A stroke is defined as a sudden onset of signs and symptoms related to focal or global cerebral deficits of brain function, lasting more than 24 h, not attributable to any apparent cause other than cerebral vasculopathy (Sacco et al., 2013). Six months post-stroke, nearly 50% of survivors have some residual motor deficits (Benjamin et al., 2017). Advances in acute stroke therapeutic management (intravenous thrombolysis, mechanical thrombectomy) have improved the prevention possibilities of long-term disability (Tong et al., 2012). Being the second cause of disability and death worldwide (GBD 2016 Stroke Collaborators, 2019), stroke has high relevance to a patient’s quality of life and significant impact on health care costs. Functional impairment, resulting in poor performance in activities of daily living, is common (Benjamin et al., 2017). Environmental conditions are required for post-stroke motor recovery (Power et al., 2011; Wenger et al., 2017). Internal processes combinations such as functional undamaged neural structures recovery and/or brain network remapping could promote impaired functions spontaneous restoration (Gazzaniga, 2005). The phenomenon behind these recovery processes is lifetime—continuous motor system neuroplasticity (Power et al., 2011; Remsik et al., 2016). Traditional rehabilitation techniques enhance motor function recovery (Kollen et al., 2006; Fleet et al., 2014; Laver et al., 2015) leveraging this motor learning circuitry, thus improving patient outcomes (Thakor, 2013). The relationship between brain activity and movements is important for motor learning, thus integrating motor system modulation and rehabilitation techniques in treatment settings could aid stroke recovery (Pfurtscheller et al., 2005; Felton et al., 2007; Schalk et al., 2008). Several neurological disorders (i.e., stroke) are associated with altered electroencephalogram (EEG) brain rhythms, which sustain motor, cognitive, and perceptive functions (Muralidharan et al., 2011; Ortner et al., 2012). EEG signal oscillations detectable in sensorimotor areas, especially in the mu (8–13 Hz) and beta (13–30 Hz) bands, present characteristic modulation during motor tasks. Interestingly, alpha and beta rhythms modulations caused by sensory stimulation, a motor act or motor imagery, are correlated with a decrease or increase in the underlying neuronal population’s synchrony (McFarland et al., 2000; Pfurtscheller et al., 2006; Nicolas-Alonso and Gomez-Gil, 2012). Modulations of sensorimotor rhythms resulting from sensory stimulation, motor act, or its imagination can be of two types, namely, event-related desynchronization (ERD) and event-related synchronization (ERS) of mu and beta rhythms (Jeannerod, 1995; Pfurtscheller and Neuper, 2001). Specifically, ERDs consist of a decrease in the amplitude of rhythms, while ERS is an increase in the amplitude of rhythms (Felton et al., 2007). Alpha (mu) and beta oscillations can be used as control rhythms for a “brain–computer interface” (BCI) system (Schalk et al., 2004). BCI systems can transform brain activity into control signals for external devices (Schalk et al., 2004; McFarland and Wolpaw, 2011; Lee et al., 2020), and can be used for tasks that require users to activate or deactivate specific brain regions (Rathee et al., 2019). Therefore, non-invasive BCI systems can facilitate recovery in patients with chronic post-stroke by linking brain activity with distal motor effectors in the peripheral nervous system (Song et al., 2014). Feedback-regulated motor imagination could be used to improve functional recovery, enhancing antagonistic ERD/ERS patterns, and, consequently, supporting stroke-affected hemisphere activation and contralateral unaffected hemisphere inhibition (Pfurtscheller and Neuper, 2006). Therefore, in the BCI system, brain activity can be transformed into control signals for external devices including “functional electrical stimulation” (FES) (McFarland and Wolpaw, 2011). Thus, non-invasive EEG-BCI-FES systems may facilitate recovery in patients with chronic post-stroke by linking brain activity with distal motor peripheral nervous system effectors and may be used as biomarkers to predict rehabilitation outcomes (Song et al., 2014, 2015).
