Randomized clinical trials have shown the benefit of acute mechanical thrombectomy (MT) in the treatment of acute ischemic stroke with large vessel occlusion (LVO) and are now considered standard care (1–6). Factors associated with the outcome following MT include non-modifiable factors, such as age or stroke symptoms severity, whereas time from onset to reperfusion is potentially modifiable (7–10). The impact on the functional outcome of time from onset to reperfusion has been studied; however, once patients are scanned and found eligible for acute MT, less is known about the impact delay from imaging to reperfusion (11–13). From studies on treatment with intravenous thrombolysis (IVT), workflow optimizations have been shown to reduce delay at every interval and improve the outcome of patients (14).
One proposed strategy for reducing time to reperfusion in MT is to bypass IVT stroke units without MT-capability (non-MT centers) for patients with suspected LVO and directly admit these selected patients to an MT-capable center (15). However, the recently published RACECAT study did not show the benefit of direct admission to an MT-capable center (16). Based on modeling data, another study suggested that in urban areas, patients with suspected LVO should be admitted directly to an MT-capable center if the transportation time did not increase more than 30 min compared to the transportation time to the nearest non-MT center (17).
In this study, we investigated the effect of relocating IVT services from a non-MT center to an MT-capable center in an urban setting with the same catchment area, and whether this organizational change was associated with reduced time to MT and with functional outcome at 90 days.
Materials and methods Study populationThe IVT services were relocated from a non-MT center to an MT-capable center on 17 April 2018; the two facilities were positioned 13 km apart in an urban area. Before the relocation of the IVT services, the non-MT center served as a primary stroke center for the catchment area (1.8 million inhabitants, census 2018), where all patients with suspected acute ischemic stroke were admitted for initial examination and potential treatment with IVT, and patients were only transferred to the MT-center if an LVO was identified. For the identification of LVO, either computed tomography (CT)-angiography or magnetic resonance (MR)-angiography was used. Prior to the relocation, no patients were acutely admitted for IVT treatment at the MT-capable center, and following the relocation, IVT was no longer provided at the non-MT center. The catchment area remained the same before and after the relocation, and only the location of IVT services was altered. Emergency Medical Services (EMS) in Denmark is an integral part of the universal healthcare system as a single provider of prehospital services for all citizens. Following dispatch, if EMS personnel suspected the patient of acute stroke, the neurologist on call at the IVT services was contacted for consultation and possible acute admission. All patients would be admitted directly to the scanning room for acute stroke imaging during the entire observation period and at both hospitals.
At both hospitals, IVT was administered in the scanner room immediately following imaging and within 4½ h after symptoms onset according to international guidelines (18). Imaging sequences acquired at the non-MT center in period 1 were transferred electronically for evaluation at the MT center. Assessment of eligibility for MT was shared by a vascular neurologist and a neurointerventionist. There was no upper age limit, but relative contraindications included pre-stroke modified Rankin Scale (mRS) score of ≥3, comorbidities that could increase the risk of procedural complications and infarct volume >1/3 of the middle cerebral artery territory, and clinical/imaging mismatch should be suggestive of salvageable tissue. The procedure was performed at the discretion of the neurointerventionist and included the use of thrombus aspiration and stent retrievers (devices used included Solitaire stent, Medtronic, Minneapolis, MN, USA; EmboTrap, Neuravi, Galway, Ireland; ERIC, MicroVention Terumo, Aliso Viejo, CA, USA; pReset, Phenox, Bochum, Germany; Capture, Medtronic, Minneapolis, MN, USA), and intraarterial thrombolytics. Patients referred for EVT from other IVT stroke units were not included in the analyses.
The observation period was divided into two periods: period 1 from 1 January 2017 to 16 April 2018, and period 2 from 17 April 2018 to 31 December 2019 (before and after the relocation). Adult patients were consecutively included, and the following data were obtained: sex (male or female), age (years), time of stroke symptoms onset registered as either known/witnessed or as when the patient last seen well (LSW), pre-stroke mRS, pre-procedural National Institute of Health Stroke Scale (NIHSS), and, at 24 h following EVT, reperfusion results in the modified treatment in cerebral infarction (mTICI) score ranging from 0 (no reperfusion) to 3 (full reperfusion) as assigned by the attending neurointerventionist, and treatment with IVT (yes/no).
