Sir,
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), previously known as “drug-related baboon syndrome,” is a benign type IV hypersensitivity drug reaction characterised by symmetrical well-demarcated erythema mainly involving the gluteal/perianal area and/or V-shaped erythema of the inguinal/perigenital area.1 The cutaneous eruption tends to develop several hours to days after exposure to the offending drug. Rarely, SDRIFE-like rashes have been reported to develop in the absence of an apparently previous drug exposure.2 Recently, isolated reports of SDRIFE-like eruptions associated with either COVID-19 infection or vaccination have been described.2-7 We report a case of SDRIFE-like eruption related exclusively with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in the absence of any associated drug.
A 29-year-old woman who was not vaccinated for COVID-19, was admitted to the emergency room with fever, cough, dyspnea and a well-demarked and symmetrical rash which was mildly pruritic, that involved the groins, inner side of thighs, axillae, antecubital fossae and the lower trunk [Figure 1]. Rash appeared one day after the onset of respiratory symptoms. She denied any topical or systemic medication prior to the onset of rash. The patient was admitted to the hospital due to hypoxemia (PO2 70 mmHg) and mild bilateral pneumonia. Reverse transcription-polymerase chain reaction analysis on nasopharyngeal swab tested positive for SARS-CoV-2. Skin biopsy from the erythematous plaque on the thigh revealed focal spongiosis associated with exocytosis, some basal cell vacuolization, and superficial dermal perivascular lymphocytic infiltrate (with few eosinophils) [Figure 2]. A complete haematological and biochemical survey disclosed leucopenia (3600 cells/mm3), lymphopenia (790 cells/mm3 22%) and elevated levels of D-dimer (1600 ng/mL). Ferritin, C-reactive protein and erythrocyte sedimentation rate levels were normal. The patient required oxygen support for one week. Treatment with 6 mg per day of intravenous dexamethasone and antipyretics (paracetamol) along with triamcinolone acetonide cream 0.1% for skin lesions during 10 days was established with good clinical response and a complete resolution of skin rash.
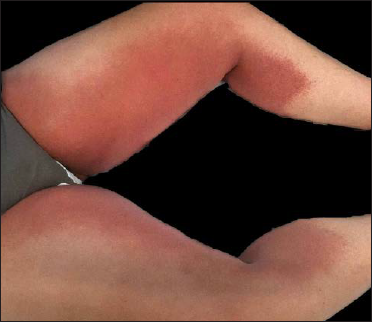
Figure 1:: Symmetrical erythemato-edematous plaques extending from the groin to the inner side of both thighs
Export to PPT
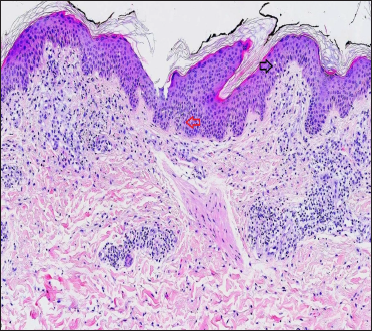
Figure 2:: Skin biopsy from an erythematous plaque on the thigh of a patient with coronavirus disease-2019 showing exocytosis (black arrow), focal spongiosis (red arrow) and superficial perivascular lymphocytic infiltrate (H&E, ×100)
Export to PPT
With a proven SARS-CoV-2 infection and a classical described rash for SDRIFE with no precipitating drug, a diagnosis of SDRIFE-like rash related to SARS-CoV-2 was established.
SDRIFE is a benign cutaneous reaction that takes place after the systemic administration of drug-related allergen. Clinically, it is manifested as a flexural symmetrically distributed skin exanthema mainly involving the buttocks and flexural areas. The face, palms, soles and mucosal surfaces are typically spared. Histological features are often non-specific. Basal cell vacuolization, often associated with necrotic keratinocytes and focal spongiosis, as well as the superficial perivascular inflammatory infiltrate, are usually observed.4 Beta-lactam antibiotics followed by non-steroidal anti-inflammatory drugs, immunoglobulin, antihypertensive, chemotherapeutic and biologic agents are the most frequently incriminated drugs.8 SDRIFE diagnosis must fulfil all the following criteria 1: sharply demarcated erythema of the gluteal/perianal area and/or V-shaped erythema of the inguinal/perigenital area, involvement of at least one other intertriginous/flexural region, the symmetry of affected areas and absence of systemic symptoms and signs in association with systemic drug exposure. Our patient fulfilled the criteria.
Furthermore, cutaneous eruptions mimicking SDRIFE have rarely been reported associated with erythrovirus B19 and Group A streptococcal infections.9,10 Recently, isolated reports of SDRIFE-like rashes associated with either SARS-CoV-2 vaccination or infection have been published.2-7 The real significance of these eruptions as a skin manifestation of COVID-19 disease remains unclear since in the vast majority of cases a concomitant or previous drug treatment was recorded.2-5,7 Additionally, SDRIFE-like reactions related to Pfizer-BioNTech COVID-19 vaccine (BNT162b2; Comirnaty) with skin biopsies showing vacuolar interface dermatitis with mild eosinophilic spongiosis have also been reported.6 The clinical and evolutive features of reported cases of SDRIFE-like eruptions associated with COVID-19 are detailed in Table 1.
Table 1:: Clinical features of previous SDRIFE-like eruptions related to SARS-CoV-2
Study Age (years)/Sex Comorbidities Rash description Duration of the rash Previous treatments SDRIFE treatment Manifestations of COVID-19 Mahé et al.2 64/F Type 2 diabetes Erythematous rash 5 days Paracetamol None Bilateral pneumonia Bevilaqua et al.7 71/F Asthma, hypothyroidism Erythematous papules converging on plaques 7 days Piperacillin/tazbactam, azithromycine,A wide range of cutaneous rashes associated to COVID-19 infection have been reported. Vesicular, morbilliform and urticarial eruptions, pseudo-chilblain lesions, livedoid and retiform purpuric rashes may appear at different times in the disease evolution and are associated with different severity, duration and prognosis.11 Our observation adds SDRIFE-like pattern as another rare cutaneous manifestation of SARS-CoV-2.
留言 (0)