To modulate and explore brain function, non-invasive brain stimulation (NIBS) could be applied. To date, there are different NIBS protocols with therapeutic applications, reflecting synaptic mechanisms of long-term potentiation (LTP) or long-term depression (LTD), even in stroke rehabilitation (Terranova et al., 2019). The NIBS after effects are short lasting (∼30–120 min) in humans (Abraham and Williams, 2003), but other mechanisms are also involved [i.e., post-tetanic potentiation (PSP) and short-term potentiation (STP)] (Ugawa, 2012). The most applied NIBS are transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS) (Paulus, 2011; Terranova et al., 2019). TMS motor-evoked potentials are obtained from the contralateral muscles of the stimulated hemisphere (Barker et al., 1985). TMS can modulate cortical excitability in different ways: (i) Inducing electrical field causing local effects immediately under the coil and/or remote effects (i.e., excitatory and inhibitory effects) (Rothwell et al., 1999) and (ii) applying a transient weak current to the brain through a pair of saline-sponged electrodes (Nitsche et al., 2008) and changing the polarity of the current. Repetitive transcranial magnetic stimulation (rTMS) produces long-term changes, reducing cortical excitability at low frequency (≤ 1 Hz), and boosting it up at high frequency (≥ 5 Hz) (Maeda et al., 2000; Siebner and Rothwell, 2003; Quartarone et al., 2005). However, it has been shown that continuous 5 Hz rTMS decreases instead of increasing corticospinal excitability (Rothkegel et al., 2010). When rTMS is administered in a complex burst pattern, i.e., theta burst stimulation, it produces more reliable effects than conventional rTMS (Huang et al., 2005; Hamada et al., 2008; Suppa et al., 2016). Another rTMS approach, namely, theta burst stimulation (TBS) (intermittent or continuous), uses 5 Hz short bursts at a repetitive high frequency mimicking the brain’s natural firing patterns (Oberman et al., 2011; Hoy et al., 2016). Compared to rTMS, intermittent TBS (iTBS) may be applied to induce greater and longer-lasting motor cortical effects on cortical excitability (Huang et al., 2005; Di Lazzaro et al., 2008). It is applied using biphasic stimulus pulses that induce an initial posterior-anterior current through M1 (Huang et al., 2005). The use of short 5-Hz high-frequency repetitive bursts that mimic the brain’s natural firing patterns would result in greater neuromodulatory potential than the standard approach. Thus, the effects on the functional brain network of patients with stroke would be greater and longer lasting in regions remote from the stimulated site (Oberman et al., 2011; Hoy et al., 2016; Suppa et al., 2016). Continuous TBS (cTBS) decreases cortical excitability, while intermittent TBS has a booster-up effect (Hamada et al., 2008). However, tDCS is mainly applied in clinical practice, while tACS and tRNS are more used in a research context (Paulus, 2011). Anodal tDCS modulates the cortical excitability of depolarizing neurons, whereas cathodal tDCS reduces the excitability of hyperpolarizing neurons (Antal et al., 2004). In 1–2 mA tDCS, electrical current is delivered over the skull through sponge electrodes, changing neurons firing frequency (Paulus, 2011); anodal stimulation induces cortical facilitation, whereas cathodal stimulation has an opposite effect (Paulus, 2011). However, despite TMS and tDCS having different mechanisms of action (acting TMS as neurostimulator and tDCS as neuromodulator), they both induce cortical excitability long-term after effects, which engage neural plasticity mechanisms (Fregni et al., 2005; Khedr et al., 2010). Transcranial alternating current stimulation (tACS) is a variant of TMS at a predetermined frequency (Alekseichuk et al., 2016). Transcranial random noise stimulation (tRNS) is another NIBS technique using a low-intensity biphasic randomly alternating current at a variable frequency (Fertonani et al., 2011). While researchers are still debating over the functional meaning of these synchronization and de-synchronization patterns of rhythmic activity, practical applications based on the accumulated knowledge are already emerging. On such a basis, this review aims to evaluate the role of brain oscillatory activity on motor function recovery in patients with post-stroke undergoing conventional rehabilitation treatment integrated with different NIBS.
Search strategy and selection criteriaA computerized literature search was performed in Pubmed, Scopus, and the Web of Science from 2012 to 2022, using the following string: “brain rhythms” and “stroke” and “EEG.” The screening process and analysis were conducted separately by 3 independent observers.
First, the articles were screened by title and abstract. The following inclusion criteria for relevant articles were used during the screening: (1) Randomized controlled trials (RCTs), (2) English language, (3) published in indexed journals over the last 10 years (2012–2022), (4) including only adult human (>18 years), and (5) dealing with brain rhythms and their analysis and applications in stroke rehabilitation. The exclusion criteria were non-English articles, reviews, non-randomized controlled studies, and trials on other nervous system diseases different from a stroke.
In the second step, the full texts of the selected articles were screened, with further exclusions according to the previously described criteria and focusing our attention on motor functions.
A flowchart is given in Figure 1.
