Idiopathic facial nerve paralysis, commonly known as Bell’s palsy (BP), is a cranial nerve VII condition leading to facial weakness (paresis) or paralysis. This acute unilateral paralysis or paresis has no identifiable cause and occurs over <72 h.1 BP has been alluded to numerous times throughout history, with descriptions of the condition found in ancient Greek, Persian and European medical texts dated as early as the 5th century BCE.2,3 Ancient Peruvian ceramic art, Razi’s “al-Hawi” and the Greek Hippocrates all indicate familiarity of these ancient cultures with BP.2–4 Many physicians throughout history, including Sydenham, Friedreich, Stalpart van der Wiel and Thomassen á Thuessink, have researched and described the condition.2,3 The name comes from Sir Charles Bell, a surgeon, who took great interest in sensory (trigeminal nerve) and motor (facial nerve) innervation of the face.2,5
Signs and symptoms of BP include ipsilateral drooping of the eyelid, dry eye, excessive tearing, drooping of the corner of the mouth, post-auricular pain, loss of taste sensation in the anterior 2 thirds of the tongue, difficulty eating, dry mouth, slavering, altered sensation and hyperacusis.2,6 The most pathognomonic sign of BP is the Bell’s phenomenon, characterized by upward rolling of the eye when attempting to close the eyelid.7Figure 1 illustrates the most common clinical signs of BP.
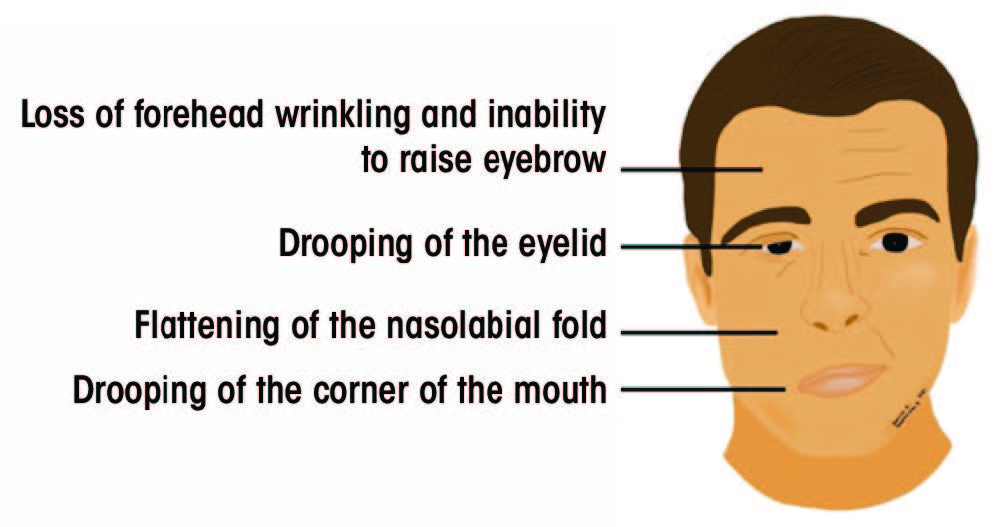
Figure 1: Signs of acute unilateral Bell’s palsy
Although the etiology of BP is unclear, most cases resolve without treatment.6 Short-term consequences of BP include the inability to close the eye, potential cornea injuries and eventual eye dryness, which can be managed clinically with a favourable prognosis.1 Long-term, incomplete recovery from BP can result in facial asymmetry, disfigurement, reduced facial movement and many other complications that greatly reduce the patient’s quality of life.1,8,9 Although various modalities are available to improve quality of life, patients with BP often experience depression, reduced quality of interpersonal relations, anxiety, decreased self-esteem and social isolation.10–12 This article aims to discuss the current evidence for the etiology, diagnosis and treatment of BP and its implications in the field of dentistry.
EpidemiologyBP is the most common cranial mononeuropathy with a reported incidence rate of 11.5–40.2 per 100 000.2,13 It is seen equally in males and females and occurs in all ages with a propensity to affect people in mid to later life.14 No race predilection exists, and both sides of the face are equally affected, although bilateral BP is rare (0.3% of cases).2,15 Various epidemiological studies have established a seasonal trend, with higher incidence in colder months of the year.2,16 Even without treatment, 70% of affected people will have complete resolution, while approximately 30% will experience partial or incomplete recovery.17 The recurrence rate of BP is estimated to be 7% and does not correlate with the prognosis.14,18 Recurrence should alert the clinician to alternative causes of facial paralysis.14 Possible risk factors for BP include hypertension, severe preeclampsia, psychological concerns, pregnancy, radiation exposure, diabetes, obesity, upper respiratory infection and migraine.1,19 Numerous cases of BP have been documented following the administration of influenza vaccines as well as many others.20 Similarly, recent studies have identified BP as a side effect of the new mRNA SARS-CoV-2 vaccine.21-23
Cranial Nerve VII AnatomyCranial nerve VII is a multifunctional nerve with significant motor, sensory and parasympathetic activities. Its nerve fibres are associated with 3 nuclei in the medulla oblongata and the pons: solitary (sensory), facial (motor) and superior salivatory (parasympathetic).24 The central motor nucleus of the facial nerve is located in the precentral gyrus of the motor cortex.25 Fibres from the central motor nucleus travel down in the corticobulbar tracts and supply the contralateral facial nucleus.25
The facial nerve is divided into 6 segments: cisternal, meatal, labyrinthine, tympanic, mastoid and extracranial.25,26 The cisternal segment includes the motor root and the nerve of Wrisberg, which advance to the internal acoustic meatus.25,26 At the meatus, these nerve segments unite to form the meatal portion of the facial nerve.25–27 Throughout the cisternal and meatal paths, CN VII travels with the vestibulocochlear cranial nerve (VIII).25–27 Further, the labyrinthine portion extends from the cochlea to the vestibule of the inner ear and joins the geniculate ganglion.25–27 The tympanic portion runs inferior to the lateral semicircular canal and gives rise to the mastoid segment of the facial nerve.25–27 The mastoid segment exits the skull through the stylomastoid foramen and becomes the extracranial portion of the nerve.25–27 This portion gives rise to the digastric and posterior auricular nerves and advances through the parotid gland to give rise to the terminal branches.25–27
Each segment gives rise to various branches (Table 1).25–28 Understanding the function of these branches is extremely helpful in determining the location of the pathology along the facial nerve. Figure 2 depicts structures innervated by the facial nerve, as well as the superficial extracranial branches.
