Adaptive decision-making is governed by the interaction of several brain circuits, each of which has unique aspects that are advantageous under particular circumstances. For instance, a classic distinction has been made between goal-directed control, involving the prefrontal cortex (PFC) and medial striatum, and habitual control systems comprised of the sensorimotor cortex and lateral striatum (Balleine and O'Doherty, 2010; Gruber and McDonald, 2012). The goal-directed system appears to implement executive functions, such as working memory and strategic planning (Fuster, 1989; Passingham and Wise, 2012). Cannabis abuse compromises the normal ability of this executive system to suppress sensorimotor responding (Knight et al., 1999; Malone and Taylor, 2006; Filbey and Yezhuvath, 2013; Rae et al., 2015). Here we report a new dissociation among executive and sensorimotor systems governing choice, which allows us to quantify their interaction while accounting for important confounding factors such as decision time and learning.
When rewards are uncertain, the most pervasive strategy in animals and humans is to repeat choices that have previously led to reward (win-stay), and to shift away from choices following reward omission (lose-shift; Kamil and Hunter, 1970; Worthy et al., 2013; Thorndike, 2017). Although the win-stay and lose-shift are complementary response strategies, they are anatomically disassociated among goal-directed and sensorimotor systems. Lesions to the rodent lateral striatum (LS), which is homologous to the human putamen and essential for sensorimotor control (Parent and Hazrati, 1995), disrupt lose-shift responding but not win-stay (Skelin et al., 2014; Gruber et al., 2017; Thapa and Gruber, 2018). A similar shifting deficit has been observed in humans with damage to putamen or insula (Danckert et al., 2011). Conversely, lesions of the rodent ventromedial striatum (VS), a key structure in goal-directed control that receives inputs from prefrontal cortex (Voorn et al., 2004), disrupts win-stay but not lose-shift responding (Gruber et al., 2017). Several other behavioral features in rodents and humans support this anatomical disassociation. The win-stay and lose-shift exhibit different temporal dynamics (Gruber and Thapa, 2016), developmental trajectories (Ivan et al., 2018), and responses to reward feedback (Banks et al., 2018). Moreover, lose-shift responding (but not win-stay) drastically increases in adult humans under cognitive load, as well as in young children (Ivan et al., 2018). These data suggest that executive function can override lose-shift responding, which can be characterized as a reflexive response by the sensorimotor striatum. This is consistent with a long history of research indicating that executive function can suppress reflexive or habitual motor responses (Chamberlain and Sahakian, 2007).
The LS/putamen receives prominent inputs from both the somatosensory and motor cortices (Brasted et al., 1999), and encodes the motor aspects of decision-making (Burton et al., 2015). Consequently, decisions and their associated motor actions are represented in egocentric (body-centered) spatial coordinates (Kesner and DiMattia, 1987; Palencia and Ragozzino, 2005). The dorsolateral caudate, which receives inputs from the dorsolateral PFC in primates, is also necessary for egocentric spatial processing (Possin et al., 2017). The VS/nucleus accumbens encodes the value of choices and can engage a wide range of spatial-motor actions when executing a single decision involving abstract representations (Burton et al., 2015). It encodes responses in both egocentric and allocentric (world-centered) spatial coordinates, likely involving its prominent inputs from the hippocampus and PFC (Voorn et al., 2004; De Leonibus et al., 2005; Possin et al., 2017). These data suggest that the control of actions by sensorimotor systems will be restricted to an egocentric framework heavily dependent on the position of targets, whereas the control by executive systems have the capacity to use abstract features of targets. This is supported by the dissociated effects of cannabis on neural structures and performance on spatial vs. non-spatial tasks, as described next.
The recreational use of psychoactive substances has complex short-term and long-term effects on the brain, some of which dissociate. Δ9- tetrahydrocannabinol (THC) administration increases dopamine release in the LS, while the VS remains unaffected (Sakurai-Yamashita et al., 1989). Behaviorally, dopamine signalling in the dorsolateral striatum is necessary for normal spatial memory, motor control, and visuospatial learning, while reward processing and goal-directed learning rely on dopamine signalling in the medial striatum (Darvas and Palmiter, 2009, 2010). This provides an explanation for the observation that THC reduces spatial processing and visuospatial memory in humans (Cha et al., 2007), particularly in females (Pope et al., 1997). Recreational drugs also differentially influence win-stay and lose-shift responding. THC and amphetamine cause large changes in lose-shift behavior in rats and humans, while the win stay is only weakly affected (Paulus et al., 2002; Wong et al., 2017a,b).