FIGURE 1
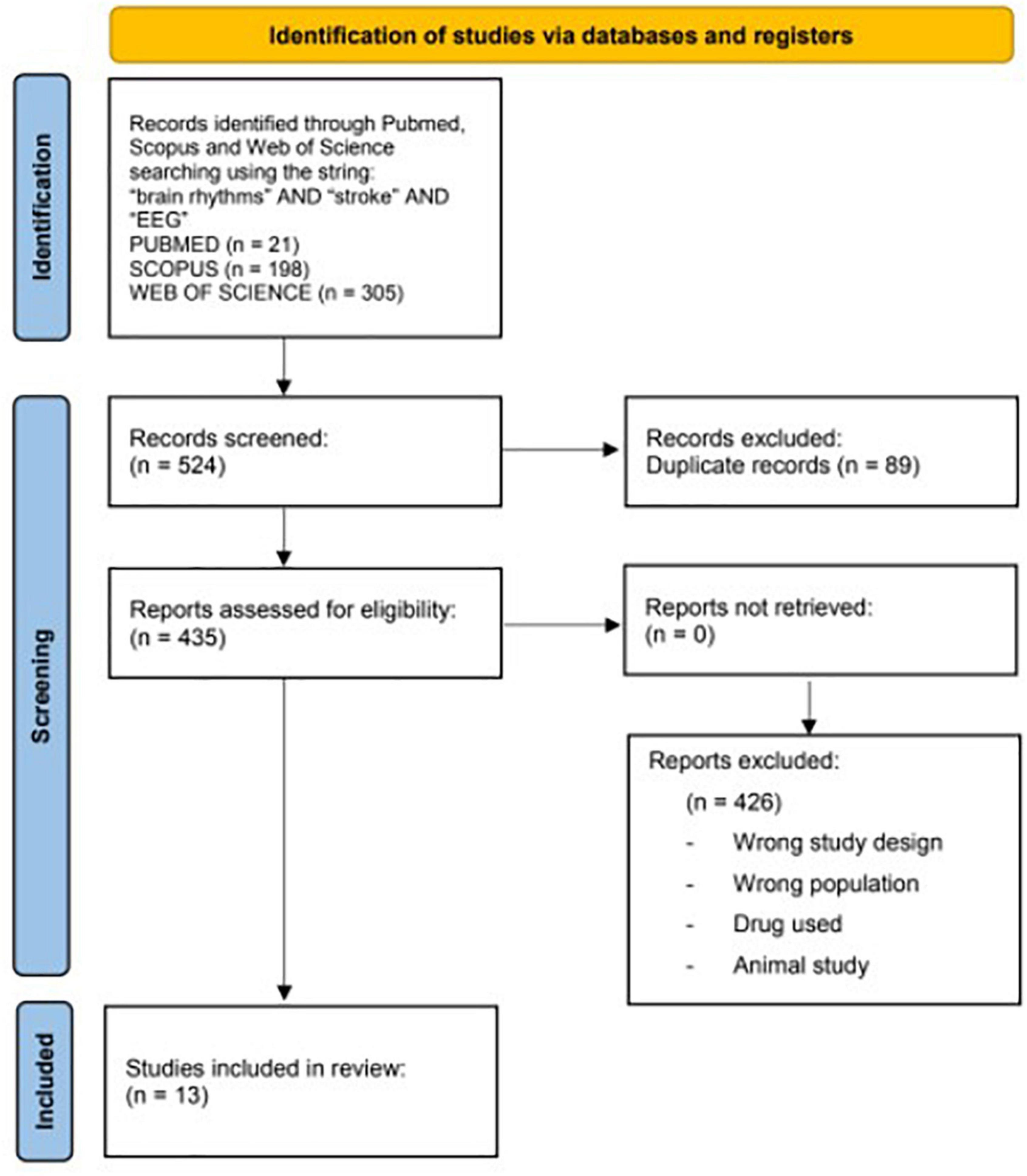
Figure 1. Preferred reporting items flowchart resuming the paper’s selection process.
The following data were retrieved: (1) treatment groups, (2) sample size and patients’ features, (3) time since stroke, (4) therapeutic protocols, (5) outcome measures, (6) time points of follow-up evaluations, and (7) summary of clinical results.
Intervention protocolsWe analyzed the RCT discussing rehabilitative interventions on motor impairment after stroke and the applied innovative NIBS and BCI systems. We grouped the studies considering the impaired involved limb/site and the applied techniques as follows:
a) Upper limb involvement applying theta burst stimulation (TBS) or repetitive transcranial magnetic stimulation rTMS or tDCS.
Four out of thirteen articles investigated upper limb functions through transcranial stimulation (Nicolo et al., 2018; Ding et al., 2021; Dionísio et al., 2021; Kuzu et al., 2021).
Dionísio et al. (2021) compared cTBS vs. placebo stimulation, finding that contralateral inhibitory stimulation led to a significant excitatory impact on the cortical oscillatory beta band patterns of the contralateral affected hemisphere. In Ding’s (2021) study, the authors found that iTBS modulates brain network functioning in stroke survivors, normalizing brain connections, re-gaining the natural oscillation frequency, and promoting motor function recovery. Kuzu et al. (2021) showed improvements in upper extremity motor function and activities of daily living by combining physical therapy and cTBS or rTMS. However, these treatments showed limited evidence of improving spasticity (Kuzu et al., 2021). Nicolo et al. (2018) demonstrated that stimulation treatments combined with physical therapy did not enhance clinical motor gains in a heterogeneous sample of patients with sub-acute stroke.