Table 1: Branches arising from segments of the facial nerve and their functions.Facial nerve segments
Branches
Function
Cisternal Meatal Labyrinthine (including geniculate ganglion) Greater superficial petrosal Parasympathetic supply to nose, palate and lacrimal gland Forms nerve of pterygoid canal with deep petrosal nerve Lesser petrosal Secretory fibres to the parotid gland External petrosal Sympathetic fibres to middle meningeal artery Tympanic Mastoid Stapedius Motor supply to stapedius Allows tolerance of loud noises Chorda tympani Sensory taste fibres to anterior 2 thirds of tongue Parasympathetic innervation of the submandibular and sublingual glands Nerve from auricular branch of vagus Sensory fibres traveling with facial nerve; headed back into the skull Extracranial Posterior auricular Motor supply to posterior and superior auricular muscles and the occipital belly of occipito-frontalis muscle Digastric Motor supply to posterior belly of digastric muscle Stylohyoid Motor supply to stylohyoid muscle Temporal Supply anterior and superior auricular muscles, muscles of the forehead (frontalis) and superior portion orbicularis oculi Zygomatic Supply orbicularis oculi, muscles of nasal aperture (e.g., nasalis) and elevator muscles of the lip (e.g., zygomaticus) Buccal Supply musculature surrounding upper and lower lip and buccinator Marginal mandibular Supply musculature of the lower lip, depressors of the lip and mentalis Cervical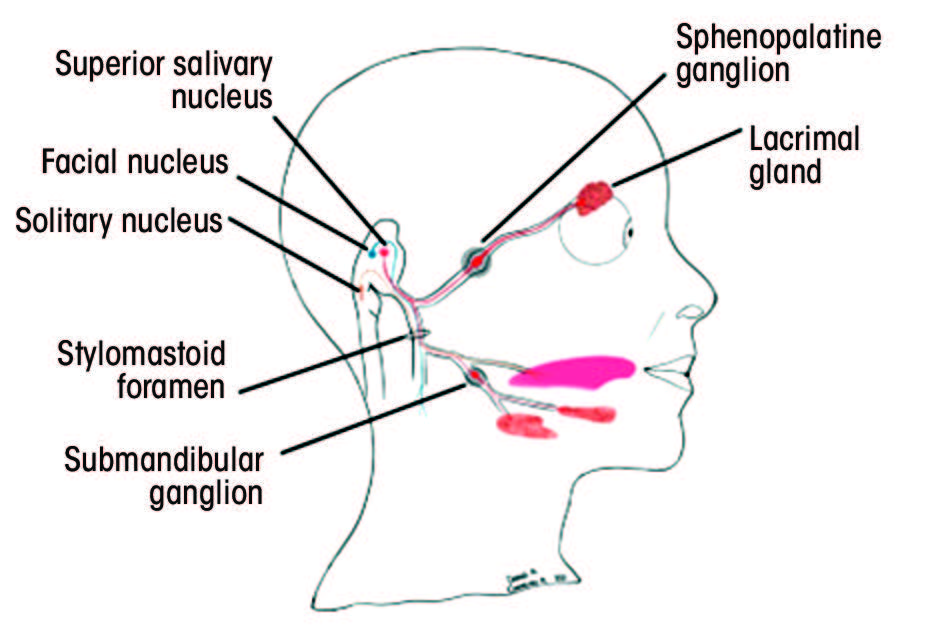
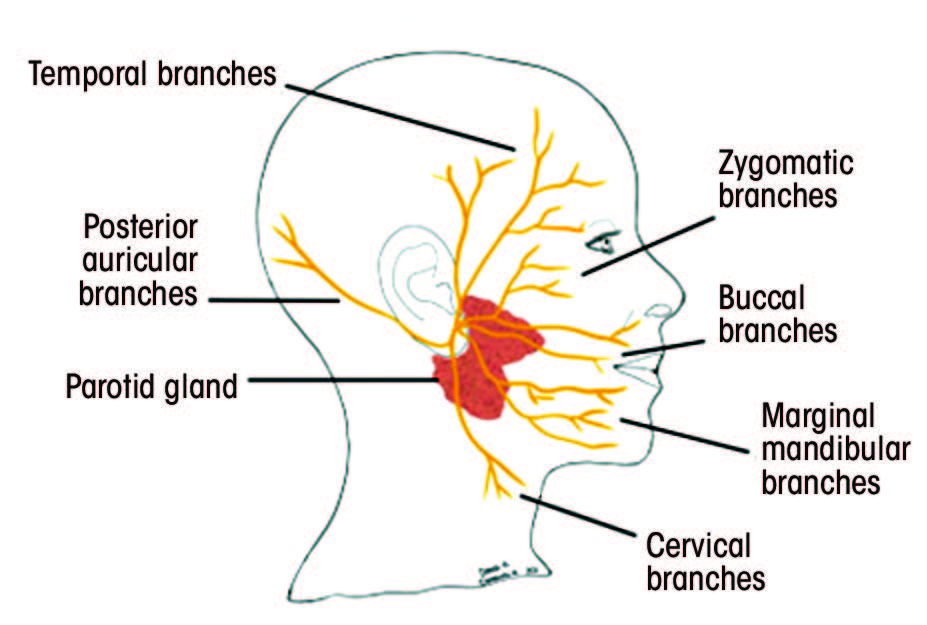
Figure 2: A. Structures innervated by the facial nerve. The red (parasympathetic), blue (motor) and orange (sensory) lines demonstrate the multifunctionality of the facial nerve. B. Superficial branches of the extracranial portion of the facial nerve.
Supranuclear pathology, more commonly known as upper motor neuron (UMN) palsy, results when neuronal fibres above the facial nucleus are disrupted.14,25 UMN palsy affects the contralateral half of the lower face, sparing the forehead and the eyebrow muscles.25 A UMN lesion of the cortex and/or corticobulbar tract spares the lacrimal and salivary secretions, and the taste function is preserved.29 Infranuclear pathology or lower motor neuron (LMN) palsy results from lesions at the facial nucleus or the exiting fascicles.29 These lesions affect the ipsilateral upper and lower face.14,25 Therefore, involvement of the forehead muscle (i.e., frontalis) and the eyebrow muscle (i.e., corrugator supercilii) provides insight into the nature of the condition. The sparing of the forehead and eyebrow muscles is attributed to the bilateral innervation of the upper third of the face and contralateral innervation of the lower 2 thirds.26 Precise location of an LMN palsy can be discerned via functional assessment of other neighbouring structures. LMN palsy involving the facial nucleus or the fascicles within the brainstem may exhibit contralateral hemiparesis, ataxia, nystagmus and CN III–CN VI palsy, as well as ophthalmoparesis.29 Conversely, LMN palsy involving the facial nerve after it exits from the brainstem may display complete ipsilateral hemifacial weakness, dysgeusia and a decrease in lacrimation and salivation.29
EtiologyThe precise etiology of BP is unknown, and its diagnosis is based on exclusion.19 Consequently, it is essential to eliminate all other potential etiologies of facial paralysis and paresis before diagnosing BP. Currently, there are numerous theories regarding the cause of BP.19
Anatomy
The anatomy of the facial nerve and its long intra-bony path have been considered as potential factors in the etiology of BP. The fallopian canal is a narrow Z-shaped canal in the temporal bone.25 Although it provides protection to the facial nerve, it predisposes the nerve to entrapment neuropathies following inflammation or edema.30 The labyrinthine segment of the facial nerve is the narrowest portion of the canal,25 and the meatal foramen—the entry point of the nerve into the labyrinthine segment—is only ~0.68 mm in diameter.30 Also, an arachnoid band of tissue around the lateral portions of the internal acoustic meatus further contributes to constriction of the area.30 Celik et al.31 showed that reduced width of the fallopian canal in the labyrinthine portion is a risk factor for BP. Similarly, Ozan and Arslan32 found increased cross-sectional area of the facial nerve and decreased cross-sectional area of the internal acoustic meatus associated with BP. Therefore, compression of the facial nerve caused by inflammation or edema along its narrow intra-bony path may cause facial nerve abnormalities.
