Cerebral venous thrombosis (CVT) is a rare yet life-threatening cerebrovascular event with an estimated incidence of <1.5 per 1,00,000 annually (1). Major risk factors include female sex and prothrombotic conditions either inherited (i.e., thrombophilia) or acquired (i.e., antiphospholipid syndrome, use of oral contraceptives, malignancy, pregnancy, and puerperium) (2). Previous studies showed that CVT accounts for only 2% of pregnancy-related stroke (3) and most often presents in the third trimester of pregnancy and the puerperium because of its hypercoagulable state. CVT in early pregnancy is extremely rare, with evidence limited to only a few published reports (4). The aims of this study were to present our experience and to summarize all available literature to further elucidate the clinical manifestations, treatment, and outcomes of CVT in early pregnancy. To the best of our knowledge, this is the largest and with the longest post-pregnancy follow-up hitherto reported.
Materials and methods Case seriesBetween May 2018 and November 2021, we retrospectively reviewed the records of all patients hospitalized in the obstetrics and gynecology department of Renji Hospital, School of Medicine, Shanghai Jiao Tong University. A total of 26 hospitalized patients received a diagnosis of pregnancy-associated CVT, and seven patients (26.9%) diagnosed with CVT in early pregnancy (<12 weeks of gestations) were identified and included in this case series. We collected data regarding patients' characteristics, laboratory and neuroimaging findings, treatment modalities, maternal outcomes, and 6-month and 1-year follow-up. Written informed consent was obtained from all the participants before the start of the study. The study was performed in compliance with the Declaration of Helsinki.
Literature reviewWe searched the Medline, Embase, and Google Scholar databases to identify all English literature published using the keywords “cerebral venous thrombosis”, “intracranial venous thrombosis”, “first-trimester pregnancy”, “early pregnancy”, alone or in combination. We reviewed all articles (including case reports, case series, and review articles), and this resulted in the inclusion of fourteen case reports with a total of 15 patients (5–18).
Results Study populationWe included seven patients in our case series, and all of them were in the first trimester of pregnancy (<12 weeks of gestations). The mean age was 32.4 ± 2.28 years (range 27–42 years), and the mean BMI was 21.67 kg/m2 (range 19.5–23.4 kg/m2). Five (71%) out of the seven patients were primiparas, and two (N1 and N2) conceived through assisted reproductive technology (ART). None of the patients reported a past or family history of venous thromboembolism (VTE), thrombophilia, or autoimmune or hematologic diseases.
All the patients presented with nausea, vomiting, and various extents of headache. Five of them were initially diagnosed with hyperemesis gravidarum (HG), and the other two presented with mild nausea and vomiting. A visual analog scale (VAS) was used to assess the severity of headache on a scale of 0 to 10, with “0” indicating no pain and “10” indicating worst pain. The headaches described by the patients were mild at the beginning with a mean score of 3.86 and progressed to 7.16 after several days. The mean time from symptom onset to admission was 5.7 days (range 3–10 days). At the time of evaluation in our institution, all the patients presented with acute neurologic symptoms including aphasia (n = 5, 71.4%), limb weakness (n = 4, 57.1%), seizures (n = 2, 28.6%), altered mental status (n = 3, 42.9%), and blurred vision (n = 2, 28.6%). The Glasgow Coma Scale (GCS) was used to assess the extent of impaired consciousness. One patient (N4) presented as a transfer from an outside hospital due to acute comatose state with a GCS score of 3. Two patients, N6 and N7, had a score of 9 and 11, respectively, consistent with moderate brain injury and presented with various neurological deficits. The remaining four patients (N1, N2, N3, and N5) scored 13 or higher consistent with mild brain injury. A summary of the patients' characteristics is shown in Table 1.
TABLE 1
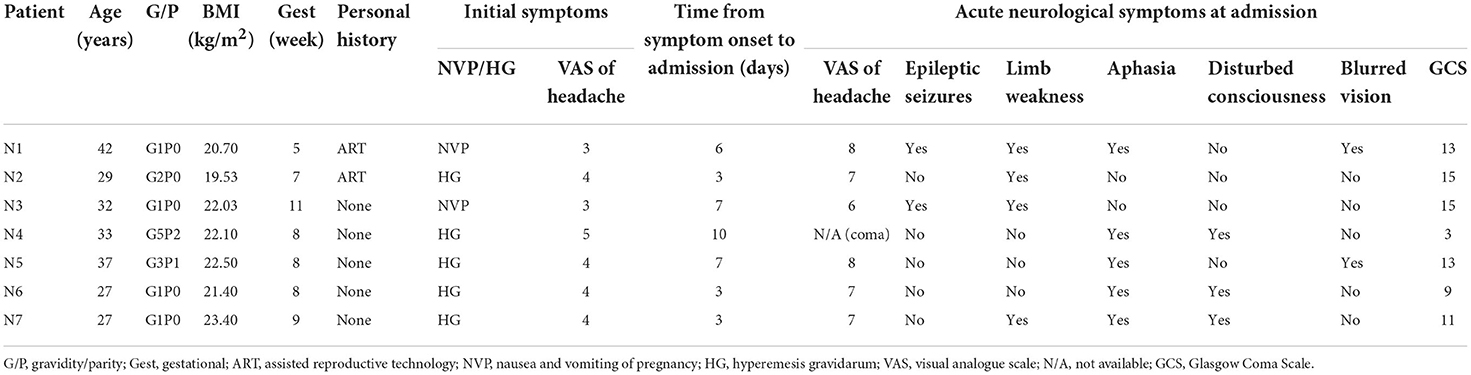
Table 1. Characteristics and clinical symptoms of included seven pregnancy cases with CVT.