Other studies have shown that tDCS enhanced perilesional beta-band oscillation coherence compared with cTBS and the sham groups (Carmichael, 2006; Murphy and Corbett, 2009). Moreover, tDCS produces and modulates the ongoing synaptic activity during motor activation determining a weak polarization of large assemblies of neurons (Carmichael, 2006; Murphy and Corbett, 2009). The modulation of inter-hemispheric driving and peri-lesional beta-band connectivity was related to functional recovery across all the patients (Carmichael, 2006; Murphy and Corbett, 2009). One of the potential mechanisms explaining these results could lie in the functional connectivity that increases with adaptive cortical plasticity induction caused by tDCS (Carmichael, 2006; Murphy and Corbett, 2009).
b) Upper limb involvement applying different kinds of stimulation.
Six out of thirteen articles investigated upper limb functions exploring mirror therapy and BCI (Bae et al., 2012; Ramos-Murguialday et al., 2013; Remsik et al., 2019; Lee et al., 2020; Ray et al., 2020; Sebastián-Romagosa et al., 2020). Some authors have shown that mirror therapy and its effect on mu-rhythm suppression positively influence brain activity (Bae et al., 2012). Other authors investigated the action observation training (AOT) effect associated with EEG-based BCI-controlled FES system on motor recovery of the upper extremity and cortical activation in patients with stroke (Remsik et al., 2019; Lee et al., 2020), showing that the AOT plus BCI-FES group had an increase in alpha and beta waves concentration, with improved functional outcome scores (Remsik et al., 2019; Lee et al., 2020), as previously reported (Prasad et al., 2010; McCrimmon et al., 2014; Chung et al., 2015). Other authors supported the importance of BCI devices to improve upper extremity functions in stroke survivors (Remsik et al., 2019). Intervention corresponds with greater mu-rhythm de-synchronization in the ipsilesional hemisphere during the impaired hand attempted movements (Remsik et al., 2019).
Different authors explored the efficacy of daily body mass index (BMI) in stroke survivors (Ramos-Murguialday et al., 2013; Ray et al., 2020).
Ray et al. (2020) evaluated the relationship between oscillatory sensorimotor brain activity and motor recovery in chronic stroke, identifying a correlation between alpha de-synchronization during the rehabilitative intervention and clinical improvement. These results showed that inter-hemispheric balance plays an important role in motor recovery using BMI associated with physiotherapy, which exerts an add-on effect on hand motor function recovery (Ramos-Murguialday et al., 2013).
Other authors investigated the correlation between EEG parameters, such as the “Brain Symmetry Index” (BSI) and the laterality coefficient (LC) (Sebastián-Romagosa et al., 2020). The authors calculated LC values in two frequency bands, namely, 8–13 Hz (α band, mu frequency rhythm) and 13–30 Hz (β band), disclosing the most relevant results in the alpha band (Sebastián-Romagosa et al., 2020). Specifically, the LC values calculated during M1 tasks with the healthy hand (LCh) were significant compared to those of the paretic hand MI tasks (LCp). These results showed that the LCh in the alpha band presented numerous significant correlations with functional scales, whereas the correlation between the LCp and the functional scales is less common (Sebastián-Romagosa et al., 2020). This could occur because the affected hemisphere does not present a normal activation pattern, but the healthy hemisphere maintains the normal patterns of de-synchronization during the ipsilateral motor movements. The ERD/ERS patterns observed in the healthy hemisphere should be more stable than those in the affected brain side (Sebastián-Romagosa et al., 2020).
c) Lower limb involvement applying iTBS
Ding et al. (2022) first reported that iTBS increase the natural frequency in the ipsilesional motor cortex and is useful in upper limb stroke rehabilitation. Unfortunately, such improvement has not been proven for the lower limbs (Lin et al., 2019).
d) Upper and lower limbs involvement applying tDCS
Kasashima et al. (2012) demonstrated that, in severe stroke hemiparesis, anodal tDCS could increase ERD in the mu-band, inducing long-term after-effects on cortical excitability through neural plasticity mechanisms (Fregni et al., 2005; Khedr et al., 2010). Such an effect makes tDCS a possible conditioning tool for BCI (Kasashima et al., 2012). Specifically, in the affected hemisphere, mu ERD increased after anodal tDCS during motor imagery, in both the stroke and healthy participants, possibly because of a decreased synchrony of the underlying neuronal population (Kasashima et al., 2012). Therefore, modulating mu ERD in BCI treatment could increase cortical excitability, normalizing stroke EEG-ERD values (Kasashima et al., 2012).
e) Upper and lower limbs involvement applying BCI
Tsuchimoto et al. (2019) analyzed a motor image-guided robotic approach in post-stroke hemiplegia using an EEG-based neurofeedback system. Specifically, the alpha band reflected the prominent component of activity in the sensory cortex and the beta band maintained activity in the motor cortex (Tsuchimoto et al., 2019). Moreover, the authors found that the integration of EEG and sensorimotor feedback led to increased co-activation of the sensory and motor cortices during the neurofeedback intervention (Tsuchimoto et al., 2019). On such a basis, alpha- and beta-band EEG may be used as physiological biomarkers of motor learning in stroke recovery (Tsuchimoto et al., 2019).