Time metrics were divided into time from ambulance departure from stroke onset location to first imaging (ambulance-imaging) and from imaging to reperfusion (imaging-reperfusion) (Figure 1). The time of ambulance departure was retrospectively collected from emergency medical service records. Time of imaging was defined as the automatic time label applied by imaging software on the first imaging sequence of either CT-angiography or MR-angiography. Reperfusion was defined as the time of satisfactory angiographic result achieved or as the time of procedure termination when no reperfusion was achieved (mTICI 0), or if no LVO was visualized on the first run of the digital subtraction angiography. Evaluation of mRS at 90 days follow-up was performed at the outpatient clinic of the MT-capable center by trained neurovascular clinicians.
FIGURE 1
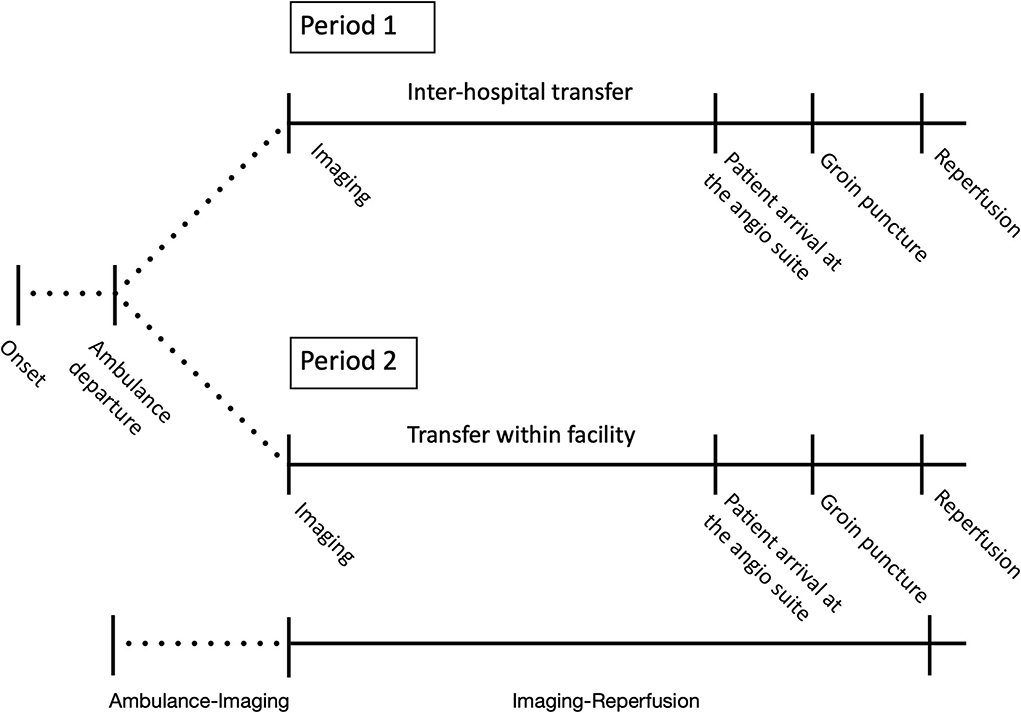
Figure 1. Changed patient flow following the relocation of IVT services. Period 1 corresponded to before the relocation of the IVT services (1 January 2017–16 April 2018) and period 2 to after the relocation (17 April 2018–31 December 2018). In period 1, IVT services were located at a non-MT center, and in period 2, IVT services were located at an MT-capable center. Time metrics were divided into time from ambulance departure from stroke onset location to first imaging after admission at the first hospital (ambulance-imaging) and from first imaging after admission to reperfusion (imaging-reperfusion). In period 1, imaging-reperfusion included inter-hospital transfer.
This study was approved by appropriate authorities, Danish Patient Safety Authority, and the Center for Regional Development (Reference R-21010059) and was exempted from approval by the ethics committee.
OutcomesThe primary analysis was for changes in time of ambulance-imaging and imaging-reperfusion between period 1 and period 2. Secondary analysis was functional outcome using mRS with favorable outcome defined as mRS 0–2 and non-favorable outcome defined as mRS 3–6. Furthermore, we analyzed the association of time in imaging-reperfusion with multivariate shift analysis of mRS at 90 days.