Laboratory and radiographic findingsAs showed in Table 2, six of the total seven patients had elevated WBC count, and all of them, were found to have D-dimer elevation (range from 0.8 to 11.6 mg/L). Serum chemistries, thyroid function, and coagulation studies were normal/non-contributory in all the patients. With regard to thrombophilia testing, only one patient (N3) was found to have a mildly reduced protein S level (43.8%, normal range 50–80%). One patient (N5) was found to have a slightly positive antinuclear antibody (ANA) with a titer of 1:160, but the remaining autoimmune panels were negative. All the patients were ruled out for any autoimmune etiologies after the interdisciplinary evaluation by obstetricians and rheumatologists. In addition, two patients (N1 and N3) with concern of meningitis underwent lumbar puncture, and the results of cerebral fluid (CSF) studies were noncontributory except for elevated opening pressure.
TABLE 2
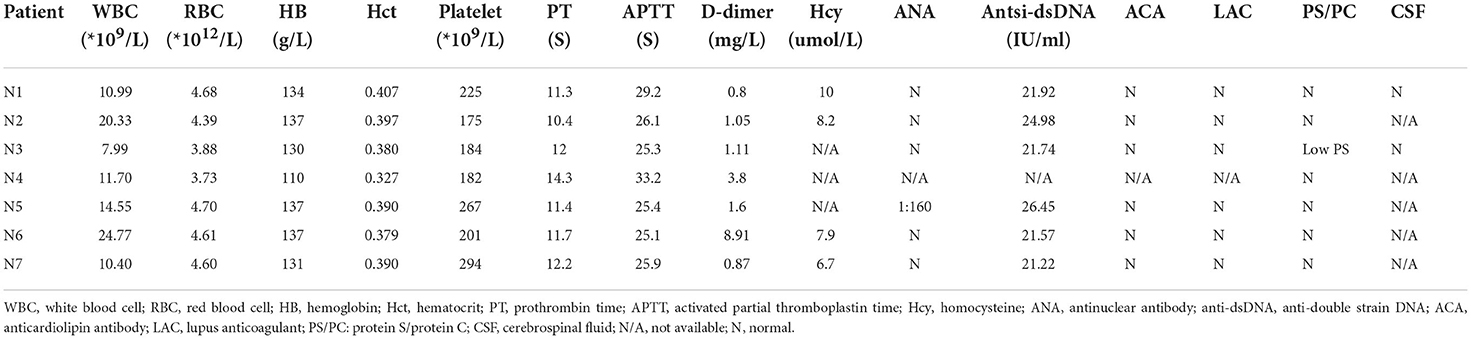
Table 2. Major relevant laboratory results of included patients with CVT.
Six patients underwent CT, and the findings demonstrated cerebral infarction (n = 3, 42.9%), intracranial hemorrhage (n = 3, 42.9%), subarachnoid hemorrhage (n = 3, 42.9%), and cerebral edema (n = 3, 42.9%); also, hyperdense lesions (n = 4, 57.1%) were found occasionally in the venous sinus, indicating thrombosis. Six patients underwent magnetic resonance venography (MRV), which demonstrated CVT in all of them. Most of the patients had multiple venous sinus involvement, and the mean number of venous sinuses involved was 3.7 (range 2–6), with the transverse sinus being the most common site (n = 7, 100%) followed by the sigmoid sinus (n = 5, 71.4%). Neuroimaging features of patients are shown in Table 3.
TABLE 3
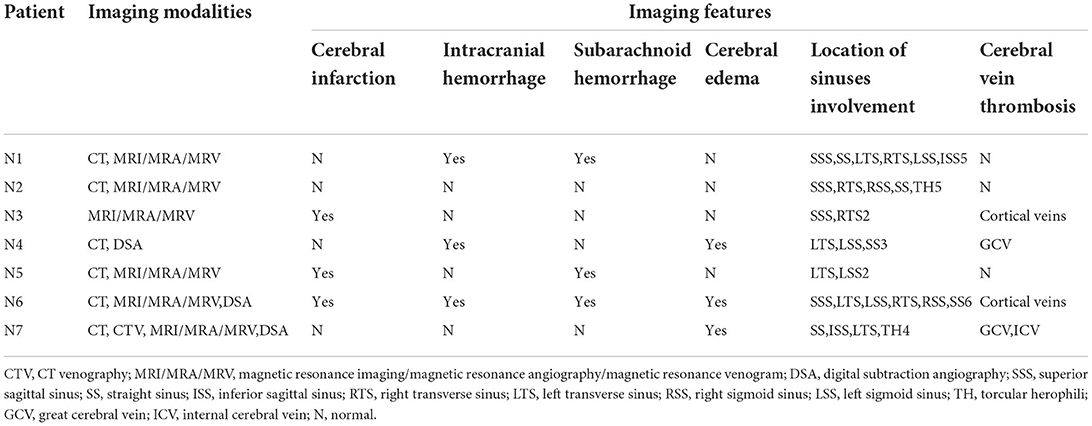
Table 3. Neuroimaging features of the included patients with CVT.