DiscussionAfter a stroke, brain oscillatory changes are likely due to the activation of inflammatory pathways and increased oxidative stress (Moskowitz et al., 2010), leading to motor, speech, and cognitive impairments (Sun et al., 2014; Hatem et al., 2016). Neuroplasticity and exercise are used to promote recovery in stroke survivors (Alia et al., 2017; Caglayan et al., 2019; Inoue et al., 2020). Thus, NIBS may modulate brain rhythms in chronic stroke. Specifically, brain oscillations are rhythmic patterns, occurring at different frequencies (i.e., delta 1–3 Hz, theta 3–7 Hz, alpha 8–12 Hz, beta 13–25 Hz, and gamma 25–100 Hz), generated by the synchronized interaction of neuronal firing (Colgin, 2016). Multiple studies found that neural oscillations after stroke influence recovery outcomes (Rabiller et al., 2015). Specifically:
a) Just after stroke:
- Alpha oscillations are lower in frequency and more synchronized (Petrovic et al., 2017).
- Beta oscillatory power is increased in both the hemispheres (Assenza et al., 2009).
- Gamma oscillations are disrupted (Buzsáki and Wang, 2012).
b) In chronic stroke:
- Alpha oscillation de-synchronization is associated with improved motor outcomes (Westlake et al., 2012; Ray et al., 2020).
- Increased beta coherence between the motor cortex and other regions in the acute phase is associated to improve functional outcomes 3 months after stroke (Nicolo et al., 2018). Higher beta power in the affected hemisphere is associated to improve motor function, whereas in the unaffected hemisphere, it correlates with worse clinical outcomes (Thibaut et al., 2017; Espenhahn et al., 2020). Thus, in stroke recovery, changes in beta oscillations across the brain may be not congruent.
- An increase in gamma power in the affected hemisphere is a good recovery target associated with improved clinical outcomes (Tecchio et al., 2007).
To date, neuromodulation approaches, i.e., FES-NIBS, are valid tools to improve stroke rehabilitation outcomes. In addition, BCI systems could support motor function enhancement by providing visual/somatosensory feedback (Pillette et al., 2020), whereas EEG could provide real-time brain rhythms feedback (Enriquez-Geppert et al., 2017). The high reproducibility of ERD/ERS could be considered a promising predictive outcome measure for evaluating patients with stroke recovery after rehabilitation.
The core articles included in this review are shown in Table 1. Specifically, it has been shown that the above-reported techniques are more effective in the chronic stroke phase, whereas in the acute/post-acute phase, their effectiveness should be better explored. However, these data need to be cautiously taken because of several study limitations. First, this review was conducted as a broad overview of a topic-related research area. Moreover, the search method depends on a non-predefined protocol, which may involve subjective selection bias. Many of these studies have been plagued by methodological problems (i.e., the heterogeneity of the population, the rehabilitation sessions and duration, the ischemic areas involved, timing from the acute event, and the different EEG-BCI-FES-NIBS applied). The Inclusion criteria of studies for review also rely on researchers’ experiences. Finally, the selected studies reported different functional outcomes and the scarcity of the data prevented performing systematic review. However, devising novel clinical trials, which consider such limitations, may be helpful to define proper treatment settings, integrating motor system modulation and rehabilitation techniques, to shed new light on the role of brain oscillations in patients with post-stroke rehabilitation.