StatisticsFor patient characteristics, continuous variables were summarized as medians with interquartile ranges (IQR), and categorical variables were presented as frequencies with percentages. For continuous variables, differences were assessed using Wilcoxon-Mann-Whitney U test. For categorical variables, the Pearson χ2 test was used to compare the distributions between groups.
The association of imaging-reperfusion in 10-min increments with shifts in functional outcome (mRS) at 90 days was analyzed by means of ordinal logistic regression adjusted for age (continuous), sex (male/female), NIHSS, general anesthesia or consciousness sedation, registration as known onset or last seen well, pretreatment with IVT (yes/no), and observational period (period 1/period 2). The level of statistical significance was set at p < 0.05. The statistical analyses were performed using IBM SPSS Statistics 28.
ResultsDuring the observation period, a total of 712 patients had groin puncture for MT, and of these, 253 patients were included for analysis as the remaining 459 patients were referred from other primary stroke centers not participating in this study. A total of 73 patients were admitted to the non-MT center in period 1, and 180 patients were directly admitted to the MT-capable center in period 2 (Table 1). Baseline characteristics were evenly distributed but differed significantly with regard to the proportion of patients receiving pre-treatment with IVT, which decreased from 49 (67.1%) in period 1 to 83 patients (46.1%) in period 2, p = 0.002 (Table 1). From period 1 to period 2, there was a significant increase in time from stroke symptoms onset to ambulance departure from a median of 60 min (IQR 30–159) to 97 min (IQR 38–352), p = 0.02, predominantly driven by LSW patients (Table 2).
TABLE 1
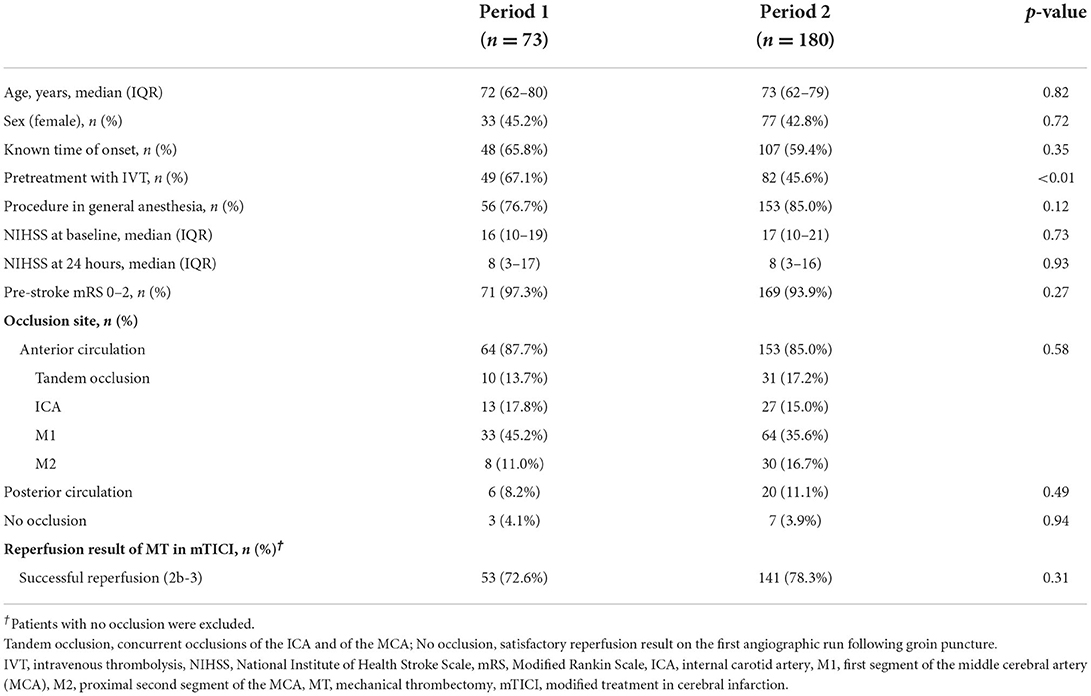
Table 1. Baseline characteristics.