Therapy and outcomesAll the patients were started on subcutaneous low-molecular-weight heparin (LMWH) at full anticoagulation doses once the diagnosis of CVT was confirmed. Six patients (85.7%) received osmotic therapy such as mannitol and diuresis to decrease intracranial pressure. Three patients (42.9%) received antiepileptic therapy.
Three patients with evidence of moderate or severe brain injury proceeded with digital subtraction angiography (DSA), and two underwent endovascular intervention. The treatment course is described below. One patient (N4) presented with a GSC score of 3 (M1V1 E1); the emergent CT revealed cerebellar hemorrhage and brain stem edema, and the subsequent DSA confirmed a complete occlusion in the great cerebral vein, straight sinus, left transverse sinus, and sigmoid sinus. A decision was made to proceed with emergent venous thrombectomy with heparin and urokinase via injection through a microcatheter positioned directly to the clot. However, the intervention failed to dissolute the clot and the patient died 4 days after the procedure. One patient (N6) presented with a GCS score of 9 (M4V1 E4) and had radiographic evidence of subarachnoid hemorrhage, left frontal and bilateral parietal lobe hemorrhage complicated by diffuse cerebral edema. The DSA confirmed a complete superficial and deep venous occlusion. Figure 1 demonstrates the radiographic features of CVT in case 6. She underwent emergent venous thrombectomy followed by decompressive craniotomy. Unfortunately, the patient died on postoperative day 4 despite the above measures. One patient (N7) with a GCS score of 11 (M6 V1 E4) underwent DSA, which showed an occluded sinus, but the circulation was compensated by reflux flow from the sylvian vein. Thus, a decision was made to not proceed with endovascular intervention.
FIGURE 1
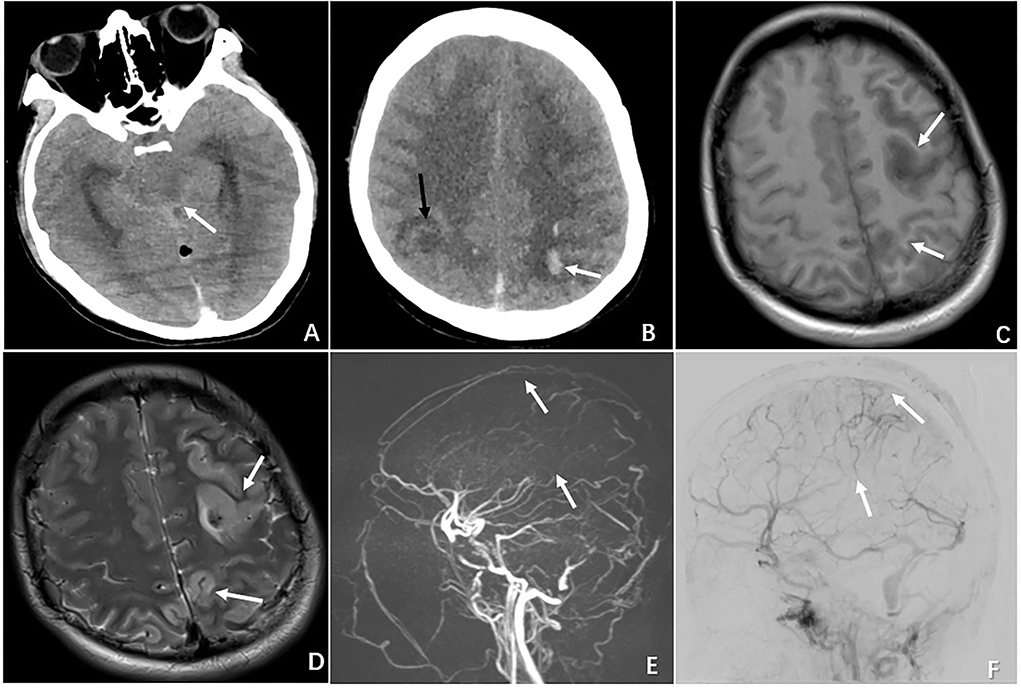
Figure 1. Radiographic features of CVT in the case of N6 who presented with severe neurological deterioration. (A) Non-contrast CT demonstrating brain swelling and ambient cistern compression (white arrow). (B) CT demonstrating multiple ischemic infarctions (black arrow) with a hemorrhagic component (white arrow). (C) MRI showing a left hyperintense lesion with a surrounding hypointense area on T1-weighted MRI. (D) MRI demonstrating the same hypointense lesion with a surrounding large hyperintense area on T2-weighted MRI. (E) MRV showing complete occlusion of the superior sagittal sinus (superior arrow) and inferior sagittal sinus (inferior arrow). (F) DSA demonstrating occlusion of the superior sagittal sinus (superior arrow) and inferior sagittal sinus (inferior arrow).