Viral Infection
Viral infection is hypothesized to be another etiological factor in BP.19 Varicella zoster virus (VZV) and herpes simplex virus (HSV) are DNA viruses that remain dormant in nervous ganglia subsequent to primary infection.33 They can reactivate anytime throughout the host’s lifespan and cause recurrent infections.33 In particular, HSV-1 remains latent in the geniculate ganglion of the facial nerve.33 Various studies have demonstrated the presence of HSV-1 DNA in the endoneural fluid of the facial nerve in BP patients, as well as the ability of HSV-1 to cause facial paralysis in animal models.34,35 Currently, HSV-1 mediated inflammation of the facial nerve in the narrow fallopian canal is widely accepted to be the mechanism responsible for most BP cases.30 Reinfection by VZV is also possible and more severe than HSV-1 because of its spread via satellite cells.30 A possible biomolecular mechanism of HSV-mediated neuronal dysfunction involves abnormal expression of p53 upregulated modulator of apoptosis (PUMA) and the innate immune signaling molecule, SARM1.19
Ischemia
The outer layer of the facial nerve consists of a periosteal membrane overlying a vascular plexus coating the epineurium of the facial nerve.36 This vascular plexus is supplied by the stylomastoid artery peripherally, with central layers supplied by the middle meningeal artery (petrosal branch), internal auditory artery and anterior inferior cerebral artery.19,24 Primary ischemia can result from vasospasms, leading to facial nerve neuropathy.19,36 Although this type of ischemia is rare, it can be appreciated in cases of diabetes mellitus and embolization of the middle meningeal artery.36 Quickly following acute ischemia, inflammation of the nerve begins with recruitment and activation of macrophages.19 Primary ischemia is also observed in animal models, with facial nerve paralysis occurring 5–15 minutes after blockage of the vascular network.37
Secondary ischemia is a consequence of primary ischemia, with initial constriction of the arterioles and subsequent dilation of the capillaries leading to production of a transudate.19,36 This transudate can compress the lymphatic capillaries, further increasing transudate production and inducing ischemia.19,36 The resultant histamine-mediated edema can lead to obstruction of venous outflow and, thus, interfere with normal arterial circulation in the vascular plexus.19,36 Secondary ischemia can progress to tertiary ischemia, where perivasculitis and endarteritis are evident.19,36 Thickening or fibrosis of the facial nerve sheath are often observed at this stage and may require surgical decompression to prevent permanent facial paralysis.36
Inflammation
Recently, more evidence has emerged regarding BP and inflammation‑induced demyelination of the facial nerve.19 Numerous studies have shown the association of a potential new marker—neutrophil to lymphocyte ratio (NLR)—with various inflammatory diseases, such as systematic lupus erythematous and hepatitis B.38,39 Similarly, a meta-analysis40 concluded that the NLR for patients with BP was higher than in patients without BP, and a higher NLR coincided with a worse prognosis. This indicates a change in the peripheral subpopulation of white blood cells as in other inflammatory demyelinating diseases, such as multiple sclerosis and Guillian-Barre syndrome.41–43 Other studies have demonstrated the role of cell-mediated immunological responses, with BP patients having higher serum levels of IL-6, IL-8 and TNF-a compared with controls.44
ManagementDiagnosis
Thorough clinical examination and medical history are essential in diagnosing BP.45 Laboratory and imaging techniques are not routinely required, but suspicion of neoplasms, prolonged facial palsy episodes, hypoacusis, nystagmus, tinnitus, sensory deficits and diplopia should be investigated further using CT scans and MRI.45,46 Sufficient clinical suspicion of Lyme disease and varicella zoster oticus demand serological work up.1,46 Electromyography (EMG) is a useful tool in determining the extent of axonal damage, thus guiding treatment.45 Electroneurography (ENG) and EMG can also be used as a prognostic indicator, determining the signs and extent of facial nerve reinnervation.46,47 It has been recommended that electrodiagnostic testing for BP cases be limited to those with complete paralysis only, as the risk of incomplete recovery is higher for such patients.1
Patient history should include a description of the onset of facial palsy, as a progressive, slowly occurring facial palsy is indicative of a neoplastic or infective etiology.48 It should be noted that the onset of BP is sudden and tends to worsen over minutes to hours.48 Alternative pathologies, including neurologic, otologic, trauma, inflammatory and congenital abnormalities, should also be excluded.28
Physical examination should include close inspection of the facial muscles with emphasis on the eyelids, nasolabial folds and the corners of the mouth.28 In BP, incomplete closure of the eyes, weakness of the orbicularis oculi muscle leading to Bell’s phenomenon (i.e., palpebral-oculogyric reflex) and lagging blink of the affected side are common.28 In addition, patients may exhibit difficulty in puffing out cheeks and pursing their lips.28 Examining taste senses using a tongue blade and salt/sugar could also assist in the diagnosis.28 Finally, the orbicularis oculi reflex test and the corneal reflex test may be conducted. The former involves gently tapping the glabella to observe involuntary blinking reflex, while the latter is carried out by stimulating the cornea with a wisp of cotton.28
Clinical practice guidelines by Baugh et al.1 provide certain criteria for diagnosing BP (Figure 3). A clinical examination algorithm for the diagnosis of BP is described in Figure 4.48 Currently, the degree of facial paralysis is determined via the House-Brackmann scale,49 which assesses various facial attributes including symmetry in rest and movement. This scale is divided into 6 grades, with a higher grade associated with a greater severity of BP and a more incomplete recovery (Table 2).49