TABLE 1
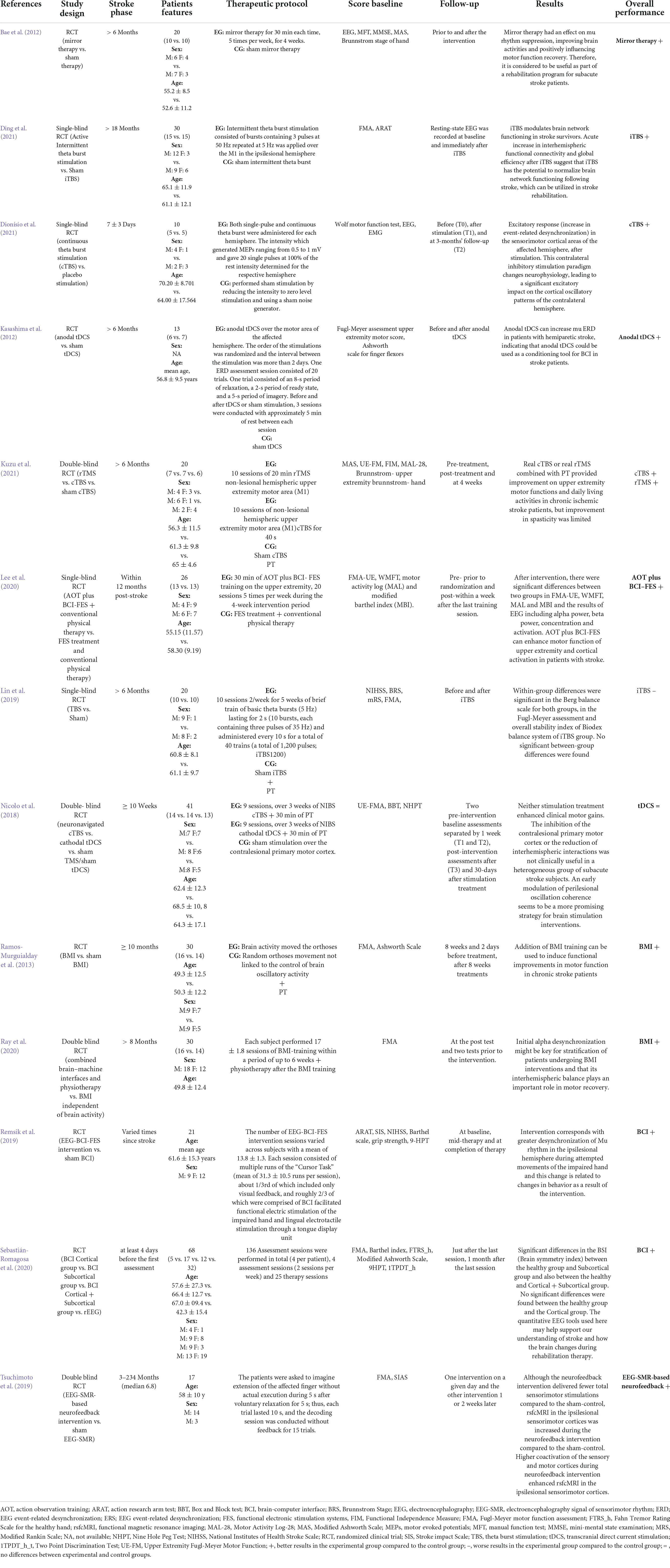
Table 1. Synopsis of all the RCTs included in the review.
Author contributionsGL and SP proposed the research idea, wrote the background and conclusion, and contributed to the literature review. AA supported the literature review and helped to revise the manuscript. SP prepared the manuscript for submission. All authors have read and approved the final version of the manuscript.
FundingThis research was funded by the Italian Ministry of Health—Current Research 2022.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAlekseichuk, I., Turi, Z., Amador de Lara, G., Antal, A., and Paulus, W. (2016). Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Curr. Biol. 26, 1513–1521. doi: 10.1016/j.cub.2016.04.035
PubMed Abstract | CrossRef Full Text | Google Scholar
Alia, C., Spalletti, C., Lai, S., Panarese, A., Lamola, G., Bertolucci, F., et al. (2017). Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front. Cell Neurosci. 11:76. doi: 10.3389/fncel.2017.00076
PubMed Abstract | CrossRef Full Text | Google Scholar
Antal, A., Nitsche, M. A., Kruse, W., Kincses, T. Z., Hoffmann, K. P., and Paulus, W. (2004). Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J. Cogn. Neurosci. 16, 521–527. doi: 10.1162/089892904323057263
PubMed Abstract | CrossRef Full Text | Google Scholar
Assenza, G., Zappasodi, F., Squitti, R., Altamura, C., Ventriglia, M., Ercolani, M., et al. (2009). Neuronal functionality assessed by magnetoencephalography is related to oxidative stress system in acute ischemic stroke. Neuroimage 44, 1267–1273. doi: 10.1016/j.neuroimage.2008.09.049
PubMed Abstract | CrossRef Full Text | Google Scholar
Bae, S. H., Jeong, W. S., and Kim, K. Y. (2012). Effects of mirror therapy on subacute stroke patients’ brain waves and upper extremity functions. J. Phys. Ther. Sci. 24, 1119–1122. doi: 10.1589/jpts.24.1119
PubMed Abstract | CrossRef Full Text | Google Scholar
Benjamin, E. J., Blaha, M. J., Chiuve, S. E., Cushman, M., Das, S. R., Deo, R., et al. (2017). Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation 135:e646. doi: 10.1161/CIR.0000000000000485
PubMed Abstract | CrossRef Full Text | Google Scholar
Caglayan, A. B., Beker, M. C., Caglayan, B., Yalcin, E., Caglayan, A., Yulug, B., et al. (2019). Acute and post-acute neuromodulation induces stroke recovery by promoting survival signaling, neurogenesis, and pyramidal tract plasticity. Front. Cell Neurosci. 13:144. doi: 10.3389/fncel.2019.00144
PubMed Abstract | CrossRef Full Text | Google Scholar
Chung, E., Park, S. I., Jang, Y. Y., and Lee, B. H. (2015). Effects of brain-computer interface-based functional electrical stimulation on balance and gait function in patients with stroke: preliminary results. J. Phys. Ther. Sci. 27, 513–516. doi: 10.1589/jpts.27.513
PubMed Abstract | CrossRef Full Text | Google Scholar
Di Lazzaro, V., Pilato, F., Dileone, M., Profice, P., Oliviero, A., Mazzone, P., et al. (2008). The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J. Physiol. 586, 3871–3879. doi: 10.1113/jphysiol.2008.152736