Of the 7 patients, two (N4 and N6) died within 5 days because of rapid deterioration. The remaining five patients and their families requested induced abortion; thus, when they were stabilized by the evaluation of a multidisciplinary team, induced abortion with consent proceeded. LMWH was held 12 h prior to the surgery, and the average time from hospitalization to abortion was 6.5 days. The mean length of stay of the surviving patients was 21.4 days. Two patients (N2 and N7) experienced complete recovery and resolution of neurological symptoms on discharge. After pregnancy termination, four patients (N1, N2, N3, and N5) received warfarin, and the doses were strictly adjusted based on an International Normalized Ratio (INR) target of 2–3. The most recently hospitalized patient (N7) received rivaroxaban (20 mg daily) upon discharge.
All the patients remained on anticoagulant therapy for 1 year. On the 6-month follow-up, all of them showed complete resolution of neurologic deficits. MRI or MRV was performed either on the 6 or 12-month follow-up visit, and all the patients had radiographic evidence of recanalization of sinuses and/or resolution of thrombi. Table 4 summarizes the treatment and outcomes of the seven patients with CVT. Figure 2 compares the radiographic findings of patients (N2, N3, N5) with CVT on admission and on discharge follow-up.
TABLE 4
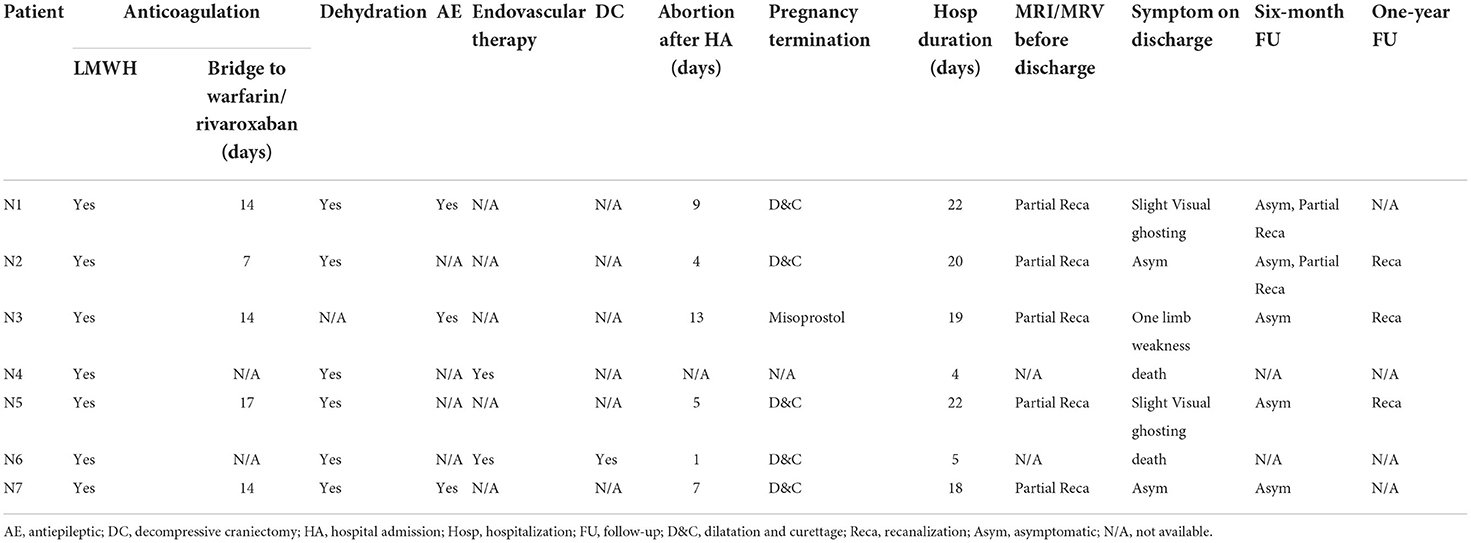
Table 4. Treatment and outcomes of the included patients with CVT.