Table 2: House-Brackmann facial nerve grading system.Grade
Symptoms
I II Slight weakness at rest (only noticeable on close inspection) No synkinesis, contracture or spasms Normal symmetry and tone motion at rest III Obvious but not disfiguring difference between sides Noticeable synkinesis, contracture and spasms Complete eye closure with effort Normal symmetry and tone motion at rest IV Obvious weakness and disfiguration of affected side Normal symmetry and tone motion at rest Incomplete eye closure V Barely perceptible motions observed Complete asymmetry VI Complete paralysis, no movement
Figure 3: Criteria for diagnosing BP
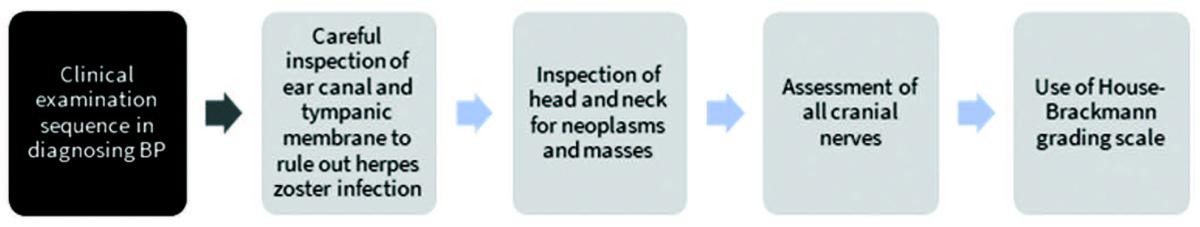
Figure 4
Various signs and symptoms may prompt the clinician to consider an alternative diagnosis. These include severe pain, hearing or vestibular abnormalities (excluding hyperacusis), history of cancer, prior insect bite and rash in or around the ear.46,50 The rate of misdiagnosis of BP is approximately 10.8%.2,5,14 The literature suggests misdiagnosis rates of up to 24% with central and secondary peripheral pathologies, such as skull base tumours being misinterpreted as BP.29 Table 3 shows conditions and syndromes that may result in facial paralysis and/or paresis.1,14,46,50 Table 4 highlights some specific examination findings that would assist in ruling out other important entities in the differential diagnosis.28
Table 3: Differential diagnosis for facial nerve palsy.Differential diagnosis
Conditions
Parenchymal lesions Multiple sclerosis, stroke, abscess, encephalitis, neoplasms Trauma Iatrogenic, basilar skull fracture, temporal bone fracture Neoplasms Schwannoma, neuroma, meningioma, metastatic, cholesteatoma, parotid perineural invasion, skin cancers Congenital Mobius syndrome, forceps trauma Meningitis Bacterial, viral (HIV, Zika, Epstein-Barr virus, polio), fungal, mycobacteria (tuberculosis, leprosy), spirochete (Lyme disease, syphilis), sarcoidosis, neoplastic Infection Ramsay Hunt syndrome, Guillain-Barre syndrome, chronic inflammatory demyelinating polyradiculoneuropathy, osteomyelitis of skull base, otitis media, parotitis, mastoiditis, amyloidosis, granulomatosis with polyangiitis, polyarteritis nodosa, Sjogren syndrome, mononucleosis Neuromuscular junction disorders/myopathy Oculopharyngeal dystrophy, myotonic dystrophy, myasthenia gravis, facioscapulohumeral muscular dystrophy Endocrine Neurovascular Other Melkersson-Rosenthal syndrome, Paget’s disease, ethylene glycol ingestion, idiopathic intracranial hypertension, hereditary neuropathy, Wernicke encephalopathy, acute lymphatic leukemia, neck carotid artery dissection Table 4: Defining characteristics of conditions associated with facial paralysis and/or paresis.Condition
Defining characteristics
Cerebrovascular event (e.g., stroke) Forehead muscles are spared, headache, ipsilateral limb weakness Guillain-Barre syndrome Ascending weakness, reflexes absent Diabetes mellitus Infection (e.g., meningitis and encephalitis) Meningeal signs, fever, pain, abnormal cerebrospinal fluid (CSF) Lyme disease Rash, malaise, arthralgia, bilateral facial palsy Ramsay Hunt syndrome Pain, vesicular eruption and/or scabs in or around the ear canal Sarcoidosis Bilateral facial palsy, elevated angiotensin-converting enzyme Neoplasms (e.g., parotid tumour) Insidious onset, palpable mass, partial involvement of facial nerve branchesTreatment
Although most cases of BP (85%) resolve spontaneously within 3 weeks and 70% of patients regain full function within 6–9 months,6,46 it is important to avoid complications and minimize the risk of incomplete recovery.51,52 Patients with milder cases of BP and those who start recovering sooner have a better prognosis.6,53 For example, 88% of patients who started recovering within the first week regained full function compared with 61% recovering within 2–3 weeks.53 Ophthalmic side effects, such as drying of the eye because of a deficit in lacrimal gland innervation, and orbicularis oculi weakness are common. If left untreated, incomplete eye closure and infrequent blinking can lead to keratitis, infection, corneal ulceration and blindness.54–56 It is crucial for patients to use eye drops regularly throughout the day and ointments at night and wear eye shields.54 Other commonly used modalities include taping of the eyes while sleeping, use of eyelid gold or platinum weights and a palpebral spring.55
The presence of underlying causes, such as viral (e.g., HSV), vascular, autoimmune and hereditary factors, should encourage clinicians to seek a different diagnosis and address such conditions promptly.57 Pharmacological management of BP without an apparent underlying cause includes the use of corticosteroids within the first 72 h of onset of symptoms.58 The anti-inflammatory properties of corticosteroids can reduce facial nerve edema and damage.1 A Cochrane review59 has demonstrated their benefits in the treatment of BP, with patients receiving corticosteroids exhibiting less postoperative sequelae, such as gustatory lacrimation (“crocodile tears”) and synkinesis. According to this systematic review, if 10 BP patients are treated with corticosteroids, 1 incomplete recovery will be avoided. Dose-dependent side effects of corticosteroids can be expected: fluid retention, hypertension, hyperglycemia, mood swings, irritability, psychosis, cataract and glaucoma, gastric and esophageal ulceration, weight gain and immunosuppression.60 However, short-term use and quick dose tapering have minimized the risk of adverse effects.59 Clinical practice guidelines recommend 50 mg of prednisolone for 10 days or 60 mg of prednisone for 5 days followed by a 5-day tapered dose.1 The literature has also demonstrated the potential benefits of high-dose corticosteroid regimens in treating severe cases of BP.58,61,62 However, extensive research is required to truly evaluate the effectiveness of high-dose corticosteroid therapy.