TABLE 2
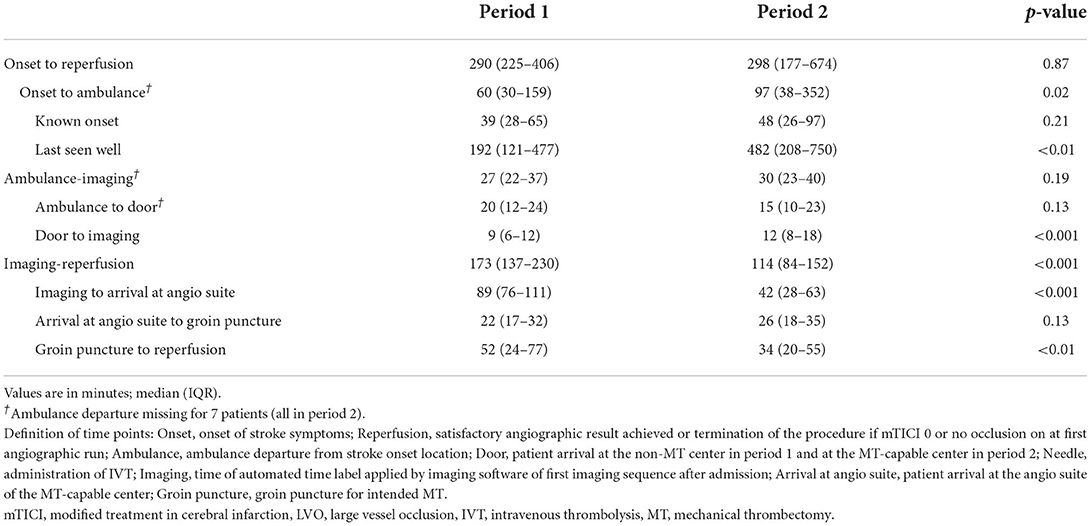
Table 2. Logistical measures.
Time from ambulance departure to imagingThe duration of the interval ambulance-imaging remained stable, median 27 min (IQR 22–37) in period 1 and 30 min (IQR 23–40) in period 2, p = 0.19 (Table 2). Median time from ambulance departure to door decreased from 20 (IQR 12–24) to 15 (IQR 10–23), although not statistically significant, p = 0.13. We observed an increase in door to imaging, median 9 min (IQR 6–12) to 12 min (IQR 8–18), p < 0.01.
Imaging to reperfusionThe duration of imaging-reperfusion decreased from period 1 to period 2, median 173 min (IQR 137–230) to 114 min (IQR 84–152), p < 0.001 (Table 2). The largest absolute reduction was from imaging to patient arrival at the angio suite from a median of 89 min (IQR 76–111) in period 1 to 42 min (IQR 28–63), p < 0.001. In period 1, this interval included the inter-hospital transfer from the non-MT center to the MT-capable center, while no inter-hospital transfer was necessary in period 2 as IVT and MT were provided within the same center. A significant reduction was also observed in procedural time (from groin puncture to reperfusion) from a median of 52 min (IQR 24–77) in period 1 to 34 min (IQR 20–55) in period 2, p < 0.01.
There was no observed difference in time from patient arrival at the angio suite to groin puncture between patients receiving general anesthesia compared to conscious sedation, median 25 min (IQR 18–34) and 24 min (IQR 16–35), p = 0.46, respectively.
In the subpopulation of patients pretreated with IVT [49 patients (67.1%) in period 1 and 82 patients (45.6%) in period 2], the time from door to needle increased from a median of 17 min (IQR 13–26) to 22 min (IQR 16–32), p = 0.022; median door to needle time was 17 min (IQR 12–26) in 2017, 16 min (IQR 13–23) in 2018, and 25 min (IQR 19–36) in 2019.
Functional outcome at 90 daysThere was no difference in the proportion of patients achieving favorable outcomes (mRS 0–2) between the two periods (54.8% in period 1 and 45.0% in period 2, p = 0.32) (Table 3). Mortality remained stable at 19.2% in period 1 and 23.3% in period 2, p = 0.34.