Because the LS is necessary for lose-shift responding, and it processes information in egocentric coordinates, we hypothesized that lose-shift responses are calculated according to the position of a target relative to the participant, rather than other visual features of target identity. We further hypothesized that frequent cannabis use will disrupt the normal positional-dependence of lose-shift and the ability of executive systems to govern sensorimotor control. We tested these hypotheses by having human participants engage in a competitive decision-making task between two choices. Crucially, the choices were visually distinct and changed their spatial configuration unpredictably between each trial. We found that following a loss, lose-shift behavior was robustly associated with a choice's previous location, rather than its visual identity. The win-stay was only weakly associated with previous choice position, and this association was eliminated by global changes in target position. Although female cannabis users exhibited reduced task performance and increased lose-shift responding, their reliance on spatial information was not different than controls. Male cannabis users, however, did exhibit a reduced reliance on spatial information. These data support the dissociation of choice among systems with different spatial propensities, and reveal a sexual dimorphism of recreational cannabis use and the function of these systems.
2. Methods 2.1. Behavioral taskDuring the experiment, participants played a competitive game colloquially called “Matching Pennies” against a computer opponent. The task display consisted of two distinct targets (a blue circle and yellow square) presented on a 15″ touchscreen monitor (Figure 1). On each trial, participants would choose either target by touching it on the screen. They would then receive visual feedback indicating “You Win” or “You Lose” for 1.5 s. On each trial, the computer algorithm attempted to predict which target would be selected. If the participant selected this target, the trial was a loss. Otherwise it was a win. The algorithm attempted to minimize the number of wins for participants. The optimal strategy for the participants is to be unpredictable in choice, in which case they win on 50% of trials. Because the win-stay and lose-shift are predictable by the computer, subjects should learn to suppress these responses as the session progresses. This task provides measures of lose-shift, win-stay, and cognitive flexibility (i.e., response entropy) as participants adapted their choices to the computer opponent.
FIGURE 1
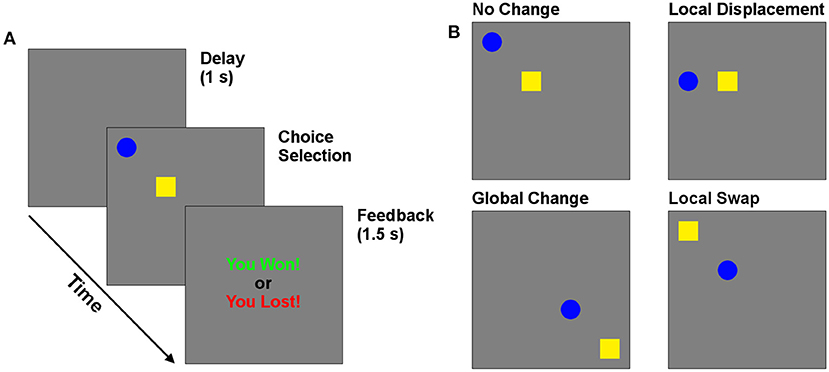
Figure 1. Behavioral task. (A) Timeline of trials in the matching pennies game. (B) Reconfiguration of targets, which could undergo local (swap and displace) and global changes in position.
The computer used four types of algorithms to detect patterns in (i) participants' choices, (ii) switching from one choice to another, (iii) choices paired with rewards (e.g., blue square after a loss), and (iv) switching paired with rewards (e.g., swapping choices after a loss). Specifically, on each trial the computer examined a subject's recent choice and reward history (e.g., shifting from the blue target to the yellow after a loss). The choice that most accurately predicted the subject's past choice history was selected as the prediction of the present choice. Patterns of choices 1–6 trials in length were considered, resulting in 24 total prediction strategies. On each trial, the best performing strategy (computed over all previous trials in the session) was used to predict participants' choices. If all strategies failed to beat the participant on ≥ 50% of past trials, the computer would select choices randomly.