FIGURE 2
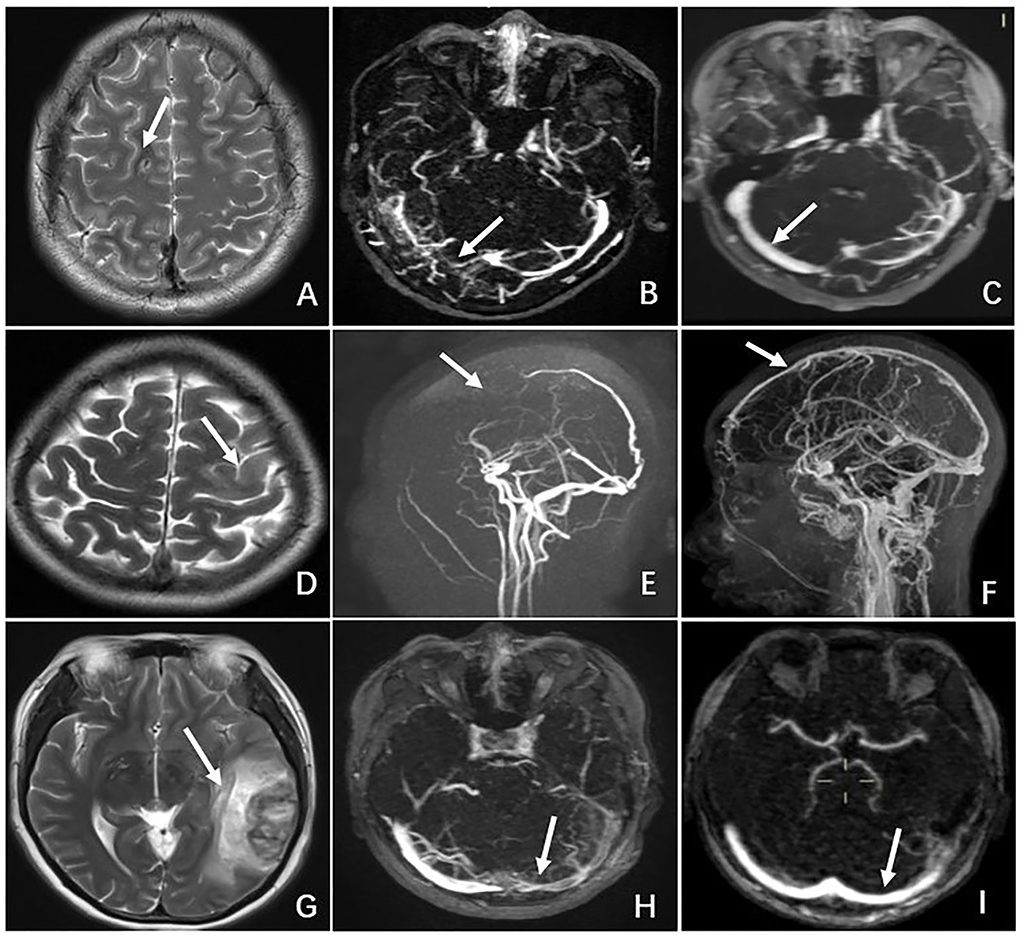
Figure 2. Comparison of radiographic features of CVT between admission and discharge follow-up in 3 cases. Patient N2: (A) MRI on admission showing a hyperintense lesion in the right frontal lobe on T2-weighted MRI. (B) MRV on admission revealing occlusion of the right transverse sinus. (C) MRV on 6-month follow-up demonstrating recanalization of the right transverse sinus. Patient N3. (D) MRI on admission showing a hyperintense lesion in the left frontal lobe on T2-weighted MRI. (E) MRV on admission revealing occlusion of the superior sagittal sinus and cortical vein. (F) MRV on 12-month follow-up demonstrating recanalization of the superior sagittal sinus and cortical vein. Patient N5. (G) MRI on admission showing a hyper-and hypo-density lesion in the left temporal lobe on T2-weighted MRI. (H) MRV on admission revealing occlusion of the left transverse sinus. (I) MRV on 6-month follow-up demonstrating recanalization of the left transverse sinus.
Literature reviewOur literature review resulted in the inclusion of 15 patients. The first 3 reported cases were diagnosed by postmortem autopsy (two cases in 1957 and one case in 1978), and the remaining 12 cases were diagnosed by neuroimaging. The mean age of the 15 patients was 28.9 ± 2.28 years. Six (40%) out of the 15 patients reported predisposing factors including history of seizure, migraine, antiphospholipid syndrome, oral contraceptive use, and heterozygous factor V Leiden mutation, or history of CVT. All the 15 patients presented with typical neurological symptoms, with headache being the most common symptom (n = 12, 80%) followed by seizures (n = 6, 40%), altered mental status (n = 3, 20%), aphasia (n = 2, 13.3%), and hemiparesis (n = 2, 13.3%). It is noteworthy that most of the patients also presented with nausea and vomiting of pregnancy (NVP) (n = 5, 33.3%) and hyperemesis gravidarum (HG, n = 3, 20%). Eleven patients (73.3%) received therapeutic anticoagulation, which included intravenous heparin, subcutaneous LMWH, warfarin, acetylsalicylic acid, and eptifibatide. One patient (6.7%) underwent catheter-directed thrombolysis and thrombectomy. Two patients (13.3%) underwent decompressive craniectomy. Eight patients (53.3%) had complete recovery and resolution of symptoms, while four patients (26.7%) showed residual neurological dysfunction including limb weakness, visual field defect, hemiplegia, and aphasia. Six (40%) patients delivered in the third trimester, and two of them underwent elective cesarean section. All the babies were born healthy. Table 5 summarizes the clinical characteristics and outcomes of the fifteen CVT cases in early pregnancy in the published literature.