Because of the possible viral etiology of BP, another pharmacological modality is the use of antiviral medications,63 prescribed alone or in combination with corticosteroids.57 A Cochrane review57 demonstrated little to no effect of combination therapy in the management of BP, even when used in severe cases. Corticosteroid alone was described as more effective than antiviral medication alone.57 However, the combination of antivirals and corticosteroid can lead to decreased incidents of BP sequelae, such as crocodile tears and motor synkinesis.57 Currently, the antiviral medications acyclovir (400 mg 5 times/day for 5 days) and valacyclovir (1000 mg/day for 5 days) are indicated if the facial palsy is a result of herpes zoster oticus, indicating Ramsay-Hunt syndrome.14 More extensive research clarifying the role of antivirals in the management of BP is required.64
Considering the proposed pathophysiology of BP, surgical decompression has been suggested as a potential modality. Although Menchetti et al.65 found no significant benefit of early surgical decompression in the management of BP, this was attributed to the low certainty of evidence. The current literature does not support the use of surgical decompression in the management of BP, because of its associated costs and the adverse effects.1,14,45,46 The decision to proceed with surgical decompression should be patient specific. Patients with complete paralysis, ENG values indicating more than 90% reduction in amplitude and EMG results showing the absence of voluntary muscle activity are often not responsive to routine management modalities and, thus, may require more aggressive interventions, such as surgical decompression.1,45
Other non-pharmacological treatments include acupuncture, physical therapy and hyperbaric oxygen therapy. The effectiveness of acupuncture has been demonstrated via various studies.66–68 A meta‑analysis of 11 randomized controlled trials associated acupuncture with increased cure rates.69 However, poor quality studies and high heterogeneity are confounders and, therefore, call for better quality studies to properly assess the effectiveness of acupuncture.69,70 Physical therapy—which entails exercises, electrotherapy, massage, relaxation and biofeedback techniques— aims to increase muscle and nerve function by decreasing swelling, increasing blood flow and increasing the amount of oxygen to the affected tissue.53,71 A Cochrane systematic review53 failed to identify high-quality evidence to support the use of physical therapy in the management of BP, and clinical practice guidelines1 have made no recommendations regarding its use. Similarly, there is a lack of evidence for the use of hyperbaric oxygen in the management of BP.51,72 Figure 5 shows the current algorithm for management of BP, as defined by the 2013 Otolaryngology Head and Neck Surgery clinical practice guidelines1 and Zandian et al.45
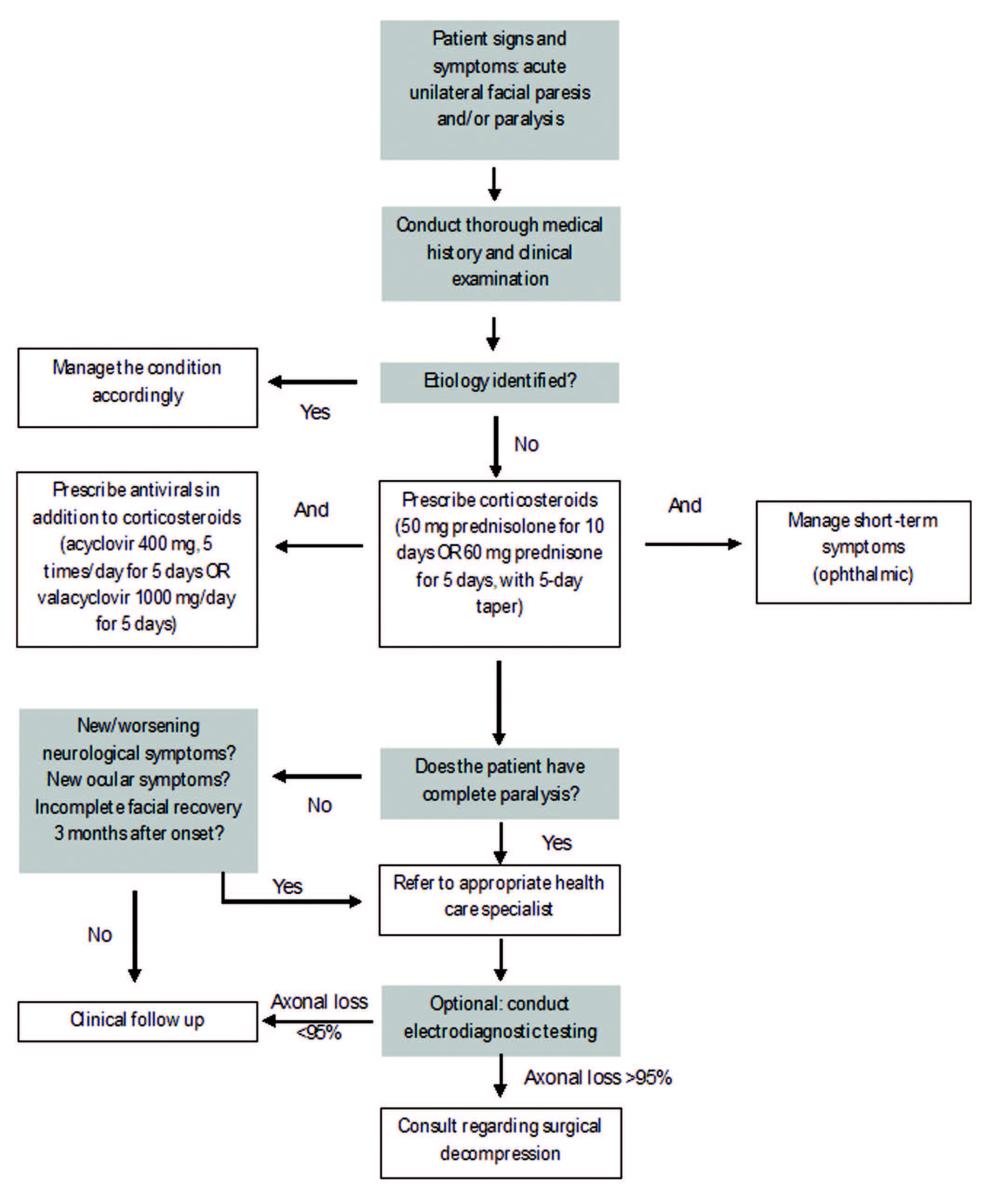
Figure 5: Algorithm for management of Bell’s palsy
Complications
In the long term, patients experiencing incomplete recovery from BP can suffer various complications. Synkinesis refers to involuntary facial muscle movement during voluntary contraction of a different region of the face.73 Although the etiology of this phenomenon is not fully understood, it is believed to be a result of aberrant reinnervation of facial muscles other than the ones originally innervated.73 Long-term treatment is still under development for synkinesis, but physiotherapy and the use of botulinum toxin have been successful.2 Crocodile tears is another common long-term sequela of BP, in which tearing of the eyes occurs while eating or drinking.74 This has been attributed to misdirection of salivary nerve fibres to the lacrimal gland.6 Incomplete recovery, aberrant contraction of the facial muscles, abnormalities in taste sensation and speech difficulties have all been documented in the literature.6 A longitudinal follow‑up study in 2021 also highlights the increased risk of ischemic stroke in BP patients.75
Dental ImplicationsDental procedures have been associated with the occurrence of BP, although this is rare.76 Development of BP following dental procedures may be immediate or delayed. Immediate-onset BP is associated with rapid recovery and involves local anesthetic complications, hematoma formation and trauma afflicted by the dental procedures.76 Vasoconstrictor agents in local anesthetics and their possible neurotoxic properties have also been associated with BP.77 Current evidence suggests that procaine and tetracaine are more harmful than bupivacaine and lidocaine, although the former 2 are not as commonly used.77 Furthermore, rapid introduction of air into an extraction socket can damage the fascial spaces, inducing stretching and inflammation of the facial nerve.76 Similarly, the needle used to administer local anesthetic can damage blood vessels around the epineurium of the facial nerve, causing hemorrhage, fibrosis and consequent compression of the nerve.77 Inferior alveolar nerve block, a common local anesthesia technique used routinely in dentistry, can damage the facial nerve by direct trauma from the needle as well as by anesthetic infiltrating the peripheral branches of the facial nerve.76 Incorrect technique (excessive posterior insertion of needle resulting in injection into the parotid gland) and anatomic variability of the facial nerve have been associated with immediate‑onset BP.76
Delayed-onset BP occurs days after the dental procedure and recovery is prolonged.78 Theories regarding viral reactivation, ischemia and inflammation have all been explored as possible etiologies for delayed-onset BP.78 Stress and local trauma in dental procedures may result in viral reactivation, particularly in patients with a prior history of HSV infection.78 This was further confirmed by a retrospective study by Gaudin et al.,78 who demonstrated prodromal symptoms related to viral reactivation experienced by BP patients following a dental procedure. Therefore, similar to other stressors, such as pregnancy, immunodeficiency and infection, a stress-induced dental procedure could also trigger viral reactivation.78 Moreover, vasospasms of the external carotid artery and activation of the sympathetic plexus of the stylomastoid artery have been implicated in ischemic events of the facial nerve.76,78 Excessive stretching of the facial nerve can also lead to direct damage of the nerve or ischemia.76 Finally, intravascular injection of local anesthetic may cause retrograde flow of the anesthetic, spreading distally and resulting in facial nerve paralysis.76 For instance, a clinical report by Cakarer et al.79 discusses the possibility of retrograde injection in the posterior superior alveolar artery traveling to the petrosal branches of the facial nerve via the middle meningeal artery before a maxillary third-molar extraction.