The effect of cue position was investigated by moving the location of one or both cues from one trial to the next. The changes came in two types—local and global. The screen was divided into four equal quadrants, each of which contained an invisible 2 × 2 grid in its center. Local changes occurred within each grid, while global changes involved shifting among quadrants. Three local manipulations are of particular interest to investigate the importance of position and cue identity. The “Control” case is when the cue positions remain in the same locations. The “Swap” case occurs when the targets swap positions (a local change). The “Displace” condition occurs when the previously selected choice moves to a previously empty position in the same 2 × 2 choice grid, while the other target remains in its previous position. Global changes occurred independently of each of these three local changes, for a total of six possible changes of target positions across subsequent trials, which were selected randomly on each trial. This manipulation allowed us to determine how the position of targets relative to each other, and to the participants, affected choice following wins and losses. In particular, this design allows us to test if participants avoid the screen position of a target following a loss, as expected by the egocentric processing framework of LS. Alternatively, they may instead avoid the target regardless of position.
2.2. ProcedureAll procedures and experimental tasks were approved by the McMaster University Research Ethics Board. One hundred and six undergraduates (53 males, mean age = 19.40, SD = 2.74) from McMaster University participated in the study in exchange for payment. After providing informed consent, participants played 600 trials of the task. They were informed that they were competing against a computer opponent and would win nothing each time the computer predicted their choice and $0.03 each time it could not, rounded up to the nearest $5 upon completion of the experiment. Participants were given no guidance as to optimal decision-making strategies.
After task completion, participants were screened with the South Oaks Gambling Screen (SOGS), the alcohol, smoking, and substance involvement screening test (ASSIST) v3.0, Adult ADHD Self-Report Scale (ASRS) v1.1, and an additional demographic questionnaire. Habitual cannabis users were defined as those meeting the criteria for brief or intensive treatment (score >3) on the ASSIST cannabis subtest. Controls were defined as those reporting zero cannabis use within the three months prior to the experiment. Total drug use was also recorded as the ASSIST score summed across all drug subtypes. Males had a mean ASSIST score of 19.11, with 32.08, 39.62, and 60.38% meeting the criteria for alcohol, cannabis, or any recreational drug use requiring intervention. Females had a mean ASSIST of 16.66, with 28.30, 30.19, and 45.28% meeting alcohol, cannabis, or general drug use criteria (see Table 1). Of the 37 subjects who met the cannabis use criteria, 18 (7 females) indicated having used cannabis once or twice in the last three months, 9 (4 females) that they used cannabis monthly, 4 (1 female) indicated weekly usage, and 6 (3 females) daily usage.
TABLE 1

Table 1. Demographic data and ASSIST questionnaire scores (±SEM) for the studied groups.
In addition, 25 subjects (11 females) reported symptoms consistent with adult ADHD, as assessed by the ASRS. Only two males reported behavior indicative of a pathological gambler. Consequently, the effects of gambling history on task performance were not assessed. Similarly, there was insufficient variability in subject age to investigate whether win-stay or lose-shift behavior changes with age.
2.3. AnalysisParticipants' responses were analyzed for proportion of lose-shift and win-stay responses, averaged over five blocks of 120 trials each, and conditioned on the type of cue shift relative to the previous trial's target positions. As a measure of behavioral flexibility, the binary response entropy (H) for each participant was calculated from four-trial choice sequences as:
H=∑i=1kPi+log2Pi (1)Where Pi is the probability of each choice sequence, and k is the total number of sequences possible (i.e., 16). For example, a participant that exhibited the choice pattern “circle-square-circle-square” to the exclusion of all other patterns, would have an entropy of 0 bits, while a participant responding randomly would have an entropy of four bits. Response entropy and task performance were averaged over the experimental session for each participant. Decision times were measured as the time to make a response following presentation of the choice selection screen. They were normalized using the inverse transform (1/RT) and averaged after removing 131 erroneous RTs of <3 ms. The inverse transform was used to normalize RTs because it produced more normalized (Gaussian) distributions than did the log or square-root transforms.