TABLE 5
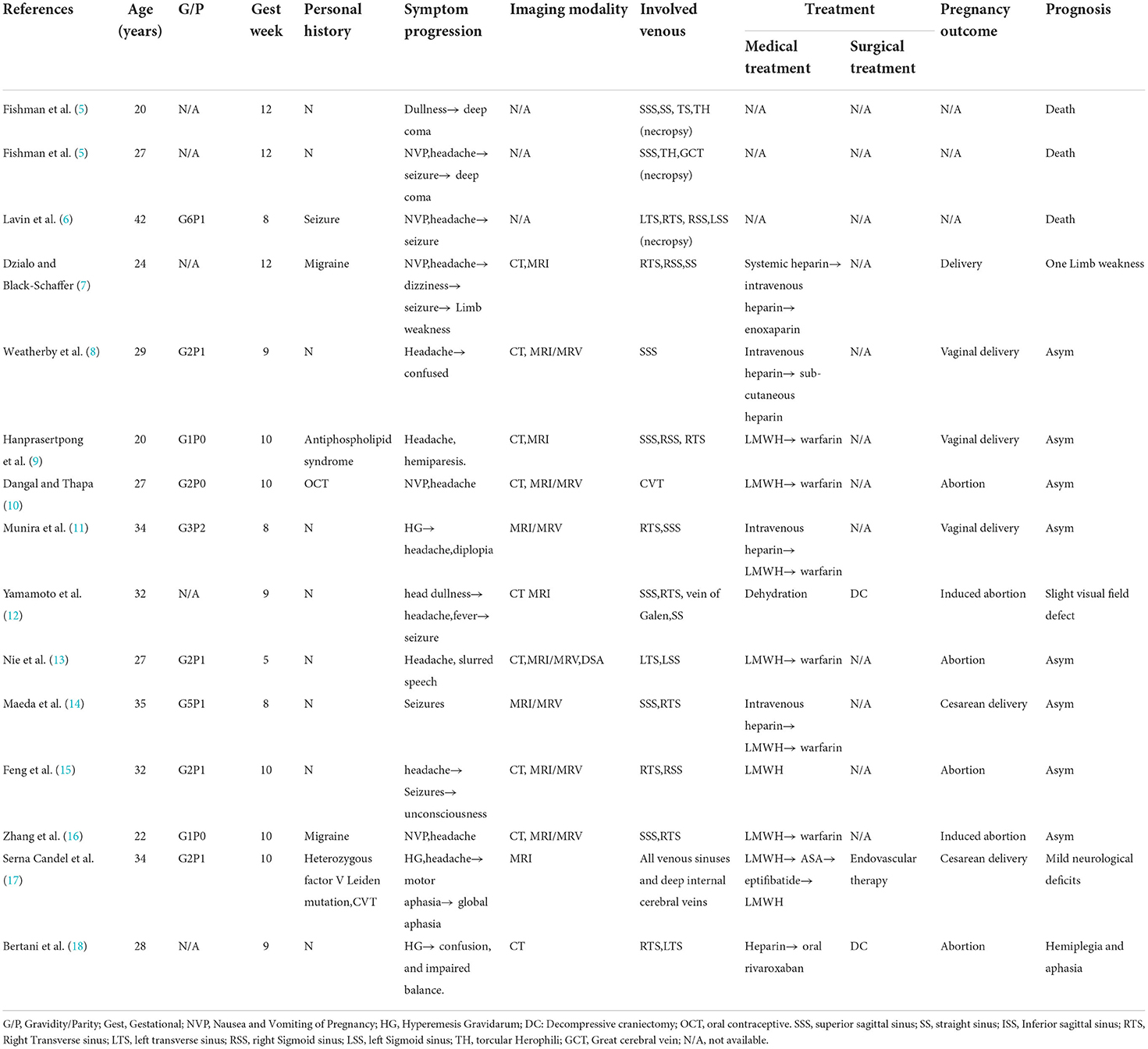
Table 5. Clinical characteristics and outcome of the 15 included patients with CVT in published literature.
DiscussionCVT is a rare case of pregnancy-related stroke, especially in early pregnancy. In the present study, we reported a retrospective case series of CVT presenting in early pregnancy complicated by nausea and vomiting in pregnancy/hyperemesis gravidarum (NVP/HG). NVP is a common condition with prevalence rates of up to 50–80% for nausea and 50% for vomiting and retching (19). The Royal College of Obstetricians and Gynecologists' (RCOG) green-top guidelines suggest that NVP/HG is a risk factor for VTE, as it often results in dehydration, malnutrition, and anemia, leading to hemoconcentration (20). Fiaschi L et al. reported a cohort study on 82,11,850 pregnancies resulting in live births or stillbirths, and women with HG had increased odds of antenatal VTE, including deep vein thrombosis (OR 1.94, 99% CI 1.57, 2.39) and pulmonary embolism (OR 2.54, 99% CI 1.89, 3.4) (21).
The pathogenesis of CVT remains to be incompletely understood, but two predominant mechanisms that may contribute to the clinical features of CVT have been proposed: (1) thrombosis of sinus results in increase in venular and capillary pressure leading to serial parenchymal insults including ischemia, edema, and hemorrhage; (2) occlusion of cerebral veins results in impaired CSF absorption leading to elevated intracranial pressure. Headache, generally indicative of an increase in intracranial pressure, is the most common symptom and presents in nearly 90% of patients with CVT (22). In our case series, all patients presented with nausea, vomiting, and various extent of headache with an average from 3.86 to 7.16 score by VAS over several days. However, headache is nonspecific and is often considered a common presentation of NVP/HG; as a result, a diagnosis of CVT is often overlooked initially in the absence of other neurological symptoms. In addition, as shown in the literature review of the 15 patients, NVP was found in 5 patients and HG was found in 2 patients, with headache being the most common symptom (n = 12, 80%). The multivariate analysis in a systematic review demonstrated that headache alone was associated with favorable outcome for CVT in pregnancy and puerperium (p = 0.04), and that coma/obtundation was associated with worse outcome (p = 0.03) (23). Likewise, our results indicated that low GCS score on admission may predict poor prognosis for patients with CVT in early pregnancy. Thus, clinicians should be prudent that persistent or aggravating headaches in patients with NVP/HG could be an initial sign of CVT, and that early diagnosis and prompt treatment are important. Delay in diagnosis can lead to irreversible and life-threatening neurological sequelae, as shown in our case series.