Other dental-related cases of BP include acute infection of a tooth leading to facial nerve paralysis. Tissue response to infection can result in cytokine release, edema and subsequent inflammation leading to compression of the facial nerve.80 Similarly, neuropraxia of the facial nerve caused by abscess or cellulitis can cause temporary facial nerve paralysis.80 Orthognathic surgery, aiming to correct skeletal deformities, has also been associated with BP, with incidence ranging from 0.10% to 0.75%.81 Furthermore, in patients with slow-resolving cases of BP, oral hygiene is significantly affected as a result of lack of self-cleansing associated with the function of orbicularis oris. This results in increased food debris in the vestibular pouch, leading to increased periodontal disease and tooth decay.82 Regular dental recalls are mandatory for such patients to maintain their oral health.82 In addition, edentulous patients with BP suffer from cheek biting, mandible deviation to the non-affected side and difficulty in pronouncing various sounds, including labiodental and bilabial fricatives.83 This causes additional complications for edentulous patients. Prosthetic face-lift devices and modifications to dentures (e.g., plumpers) have been investigated to support weakened muscles and improve esthetics and stability for such patients.83
ConclusionIt is evident that BP can significantly affect a patient’s quality of life, as the human face is a vital structure in displaying emotions, communicating and carrying out essential functions. It is important for health care professionals to recognize this debilitating condition, diagnose it early and differentiate it from other potentially life-threatening underlying causes. Although most cases resolve spontaneously, early diagnosis and prompt management contribute to a favourable prognosis. In a small subset of patients, permanent complications may arise, requiring a multidisciplinary approach involving neurologists, ophthalmologists and physicians. Therefore, it is crucial for clinicians to understand the etiology, the signs and symptoms and the correct management of BP patients.
THE AUTHORS

Dr. Danesh is a periodontics resident at Nova Southeastern University. He completed his DDS at the University of Toronto.

Dr. Ouanounou is an associate professor, department of clinical sciences (pharmacology & preventive dentistry), faculty of dentistry, University of Toronto.
Corresponding author: Dr. Aviv Ouanounou, Dept. of Clinical Sciences, Pharmacology, Faculty of Dentistry, University of Toronto, 124 Edward St., Room 370, Toronto ON M5G 1G6. Email: aviv.ouanounou@dentistry.utoronto.ca
The authors have no declared financial interests.
This article has been peer reviewed.
References Baugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, et al. Clinical practice guideline: Bell’s palsy. Otolaryngol Head Neck Surg. 2013;149(3 suppl):S1-27. Eviston TJ, Croxson GR, Kennedy PGE, Hadlock T, Krishnan AV. Bell’s palsy: aetiology, clinical features and multidisciplinary care. J Neurol Neurosurg Psychiatry. 2015;86(12):1356-61. Sajadi MM, Sajadi MRM, Tabatabaie SM. The history of facial palsy and spasm: Hippocrates to Razi. Neurology. 2011;77(2):174-8. Canalis RF, Cino L. Ceramic representations of facial paralysis in ancient Peru. Otol Neurotol. 2003;24(5):828-31. Warner MJ, Hutchison J, Varacallo M. Bell palsy. In: StatPearls. Treasure Island, Fla.: StatPearls Publishing; 2022. Available: https://www.ncbi.nlm.nih.gov/books/NBK482290/ (accessed 2022 July 24). Somasundara D, Sullivan F, Cheesbrough GF. Management of Bell’s palsy. Aust Prescr. 2017;40(3):94-7. Nagaraj T, Sahu P, Nigam H, Sumana CK. Bell’s palsy: Two case reports and review of literature. J Med Radiol Pathol Surg. 2018;5(1):12–5. Available: https://www.proquest.com/openview/9b2a6f71caa58b4166dc0263a129cc2d/ (accessed 2022 July 24). Luijmes RE, Pouwels S, Beurskens CHG, Kleiss IJ, Siemann I, Ingels KJAO. Quality of life before and after different treatment modalities in peripheral facial palsy: a systematic review. Laryngoscope. 2017;127(5):1044-51. Bylund N, Hultcrantz M, Jonsson L, Marsk E. Quality of life in Bell’s palsy: correlation with Sunnybrook and House-Brackmann over time. Laryngoscope. 2021;131(2):E612-8. Saadi R, Shokri T, Schaefer E, Hollenbeak C, Lighthall JG. Depression rates after facial paralysis. Ann Plast Surg. 2019;83(2):190-4. Cuenca-Martínez F, Zapardiel-Sánchez E, Carrasco-González E, La Touche R, Suso-Martí L. Assessing anxiety, depression and quality of life in patients with peripheral facial palsy: a systematic review. PeerJ. 2020;8:e10449. Lee SY, Kong IG, Oh DJ, Choi HG. Increased risk of depression in Bell’s palsy: two longitudinal follow-up studies using a national sample cohort. J Affect Disord. 2019;251:256-62. Kurth I. Peripheral neuropathies. Editorial. Medizinische Genetik. 2020;32(3): 193-4. Reich SG. Bell’s palsy. Continuum (Minneap Minn). 2017;23(2):447-66. Yilmaz NDS, Gur OE, Kucuktepe U, Ensari N, Yilmaz MD. Seasonal distribution of the incidence of Bell’s palsy. Med Sci. 2019;8(3):750‑3. Available: http://www.medicinescience.org/wp-content/uploads/2021/12/53-1545908140.pdf (accessed 2022 July 25). Erdur H, Ernst S, Ahmadi M, Albers AE, Marzinzik F, Somasundaram R, et al. Evidence for seasonal variation of Bell’s palsy in Germany. Neuroepidemiology. 2018;51(3-4):128-30. Kim SH, Jung J, Lee JH, Byun JY, Park MS, Yeo SG. Delayed facial nerve decompression for Bell’s palsy. Eur Arch Otorhinolaryngol. 