TABLE 3
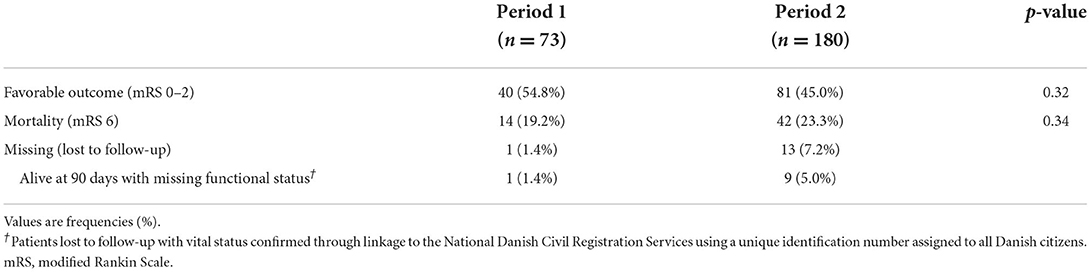
Table 3. Functional outcome at 90 days.
In multivariate analysis, every 10-min increase in time from imaging to reperfusion was associated with a shift toward poor functional outcome, aOR = 0.95 (95% CI: 0.93–0.98), p < 0.001 (Table 4). Only two other variables were also significantly associated with functional outcomes in multivariate analysis; NIHSS at baseline, aOR = 0.90 (95% CI: 0.87–0.93), p < 0.001, and age, aOR = 0.96 (95% CI: 0.94–0.98), p < 0.001.
TABLE 4
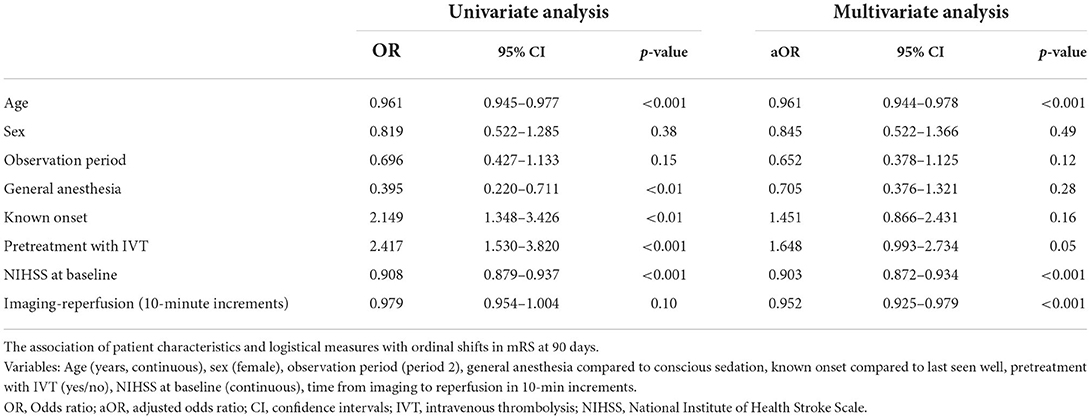
Table 4. Delay from imaging to reperfusion and functional outcome.
A subanalysis in patients with a known time of onset [48 patients (65.8%) in period 1 and 107 patients (59.4%) in period 2], every 10-min delay from onset to reperfusion, increased the risk of poor outcome, aOR = 0.97 (95% CI: 0.95–0.99), p = 0.03; also in this population, time from imaging to reperfusion was significantly associated with outcome, aOR = 0.95 (95% CI: 0.92–0.98), p < 0.01.
DiscussionThis study shows that relocation of the IVT services from a non-MT center to an MT-capable center, located 13 km apart in an urban environment, with no change in the catchment area, was significantly associated with reduced delay to reperfusion without influencing prehospital ambulance transportation time. A reduction in the median time from imaging to patient arrival at the angio suite from 89 to 42 min was the main driver of the reduction from imaging to reperfusion. Multivariate analyses showed that increasing delay from imaging to reperfusion was significantly associated with the risk of poor functional outcome at 90 days.
Previous studies have shown that outcome following MT is time-dependent but predominantly focused on the total duration from stroke symptoms onset to reperfusion (11–13, 19). However, once a patient is scanned and is eligible for MT with potentially salvageable tissue, every effort should be done to reduce delay to MT, irrespective of when stroke symptoms started—“the clock is re-set.”
The organizational change of relocating IVT services from a non-MT center to an MT-capable center, thereby avoiding inter-hospital transfer, could potentially increase prehospital transportation time and negate any time saved. Few studies have reported inter-hospital transfer in MT but in the American STRATIS registry the authors reported longer time from stroke symptoms onset to reperfusion for patients first admitted to a non-MT center (median 311 min) than for patients directly admitted to an MT-capable center (median 202 min); prehospital transportation time was unchanged, like in our study, which is likely generalizable to other urban areas with short inter-hospital distances (19).