The differences of marginal means of derived quantities (decision time, lose-shift, etc.) were tested by analysis of variance (for categorical factors) or co-variance (for continuous factors) using repeated-measure, mixed-effects models. Each model utilized a maximal random-effects structure and was fit in R using the lme4 package (Bates et al., 2014). A maximal model ensured that variations in effects between participants, and between trial blocks within each participant, were properly controlled (Barr et al., 2013). Degrees of freedom and p-values were calculated using the Welch-Satterthwaite equation and type-III sums of squares. The effects of local changes in position were assessed via planned paired t-tests comparing the effects of spatial swaps and displacement relative to the no-change condition. For all statistical comparisons, t-tests were only employed following significant ANOVA results, with statistical significance being assessed with a firm p < 0.05 cutoff. As such, no statistical corrections for multiple comparisons were employed. Additional statistical characteristics (e.g. Cohen's D, confidence intervals) are reported in Table 2 for analyses of primary importance to our interpretation of the data.
TABLE 2
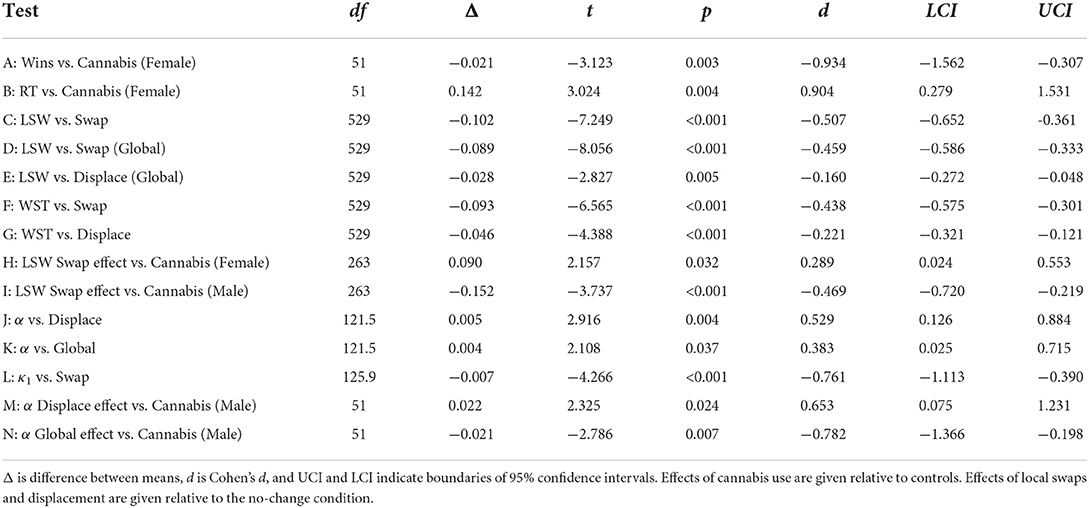
Table 2. Table summarizing key significant effects.
We also used the Q-learning with forgetting (Barraclough et al., 2004) reinforcement learning model to examine the effects of cannabis use, local changes, and global changes on reward sensitivity, choice stochasticity, and learning rates. In this model the probability of selecting one of the two choices (C) on a given trial (t) are calculated according to the softmax equation (Sutton and Barto, 2018).
P(Ct=i|Qi,Qj)=exp[β×Qi(t)]exp[β×Qi(t)]+exp[β×Qj(t)], (2)where Qi and Qj are the value each subject assigns to choices i and j. β refers to the inverse temperature that balances exploiting known action-reward associations with exploring more of the state/action space. As such, larger values of β indicate a greater tendency to choose the most highly valued action. The values of each choice are updated from rewards (R) according to the following rules:
Qi(t)=,,,]},,,]},,,]},,,,,]}],"socialLinks":[,"type":"Link","color":"Grey","icon":"Facebook","size":"Medium","hiddenText":true},,"type":"Link","color":"Grey","icon":"Twitter","size":"Medium","hiddenText":true},,"type":"Link","color":"Grey","icon":"LinkedIn","size":"Medium","hiddenText":true},,"type":"Link","color":"Grey","icon":"Instagram","size":"Medium","hiddenText":true}],"copyright":"Frontiers Media S.A. All rights reserved","termsAndConditionsUrl":"https://www.frontiersin.org/legal/terms-and-conditions","privacyPolicyUrl":"https://www.frontiersin.org/legal/privacy-policy"}'>
留言 (0)