficial effects of medicinal plants associated with conventional drugs for the treatment of psoriasis.
The sample sizes of the studies included were smaller than those typically found in large intervention trials. However, all of the research outcomes included (improvement in quality of life of psoriasis patients and significant changes in PASI score) were clinically significant.
The effectiveness of medicinal plants in patients with psoriasis is shown by the GRADE framework certainty of the evidence assessment.
ConclusionThis systematic review identified studies with a low risk of bias and a considerable level of evidence. Limitations included the small sample size and short time to assess therapeutic efficacy.
The studies in this meta-analysis showed that medicinal plants used as topical or oral products may be used either alone or in combination with other forms of treatment depending on the severity of psoriasis. Medicinal plants may enhance the quality of life of the patient by ameliorating psoriasis plaques as assessed by clinical metrics.
More studies are needed to complement the understanding of the therapeutic efficacy of these medicinal plants.
留言 (0)