The mainstay of treatment for CVT is anticoagulation with intravenous heparin or subcutaneous LMWH. In our case series, all the patients received subcutaneous LMWH with full anticoagulation doses once the diagnosis was confirmed. Warfarin and new oral anticoagulants (NOAC) are contraindicated in pregnancy because of potential teratogenic effects and risk of fetal hemorrhage (24). There is no definitive evidence regarding the optimal duration of anticoagulant therapy. In our case series, 5 patients remained on anticoagulant therapy for 1 year to reduce the risk of recurrent CVT: 4 patients received warfarin with an INR goal of 2–3, and the most recently hospitalized patient received rivaroxaban (20 mg daily). No adverse effects from the anticoagulant therapy were reported, and all the patients showed radiographic evidence of recanalization or thrombus resolution on the 12-month follow-up.
Endovascular treatment of CVT remains controversial and is usually reserved for patients who are comatose or deteriorating despite anticoagulation. A systemic review of 183 cases of CVT undergoing mechanical thrombectomy revealed that 40% of the patients were in a comatose state at the time of the procedure. Following mechanical thrombectomy, 9% of the patients had worsening or new intracranial hemorrhage, 69% had complete recanalization, and 35% had complete recovery (25). Decompressive craniectomy has shown to be beneficial to critically ill patients with radiological and clinical features of mass effect, signs of brainstem dysfunction, and/or refractory intracranial hypertension (26). However, evidence regarding pregnant or puerperal populations is limited. In our case series, venous thrombectomy was performed on two patients, both experienced rapid deterioration and died. One patient subsequently proceeded with decompressive craniotomy.
We do not have enough evidence to provide the fetal outcomes of CVT in early pregnancy in our study. A shared decision was made after a thorough discussion of the diagnoses, treatment options, maternal and fetal outcomes, and all the five patients decided to proceed with pregnancy termination. Based on the literature review, 6 (40%) out of the 15 patients delivered in the third trimester and all the babies were born healthy. We believe that CVT is not an absolute indication for pregnancy termination, and it has been reported that in women with prior cerebral venous thrombosis, recurrent venous thrombotic events during subsequent pregnancies are infrequent (27, 28). Thus, the decision-making on continuing or terminating a pregnancy should be a shared process and based on the patient's ideologies. A multidisciplinary team of specialists with expertise should be involved in management of CVT in early pregnancy given the complexity and acuity of its nature.
ConclusionIn summary, CVT in early pregnancy represents a diagnostic challenge given its rarity and nonspecific overlapping clinical features with HG, which could lead to delay in diagnosis and result in rapid deterioration. Urgent MRI/MRV remains the cornerstone for diagnosis, and anticoagulation is the mainstay of treatment. CVT in early pregnancy is not an absolute indication for pregnancy termination. A multidisciplinary team of specialists with expertise should be involved in management of CVT in early pregnancy. Larger patient series with a longer follow-up are warranted to draw more definitive conclusions on the subject.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statementEthical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients or patients legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributionsCW, JL, and NZ conceived and designed the study and interpreted the results. CW collected and analyzed the data. XH: figure preparation. CW and KL contributed to the writing of the manuscript. All authors contributed to the article, read, and approved the final version of the manuscript.
FundingThis study was supported by the Natural Science Foundation of Science and Technology Commission of Shanghai Municipality (No. 22ZR1438700).
AcknowledgmentsWe acknowledge multidisciplinary members of the Obstetrics, Interventional Radiology, Neurosurgery, Intensive Care Unit, and Anesthesia teams for their dedication and support. All authors would like to thank the patients who shared their time and agreed to participate in this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AbbreviationsART, assisted reproductive technology; CVT, cerebral venous thrombosis; DSA, digital subtraction angiography; VAS, visual analog scale; GCS, glasgow coma scale; HG, hyperemesis gravidarum; LMWH, low-molecular-weight heparin; MRI, magnetic resonance imaging; MRV, magnetic resonance venography; VTE, venous thromboembolism.