The non-MT center, where IVT treatment was provided in period 1, and the MT-capable center were organized under the same hospital administration with staff working at both facilities. There was an increase in median time from door to needle (IVT treatment) between the periods, mainly driven by data from 2019. This corresponds to data from the Danish Stroke Registry on all patients treated with IVT in the capital region of Denmark, where the median door-to-needle time increased from 19 min in 2018 to 24 min in 2019 (20). This is likely driven by the implementation of results from the WAKE-UP trial, which enabled IVT treatment in patients with unknown time of onset based on MRI, which is more time-consuming compared to CT (21). The IVT services were relocated to the MT-capable center in 2018, before the results of the WAKE-UP trial, and in that year, the door-to-needle-time was unchanged suggesting that the relocation of IVT services did not negatively impact door-to-needle time.
Changes in patients' admittance and treatment with mechanical thrombectomyThe comparison of the two periods may be biased due to changes in patient characteristics and the provision of healthcare services. During our observation period, trial results extended the time window for MT, and patients with unknown time of stroke symptoms onset were examined for potential IVT treatment based on MRI, which increased the number of patients being acutely admitted (21–23). This change in admission policy is consistent with data from our study as the proportion of patients treated with IVT decreased significantly, and there was a significant increase in time from stroke symptoms onset to ambulance departure. In our analysis, we adjusted for pretreatment with IVT, which had no effect on the outcome. From period 1 to period 2, the number of patients treated with MT increased, while the catchment area was unchanged suggesting improved awareness of the eligibility criteria for MT and extension of time for MT rather than changes in LVO occurrence. This could possibly influence the outcome of patients in period 2 negatively with more patients arriving in the late time window. We, therefore, included adjustment for the study period to reduce the risk of residual bias according to when the MT procedure was performed, but this variable was not significantly associated with the mRS at 90 days.
In time metrics analysis, time from groin puncture to reperfusion also decreased significantly from 52 to 34 min, without a change in NIHSS severity at baseline or location of vessel occlusion, and with the same reperfusion results. Increased experience by neurointerventionists is a likely contributing factor in reducing the overall delay to reperfusion (24). However, in our study, this interval constituted less than one-third of the overall time reduction from imaging to reperfusion.
Strengths and limitationsStrengths of our study include the consecutive, observational design with complete registration and follow-up regarding the vital status of all MT-treated patients for the catchment area, which is mandatory by the Danish Health Authorities, and MT is only performed as a public health service. We have detailed information on time from ambulance departure to reperfusion.
However, our study also has limitations, as patient composition changed over time following the publication of results from WAKE-UP and trials on MT in extended time windows (21–23). We find it unlikely that these changes could influence the time registrations that were based on automatic and standardized data registrations only. Although the observation period was not significantly associated with functional outcomes in our analysis, it is still possible that other factors not accounted for could contribute to the observed changes in outcome. However, other studies have shown that increasing time to reperfusion is associated with a worse outcome, and results were consistent in the subanalysis of patients with a known time of stroke symptoms onset. The study is not a randomized clinical trial, but an analysis of real-life data using the same catchment area before and after an organizational change, which did not impact prehospital transportation time, but in other settings, such changes may cause unwanted delay. The reperfusion results were assessed by the attending neurointerventionist, and the evaluation of functional outcome was performed by clinicians who had full access to the patient's files, but none of this would impact the time registrations. Finally, our data do not allow for the estimation of numbers needed to treat as a result of the reduction in delay of imaging-reperfusion.
In conclusion, in our population, relocating IVT services to an MT-capable center was the main cause of reduced delay to reperfusion in patients with acute ischemic stroke and LVO, without influencing prehospital transportation time. Time from imaging to reperfusion was a significant predictor of functional outcome at 90 days following MT, and our study suggests that this organizational change may likely benefit patients eligible for acute MT in other urban areas if prehospital time is not prolonged.
Data availability statementThe datasets presented in this article are not readily available because individual patient data cannot be made accessible according to Danish law. Requests to access the datasets should be directed to nicolaj.groenbaek.laugesen.01@regionh.dk.
Ethics statementEthical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributionsNL and TT co-authored the manuscript draft with critical revision by KH, JH, and HI. Study was conceptualized and designed by NL, TT, and KH. Approval of data collection by KH. Data was acquired by NL and JH. Data was analyzed by NL. All authors contributed to the article and approved the submitted version.