2016;273(7):1755-60. Dong SH, Jung AR, Jung J, Jung SY, Byun JY, Park MS, et al. Recurrent Bell’s palsy. Clin Otolaryngol. 2019;44(3):305-12. Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X. The etiology of Bell’s palsy: a review. J Neurol. 2020;267(7):1896-905. Dudley MZ, Salmon DA, Halsey NA, Orenstein WA, Limaye RJ, O’Leary ST, et al. The clinician’s vaccine safety resource guide: optimizing prevention of vaccine-preventable diseases across the lifespan. Cham, Switzerland: Springer; 2018. Ozonoff A, Nanishi E, Levy O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21(4):450-2. Repajic M, Lai XL, Xu P, Liu A. Bell’s palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021;13:100217. Cirillo N. Reported orofacial adverse effects of COVID‐19 vaccines: the knowns and the unknowns. J Oral Pathol Med. 2021;50(4):424-7. Takezawa K, Townsend G, Ghabriel M. The facial nerve: anatomy and associated disorders for oral health professionals. Odontology. 2018;106(2):103-6. Chhabda S, St Leger D, Lingam RK. Imaging the facial nerve: a contemporary review of anatomy and pathology. Eur J Radiol. 2020;126:108920. Ho ML, Juliano A, Eisenberg RL, Moonis G. Anatomy and pathology of the facial nerve. AJR Am J Roentgenol. 2015;204(6):W612-9. Yang SH, Park HK, Yoo DS, Joo W, Rhoton A. Microsurgical anatomy of the facial nerve. Clin Anat. 2021;34(1):90-102. Patel DK, Levin KH. Bell palsy: clinical examination and management. Cleve Clin J Med. 2015;82(7):419-26. George E, Richie MB, Glastonbury CM. Facial nerve palsy: clinical practice and cognitive errors. Am J Med. 2020;133(9):1039-44. Jain S, Kumar S. Bell’s palsy: a need for paradigm shift? Ann Otol Neurotol. 2018;01(01):034–9. Celik O, Eskiizmir G, Pabuscu Y, Ulkumen B, Toker GT. The role of facial canal diameter in the pathogenesis and grade of Bell’s palsy: a study by high resolution computed tomography. Braz J Otorhinolaryngol. 2017;83(3):261-8. Available: http://dx.doi.org/10.1016/j.bjorl.2016.03.016 Ozan Sanhal E, Arslan H. Evaluation of the facial nerve and internal auditory canal cross-sectional areas on three-dimensional fast imaging employing steady-state acquisition magnetic resonance imaging in Bell’s palsy. Turkish J Med Sci. 2018;48(3):525-30. McElwee M, Vijayakrishnan S, Rixon F, Bhella D. Structure of the herpes simplex virus portal-vertex. PLoS Biol. 2018;16(6):e2006191. Fujiwara T, Matsuda S, Tanaka J, Hato N. Facial paralysis induced by ear inoculation of herpes simplex virus in rat. Auris Nasus Larynx. 2017;44(1):58-64. Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124(1 Pt 1):27–30. Grewal DS. Bell’s palsy-tertiary ischemia: an etiological factor in residual facial palsy. Indian J Otolaryngol Head Neck Surg. 2018;70(3):374-9. Chen H, Liu C, Yin J, Chen Z, Xu J, Wang D, et al. Mitochondrial cyclophilin D as a potential therapeutic target for ischemia-induced facial palsy in rats. Cell Mol Neurobiol. 2015;35(7):931-41. Zhao Z, Liu J, Wang J, Xie T, Zhang Q, Feng S, et al. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int Immunopharmacol. 2017;51:1-8. Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372-6. Oya R, Takenaka Y, Imai T, Sato T, Oshima K, Ohta Y, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as prognostic hematologic markers of Bell’s palsy: a meta-analysis. Otol Neurotol. 2019;40(5):681-7. Hashim NA, Mohamed WS, Emad EM. Neutrophil–lymphocyte ratio and response to plasmapheresis in Guillain–Barré syndrome: a prospective observational study. Egypt J Neurol Psychiatry Neurosurg. 2020;56:17. Available: https://ejnpn.springeropen.com/articles/10.1186/s41983-020-0154-z (accessed 2022 July 25). Demirci S, Demirci S, Kutluhan S, Koyuncuoglu HR, Yurekli VA. The clinical significance of the neutrophil-to-lymphocyte ratio in multiple sclerosis. Int J Neurosci. 2016;126(8):700-6. D’Amico E, Zanghì A, Romano A, Sciandra M, Palumbo GA, Patti F. The neutrophil-to-lymphocyte ratio is related to disease activity in relapsing remitting multiple sclerosis. Cells. 2019;8(10):1114. Bucak A, Ulu S, Oruc S, Yucedag F, Tekin MS, Karakaya F, et al. Neutrophil-to-lymphocyte ratio as a novel-potential marker for predicting prognosis of Bell palsy. Laryngoscope. 2014;124(7):1678-81. Zandian A, Osiro S, Hudson R, Ali IM, Matusz P, Tubbs SR, et al. The neurologist’s dilemma: a comprehensive clinical review of Bell’s palsy, with emphasis on current management trends. Med Sci Monit. 2014;20:83-90. Heckmann JG, Urban PP, Pitz S, Guntinas-Lichius O, Gágyor I. The diagnosis and treatment of idiopathic Bell’ palsy. Dtsch Arztebl Int. 2019;116(41):692-702. Available: https://www.aerzteblatt.de/int/archive/article/210242 (accessed 2022 July 25). Urban E, Volk GF, Geißler K, Thielker J, Dittberner A, Klingner C, et al. Prognostic factors for the outcome of Bells’ palsy: a cohort register-based study. Clin Otolaryngol. 2020;45(5):754-61. Phan NT, Panizza B, Wallwork B. A general practice approach to Bell’s palsy. Aust Fam Physician. 2016;45(11):794-7. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146-7. Fuller G, Morgan C. Bell’s palsy syndrome: mimics and chameleons. Pract Neurol. 2016;16(6):439-44. McCaul JA, Cascarini L, Godden D, Coombes D, Brennan PA, Kerawala CJ. Evidence based management of Bell's palsy. Br J Oral Maxillofacial Surg. 2014;52(5):387-91. Jalali MM, Soleimani R, Soltanipour S, Jalali SM. Pharmacological treatments of Bell’s palsy in adults: a systematic review and network meta-analysis. Laryngoscope. 