PubMed Abstract | CrossRef Full Text | Google Scholar
Ding, Q., Chen, S., Chen, J., Zhang, S., Peng, Y., Chen, Y., et al. (2022). Intermittent theta burst stimulation increases natural oscillatory frequency in ipsilesional motor cortex post-stroke: a transcranial magnetic stimulation and electroencephalography study. Front. Aging Neurosci. 14:818340. doi: 10.3389/fnagi.2022.818340
PubMed Abstract | CrossRef Full Text | Google Scholar
Ding, Q., Zhang, S., Chen, S., Chen, J., Li, X., Chen, J., et al. (2021). The effects of intermittent theta burst stimulation on functional brain network following stroke: an electroencephalography study. Front. Neurosci. 15:755709. doi: 10.3389/fnins.2021.755709
PubMed Abstract | CrossRef Full Text | Google Scholar
Dionísio, A., Gouveia, R., Castelhano, J., Duarte, I. C., Santo, G. C., Sargento-Freitas, J., et al. (2021). The role of continuous theta burst TMS in the neurorehabilitation of subacute stroke patients: a placebo-controlled study. Front. Neurol. 12:749798. doi: 10.3389/fneur.2021.749798
PubMed Abstract | CrossRef Full Text | Google Scholar
Enriquez-Geppert, S., Huster, R. J., and Herrmann, C. S. (2017). EEG-neurofeedback as a tool to modulate cognition and behavior: a review tutorial. Front. Hum. Neurosci. 11:51. doi: 10.3389/fnhum.2017.00051
PubMed Abstract | CrossRef Full Text | Google Scholar
Espenhahn, S., Rossiter, H. E., van Wijk, B. C. M., Redman, N., Rondina, J. M., Diedrichsen, J., et al. (2020). Sensorimotor cortex beta oscillations reflect motor skill learning ability after stroke. Brain Commun. 2:fcaa161. doi: 10.1093/braincomms/fcaa161
PubMed Abstract | CrossRef Full Text | Google Scholar
Felton, E. A., Wilson, J. A., Williams, J. C., and Garell, P. C. (2007). Electrocorticographically controlled brain-computer interfaces using motor and sensory imagery in patients with temporary subdural electrode implants. Report of four cases. J. Neurosurg. 106, 495–500. doi: 10.3171/jns.2007.106.3.495
PubMed Abstract | CrossRef Full Text | Google Scholar
Fertonani, A., Pirulli, C., and Miniussi, C. (2011). Random noise stimulation improves neuroplasticity in perceptual learning. J. Neurosci. 31, 15416–15423. doi: 10.1523/JNEUROSCI.2002-11.2011
PubMed Abstract | CrossRef Full Text | Google Scholar
Fleet, A., Page, S. J., MacKay-Lyons, M., and Boe, S. G. (2014). Modified constraint-induced movement therapy for upper extremity recovery post stroke: what is the evidence? Top. Stroke Rehabil. 21, 319–331. doi: 10.1310/tsr2104-319
PubMed Abstract | CrossRef Full Text | Google Scholar
Fregni, F., Boggio, P. S., Mansur, C. G., Wagner, T., Ferreira, M. J., Lima, M. C., et al. (2005). Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 16, 1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e
CrossRef Full Text | Google Scholar
GBD 2016 Stroke Collaborators (2019). Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 439–458.