FundingThis study was supported by a research grant to NL from Copenhagen University Hospital, Rigshospitalet (No. E-24087-01) and support to TT from the Novo Nordic Foundation (No. 65517). Funding sources were not involved in study design, data collection, manuscript writing, or the decision of publication.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.989607/full#supplementary-material
References1. Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Jovin T, Chamorro A, Cobo E, de Miquel M, Molina C, Rovira A, et al. Thrombectomy within eight hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 22:36. doi: 10.1056/NEJMoa1503780
PubMed Abstract | CrossRef Full Text
5. Campbell BC V, Yassi N, Yan B, Oxley TJ, Wu TY, Davis SM, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic Stroke Endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. (2019) 4:6. doi: 10.1177/2396987319832140
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Goyal M, Menon BK, Van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Rost NS, Bottle A, Lee JM, Randall M, Middleton S, Shaw L, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. (2016) 5:1–7. doi: 10.1161/JAHA.115.002433
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Hassan AE, Shariff U, Saver JL, Goyal M, Liebeskind D, Jahan R, et al. Impact of procedural time on clinical and angiographic outcomes in patients with acute ischemic stroke receiving endovascular treatment. J Neurointerv Surg. (2019) 11:984–8. doi: 10.1136/neurintsurg-2018-014576
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Heit JJ, Mlynash M, Christensen S, Kemp SM, Lansberg MG, Marks MP, et al. What predicts poor outcome after successful thrombectomy in late time windows? J Neurointerv Surg. (2021) 13:421–5. doi: 10.1136/neurintsurg-2020-016125
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Jahan R, Saver JL, Schwamm LH, Fonarow GC, Liang L, Matsouaka RA, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. (2019) 322:252–63. doi: 10.1001/jama.2019.8286
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Saver JL, Goyal M, Van Der Lugt A, Menon BK, Majoie CBLM, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA. (2016) 316:1279–88. doi: 10.1001/jama.2016.13647
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Mulder M, Jansen I, Goldhoorn R, Venema E, Chalos V, Compagne K. et al. Time to endovascular treatment and outcome in acute ischemic stroke: MR CLEAN registry results. Circulation. (2018) 138:232–40. doi: 10.1161/CIRCULATIONAHA.117.032600
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg P, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. (2012) 79:306–13. doi: 10.1212/WNL.0b013e31825d6011
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Schwamm LH. Optimizing prehospital triage for patients with stroke involving large vessel occlusion: the road less traveled. JAMA Neurol. (2018) 75:1467–9. doi: 10.1001/jamaneurol.2018.2323
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Pérez De La Ossa N, Abilleira S, Jovin TG, García-Torne Á, Jimenez X, Urra X, et al. Effect of direct transportation to thrombectomy-capable center vs local stroke center on neurological outcomes in patients with suspected large-vessel occlusion stroke in nonurban areas: the RACECAT Randomized Clinical Trial. JAMA 327:1782–94. doi: 10.1001/jama.2022.4404
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Schlemm L, Endres M, Nolte CH. Bypassing the closest stroke center for thrombectomy candidates what additional delay to thrombolysis is acceptable? Stroke. (2020) 867–75. doi: 10.1161/STROKEAHA.119.027512
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Berge E, Whiteley W, Audebert H, Marchis GM De, Fonseca AC, Padiglioni C, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. (2021) 6:I. doi: 10.1177/2396987321989865
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Froehler MT, Saver JL, Zaidat OO, Jahan R, Aziz-Sultan MA, Klucznik RP, et al. Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke). Circulation. (2017) 136:2311–21. doi: 10.1161/CIRCULATIONAHA.117.028920
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. (2018) 379:611–22. doi: 10.1056/NEJMoa1804355
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2017) 372:2296–306.
PubMed Abstract | Google Scholar
23. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Olthuis SG, den Hartog SJ. van Kuijk, SJ, Staals J, Benali F, van der Leij C, et al. Influence of the interventionist's experience on outcomes of endovascular thrombectomy in acute ischemic stroke: results from the MR CLEAN Registry. J NeuroIntervent Surg. (2022) 0:1–7. doi: 10.1136/neurintsurg-2021-018295
留言 (0)