2021;131(7):1615-25. Teixeira LJ, Valbuza JS, Prado GF. Physical therapy for Bell's palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2011;(12):CD006283. Sthapit PR, Kaiti R, Khanal K, Shrestha P. Ocular manifestations in cases of Bell’s palsy. IP Int J Ocular Oncol Oculoplasty 2017;3(4):277-80. Available: https://www.researchgate.net/profile/Raju-Kaiti/publication/323389468_Ocular_manifestations_in_cases_of_Bell's_Palsy/links/5ecf1b90299bf1c67d23bedf/Ocular-manifestations-in-cases-of-Bells-Palsy.pdf (accessed 2022 July 25). MacIntosh PW, Fay AM. Update on the ophthalmic management of facial paralysis. Surv Ophthalmol. 2019;64(1):79-89. Joseph SS, Joseph AW, Smith JI, Niziol LM, Musch DC, Nelson CC. Evaluation of patients with facial palsy and ophthalmic sequelae: a 23-year retrospective review. Ophthalmic Epidemiol. 2017;24(5):341-5. Gagyor I, Madhok VB, Daly F, Sullivan F. Antiviral treatment for Bell's palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2019;9(9):CD001869. Fujiwara T, Namekawa M, Kuriyama A, Tamaki H. High-dose corticosteroids for adult Bell’s palsy: systematic review and meta-analysis. Otol Neurotol. 2019;40(8):1101-8. Madhok VB, Gagyor I, Daly F, Somasundara D, Sullivan M, Gammie F, Sullivan F. Corticosteroids for Bell's palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2016;7(7):CD001942. Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs. 2019;38(5):336-9. Furukawa T, Abe Y, Ito T, Kubota T, Kakehata S. Benefits of high-dose steroid + hespander + mannitol administration in the treatment of Bell’s palsy. Otol Neurotol. 2017;38(2):272–7. Fujiwara T, Haku Y, Miyazaki T, Yoshida A, Sato SI, Tamaki H. High-dose corticosteroids improve the prognosis of Bell’s palsy compared with low-dose corticosteroids: a propensity score analysis. Auris Nasus Larynx. 2018;45(3):465-70. Kasle DA, Torabi SJ, Savoca E, Tower JI, Hildrew D. Variations in the management of acute Bell’s palsy. Am J Otolaryngol. 2020;41(1):102299. Thielker J, Geissler K, Granitzka T, Klingner CM, Volk GF, Guntinas-Lichius O. Acute management of Bell’s palsy. Curr Otorhinolaryngol Rep. 2018 Jun;6(2):161-70. Menchetti I, McAllister K, Walker D, Donnan PT. Surgical interventions for the early management of Bell’s palsy. Cochrane Database Syst Rev. 2021;1(1):CD007468. Öksüz CE, Kalaycıoğlu A, Uzun Ö, Kalkışım ŞN, Zihni NB, Yıldırım A, et al. The efficacy of acupuncture in the treatment of Bell’s palsy sequelae. J Acupunct Meridian Stud. 2019;12(4):122-30. Parthasarathy S, Hanifah M. Efficacy of electro acupuncture in Bell’s palsy — a clinical interventional pilot trial in Indian patients. Inter J Contemporary Med Res. 2019;6(6):F15-8. Available: http://dx.doi.org/10.21276/ijcmr.2019.6.6.21 (accessed 2022 July 28). Zhu J, Arsovska B, Kozovska K. Acupuncture treatment in Bell’s palsy. IOSR J Dent Med Sci. 2018;17(1):47-9. Available: http://eprints.ugd.edu.mk/19683/1/Acupuncture%20treatment%20in%20Bell%E2%80%99s%20palsy.pdf (accessed 2022 July 28). Zhang R, Wu T, Wang R, Wang D, Liu Q. Compare the efficacy of acupuncture with drugs in the treatment of Bell’s palsy. Medicine (Baltimore). 2019;98(19):e15566. Dimitrova A, Murchison C, Oken B. Acupuncture for the treatment of peripheral neuropathy: a systematic review and meta-analysis. J Altern Complement Med. 2017;23(3):164-79. van Landingham SW, Diels J, Lucarelli MJ. Physical therapy for facial nerve palsy: applications for the physician. Curr Opin Ophthalmol. 2018;29(5):469-75. Mathieu D, Marroni A, Kot J. Tenth European Consensus Conference on Hyperbaric Medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb Med. 2017;47(1):24-32. Maria CM, Kim J. Individualized management of facial synkinesis based on facial function. Acta Otolaryngol. 2017;137(9):1010-5. Pashov A. Paradigm shift in rehabilitation of long-standing Bell’s palsy during later stages of recovery. Fundam Appl Res Pract Leading Scientific Schools. 2018;26(2):294-8. Available: https://farplss.org/index.php/journal/article/view/348/317 (accessed 2022 July 28). Lee SY, Lim JS, Oh DJ, Park B, Park IS, Choi HG. Increased risk of ischemic stroke in patients with Bell’s palsy: a longitudinal follow-up study using a national sample cohort. Auris Nasus Larynx. 2021;48(2):194-200. Jenyon T, Panthagani J, Green D. Transient facial nerve palsy following dental local anaesthesia. BMJ Case Rep. 2020;13(9):e234753. Misirlioglu M, Adisen M, Okkesim A, Akyil Y. Facial nerve paralysis after dental procedure. J Oral Maxillofac Radiol. 2016;4(3):80. Available: https://www.joomr.org/text.asp?2016/4/3/80/196356 (accessed 2022 July 28). Gaudin RA, Remenschneider AK, Phillips K, Knipfer C, Smeets R, Heiland M, et al. Facial palsy after dental procedures – is viral reactivation responsible? J Craniomaxillofac Surg. 2017;45(1):71-5. Cakarer S, Can T, Cankaya B, Erdem MA, Yazici S, Ayintap E, et al. Peripheral facial nerve paralysis after upper third molar extraction. J Craniofac Surg. 2010;21(6):1825-7. Al-Muharraqi MA, O’Sullivan EC. Unilateral facial nerve paralysis following an infected lower third molar. Int J Oral Maxillofac Surg. 2010;39(2):192-5. Bisatto NV, Andriola F de O, Barreiro BOB, Maahs TP, Pagnoncelli RM, Fritscher GG. Facial nerve palsy associated with orthognathic surgery. J Craniofac Surg. 2020;31(6):e546-9. Tolstunov L, Belaga GA. Bell’s palsy and dental infection: a case report and possible etiology. J Oral Maxillofac Surg. 2010;68(5):1173-8. Gupta R, Luthra RP, Aggarwal B. Bell’s palsy and its prosthodontic significance. Review. J Appl Dent Med Sci. 2018;4(4):58-62. Available: https://joadms.org/download/article/337/35012019_55/1552613421.pdf (accessed 2022 July 28).
留言 (0)