References2. Pizzi M, Alejos D, Siegel J, Kim B, Miller D, Freeman W. Cerebral venous thrombosis associated with intracranial hemorrhage and timing of anticoagulation after hemicraniectomy. J Stroke Cerebrovasc Dis. (2016) 25:2312–6. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.025
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Saposnik G, Barinagarrementeria F, Brown R, Bushnell C, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2011) 42:1158–92. doi: 10.1161/STR.0b013e31820a8364
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Gioti I, Faropoulos K, Picolas C, Lambrou M. Decompressive craniectomy in cerebral venous sinus thrombosis during pregnancy: a case report. Acta Neurochir. (2019) 161:1349–52. doi: 10.1007/s00701-019-03921-5
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Weatherby S, Edwards N, West R, Heafield M. Good outcome in early pregnancy following direct thrombolysis for cerebral venous sinus thrombosis. J Neurol. (2003) 250:1372–3. doi: 10.1007/s00415-003-0173-6
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Hanprasertpong T, Hanprasertpong J, Riabroi K. Cerebral venous sinus thrombosis in early pregnancy: an unusual presentation of primary antiphospholipid syndrome. J Obstet Gynaecol Res. (2009) 35:1125–8. doi: 10.1111/j.1447-0756.2009.01088.x
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Munira Y, Sakinah Z, Zunaina E. Cerebral venous sinus thrombosis presenting with diplopia in pregnancy: a case report. J Med Case Rep. (2012) 6:336. doi: 10.1186/1752-1947-6-336
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Yamamoto J, Kakeda S, Takahashi M, Idei M, Nakano Y, Soejima Y, et al. Severe subarachnoid hemorrhage associated with cerebral venous thrombosis in early pregnancy: a case report. J Emerg Med. (2013) 45:849–55. doi: 10.1016/j.jemermed.2013.05.063
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Nie Q, Guo P, Ge J, Qiu Y. Cerebral venous sinus thrombosis with cerebral hemorrhage during early pregnancy. Neurosciences. (2015) 20:48–51.
PubMed Abstract | Google Scholar
14. Maeda Y, Satoh K, Haboshi T, Hanaoka M, Shimada K, Matsuzaki K, et al. A case report of congenital protein C deficiency with cerebral venous sinus thrombosis during early pregnancy period. No shinkei geka. Neurological Surgery. (2017) 45:913–18. doi: 10.11477/mf.1436203617
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Feng X, Zhao T, Liu J, Zhou C. Cerebral venous sinus thrombosis with cerebral hemorrhage presenting with status epilepticus in early pregnancy. Clin Lab. (2018) 64:611–4. doi: 10.7754/Clin.Lab.2017.171110
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Zhang X, Zhang Z, Li N. An early pregnant Chinese woman with cerebral venous sinus thrombosis succeeding in induction of labor in the second trimester. Chin Med Sci J Chung-kuo i hsueh k'o hsueh tsa chih. (2018) 33:267–71.
PubMed Abstract | Google Scholar
17. Serna Candel C, Hellstern V, Beitlich T, Aguilar Pérez M, Bäzner H, Henkes H. Management of a decompensated acute-on-chronic intracranial venous sinus thrombosis. Ther Adv Neurol Disord. (2019) 12:1756286419895157. doi: 10.1177/1756286419895157
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Bertani R, Rodrigues R, Koester S, Vasconcelos F., Monteiro R. Complicated cerebral venous thrombosis during the first trimester of pregnancy. Cureus. (2020) 12:e10683. doi: 10.7759/cureus.10683
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Revell B, Smith R. Thrombosis and embolism in pregnancy and the puerperium, reducing the risk: what proportion of patients reach the threshold for thromboprophylaxis? Obstetric Med. (2011) 4:12–4. doi: 10.1258/om.2010.100042
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Fiaschi L, Nelson-Piercy C, Gibson J, Szatkowski L., Tata L. Adverse maternal and birth outcomes in women admitted to hospital for hyperemesis gravidarum: a population-based cohort study. Paediatr Perin Epidemiol. 32 (2018) 40–51. doi: 10.1111/ppe.12416
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Duman T, Uluduz D, Midi I, Bektas H, Kablan Y, Goksel B, et al. A multicenter study of 1144 patients with cerebral venous thrombosis: the VENOST study. J Stroke Cerebrovasc Dis. (2017) 26:1848–57. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.020
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Kashkoush A, Ma H, Agarwal N, Panczykowski D, Tonetti D, Weiner G, et al. Cerebral venous sinus thrombosis in pregnancy and puerperium: a pooled, systematic review. J Clin Neurosci Off J Neurosurg Soc Aust. 39 (2017) 9–15. doi: 10.1016/j.jocn.2017.02.046
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Durmu? B, Yperzeele L, Zuurbier S. Cerebral venous thrombosis in women of childbearing age: diagnosis, treatment, and prophylaxis during a future pregnancy. Ther Adv Neurol Disord. (2020) 13:1756286420945169. doi: 10.1177/1756286420945169
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Siddiqui F, Dandapat S, Banerjee C, Zuurbier S, Johnson M, Stam J, et al. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke. (2015) 46:1263–8. doi: 10.1161/STROKEAHA.114.007465
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Ferro J, Crassard I, Coutinho J, Canhão P, Barinagarrementeria F, Cucchiara B, et al. Decompressive surgery in cerebrovenous thrombosis: a multicenter registry and a systematic review of individual patient data. Stroke. (2011) 42:2825–31. doi: 10.1161/STROKEAHA.111.615393
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Aguiar de Sousa D., Canhão P., Crassard I., Coutinho J., Arauz A., Conforto A., et al. Safety of pregnancy after cerebral venous thrombosis: results of the ISCVT (International Study on Cerebral Vein and Dural Sinus Thrombosis)-2 PREGNANCY Study. Stroke. (2017) 48: 3130–33. doi: 10.1161/STROKEAHA.117.018829
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Aguiar de Sousa D, Canhão P., Ferro J. Safety of pregnancy after cerebral venous thrombosis: systematic review update. J Neurol. (2018) 265: 211–12. doi: 10.1007/s00415-017-8666-x
留言 (0)