Hamada, M., Terao, Y., Hanajima, R., Shirota, Y., Nakatani-Enomoto, S., Furubayashi, T., et al. (2008). Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J. Physiol. 586, 3927–3947. doi: 10.1113/jphysiol.2008.152793
PubMed Abstract | CrossRef Full Text | Google Scholar
Hatem, S. M., Saussez, G., Della Faille, M., Prist, V., Zhang, X., Dispa, D., et al. (2016). Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 10:442. doi: 10.3389/fnhum.2016.00442
PubMed Abstract | CrossRef Full Text | Google Scholar
Hoy, K. E., Bailey, N., Michael, M., Fitzgibbon, B., Rogasch, N. C., Saeki, T., et al. (2016). Enhancement of working memory and task-related oscillatory activity following intermittent theta burst stimulation in healthy controls. Cerebr. Cortex 26, 4563–4573. doi: 10.1093/cercor/bhv193
PubMed Abstract | CrossRef Full Text | Google Scholar
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P., and Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. doi: 10.1016/j.neuron.2004.12.033
PubMed Abstract | CrossRef Full Text | Google Scholar
Inoue, T., Okamura, M., Kitahara, M., Takamatsu, Y., Sakakima, H., and Maejima, H. (2020). Exercise plus pharmacological neuromodulation of synaptic inhibition enhance motor function recovery after ischemic stroke. Neuroscience 430, 12–24. doi: 10.1016/j.neuroscience.2020.01.012
PubMed Abstract | CrossRef Full Text | Google Scholar
Jeannerod, M. (1995). Mental imagery in the motor context. Neuropsychologia 33, 1419–1432. doi: 10.1016/0028-3932(95)00073-C
CrossRef Full Text | Google Scholar
Kasashima, Y., Fujiwara, T., Matsushika, Y., Tsuji, T., Hase, K., Ushiyama, J., et al. (2012). Modulation of event-related desynchronization during motor imagery with transcranial direct current stimulation (tDCS) in patients with chronic hemiparetic stroke. Exp. Brain Res. 221, 263–268. doi: 10.1007/s00221-012-3166-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Khedr, E. M., Etraby, A. E., Hemeda, M., Nasef, A. M., and Razek, A. A. (2010). Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol. Scand. 121, 30–37. doi: 10.1111/j.1600-0404.2009.01195.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Kollen, B., Kwakkel, G., and Lindeman, E. (2006). Functional recovery after stroke: a review of current developments in stroke rehabilitation research. Rev. Recent. Clin. Trials 1, 75–80. doi: 10.2174/157488706775246111
PubMed Abstract | CrossRef Full Text | Google Scholar
Kuzu, Ö, Adiguzel, E., Kesikburun, S., Yaşar, E., and Yılmaz, B. (2021). The effect of sham controlled continuous theta burst stimulation and low frequency repetitive transcranial magnetic stimulation on upper extremity spasticity and functional recovery in chronic ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 30:105795. doi: 10.1016/j.jstrokecerebrovasdis.2021.105795
PubMed Abstract | CrossRef Full Text | Google Scholar
Laver, K., George, S., Thomas, S., Deutsch, J. E., and Crotty, M. (2015). Virtual reality for stroke rehabilitation: an abridged version of a Cochrane review. Eur. J. Phys. Rehabil. Med. 51, 497–506. doi: 10.1002/14651858.CD008349.pub3
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, S. H., Kim, S. S., and Lee, B. H. (2020). Action observation training and brain-computer interface controlled functional electrical stimulation enhance upper extremity performance and cortical activation in patients with stroke: a randomized controlled trial. Physiother. Theory Pract. [Epub ahead of print]. doi: 10.1080/09593985.2020.1831114
PubMed Abstract | CrossRef Full Text | Google Scholar
Lin, L. F., Chang, K. H., Huang, Y. Z., Lai, C. H., Liou, T. H., and Lin, Y. N. (2019). Simultaneous stimulation in bilateral leg motor areas with intermittent theta burst stimulation to improve functional performance after stroke: a feasibility pilot study. Eur. J. Phys. Rehabil. Med. 55, 162–168. doi: 10.23736/S1973-9087.18.05245-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., and Pascual-Leone, A. (2000). Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 133, 425–430. doi: 10.1007/s002210000432
PubMed Abstract | CrossRef Full Text | Google Scholar
McCrimmon, C. M., King, C. E., Wang, P. T., Cramer, S. C., Nenadic, Z., and Do, A. H. (2014). Brain-controlled functional electrical stimulation for lower-limb motor recovery in stroke survivors. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 1247–1250. doi: 10.1109/EMBC.2014.6943823
PubMed Abstract | CrossRef Full Text | Google Scholar
McFarland, D. J., Miner, L. A., Vaughan, T. M., and Wolpaw, J. R. (2000). Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topogr. 12, 177–186. doi: 10.1023/A:1023437823106
CrossRef Full Text | Google Scholar
Muralidharan, A., Chae, J., and Taylor, D. M. (2011). Extracting attempted hand movements from EEGs in people with complete hand paralysis following stroke. Front. Neurosci. 5:39. doi: 10.3389/fnins.2011.00039
PubMed Abstract | CrossRef Full Text | Google Scholar
Nicolo, P., Magnin, C., Pedrazzini, E., Plomp, G., Mottaz, A., Schnider, A., et al. (2018). Comparison of neuroplastic responses to cathodal transcranial direct current stimulation and continuous theta burst stimulation in subacute stroke. Arch. Phys. Med. Rehabil. 99, 862.e1–872.e1. doi: 10.1016/j.apmr.2017.10.026
PubMed Abstract | CrossRef Full Text | Google Scholar
Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori, A., Lang, N., Antal, A., et al. (2008). Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 1, 206–223. doi: 10.1016/j.brs.2008.06.004
PubMed Abstract | CrossRef Full Text | Google Scholar
Oberman, L., Edwards, D., Eldaief, M., and Pascual-Leone, A. (2011). Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. J. Clin. Neurophysiol. 28, 67–74. doi: 10.1097/WNP.0b013e318205135f
留言 (0)