Essential tremor (ET) is a pathological tremor that affects an estimated ~1% of the population, or 4.6% of those aged 65 years and above (Louis and Ferreira, 2010). ET is characterised as an action tremor—a tremor typically produced by voluntary contraction of muscles and present during sustained posture or voluntary movement (Thenganatt and Jankovic, 2016; Bhatia et al., 2018).
ET tremor frequency in humans is usually around 6–12 Hz and the tremor typically affects the arms and hands but can also affect the head and voice. The pathophysiology underlying ET is unclear, although it is increasingly recognised as a heterogenous disease or syndrome, where there may be several underlying aetiologies producing the clinical phenotype of ET. Converging research suggests that a common feature is abnormalities in activity within the cerebellum which are propagated through the cerebello-thalamo-cortical network. A cardinal symptom of cerebellar dysfunction is intention tremor; a tremor that worsens with goal-directed movement, resulting from disturbances to cerebellar output pathways (Holmes, 1939; Fahn, 1984) and intention tremor has been associated with severe or advanced cases of ET (Deuschl et al., 2000; Louis et al., 2009; Sternberg et al., 2013). Similarly, ataxia—another cardinal sign of cerebellar dysfunction—can be present in ET (Singer et al., 1994; Stolze et al., 2001; Duval et al., 2006; Arkadir and Louis, 2013). Patients with ET have also shown impaired performance on cerebellar-dependent behaviours, such as eye-blink conditioning (Kronenbuerger et al., 2007, 2008), and visuo-motor adaptation (Hanajima et al., 2016). Neuropathological changes have also been observed in the cerebellum of ET patients, including Purkinje cell loss, changes in Purkinje cell morphology and connectivity (e.g., Kuo et al., 2011; 2017a; Yu et al., 2012; Babij et al., 2013; Louis et al., 2014) and redistribution of climbing fibre synapses on the Purkinje cell dendritic arbour, with a greater number of climbing fibre synapses found on distal dendritic spines (Lin et al., 2014; Kuo et al., 2017b; Lee D. et al., 2018; Pan et al., 2020). Clinical studies describing cerebellar abnormalities have, therefore, been closely associated with ET.
A major outflow path of the cerebellum is its projection to the sensorimotor cortex via the ventrolateral thalamus (VL), including a subregion termed the ventral intermediate nucleus (VIM, Lierse, 1993). Hua et al. (1998) identified cells within VIM that fire periodically at rates that correlate with tremors. Furthermore, deep brain stimulation of VIM can provide symptomatic relief to ET (Vaillancourt et al., 2003). And thalamic stimulation at a near-to-tremor frequency can entrain the frequency of the tremor, as well as amplify or suppress tremor amplitude, depending on the phase of tremor oscillation when the stimulation is applied (Cagnan et al., 2013). Taken together these findings suggest the thalamus plays an important role in propagating tremor oscillations through the cerebello-thalamo-cortical pathway.
Harmaline is a pharmacological model of tremor, that involves the administration of the β-carboline alkaloid harmaline, which is a reversible inhibitor of monoamine oxidase-A (Hoon et al., 1997). Harmaline-induced tremor is characterised as an action tremor, akin to ET, with the frequency of tremor varying slightly across species (10–16 Hz in mice, 8–12 Hz in rats, 7–12 Hz in monkeys, and 10–16 Hz in pigs; Lamarre et al., 1975; Martin et al., 2005; Lee J. et al., 2018). Harmaline tremor shares similar underlying neural pathways as ET (Handforth, 2012). For example, electrical stimulation within the thalamus in harmaline-treated mice and rats significantly reduces the amplitude of harmaline-induced tremor, akin to the effects of deep brain stimulation observed in ET patients (Bekar et al., 2008; Lee and Chang, 2019). Harmaline induces a temporary increase in the frequency of rhythmic activity in the brainstem inferior olivary (IO) complex, predominantly within the caudal medial accessory olive and caudal dorsal accessory olive (De Montigny and Lamarre, 1973; Lamarre et al., 1975). This in turn, increases the regularity and synchrony of climbing fibre evoked complex spikes in the cerebellar cortex, particularly within the vermis and paravermis, with a complete suppression of simple spikes (De Montigny and Lamarre, 1973; Bernard et al., 1984).
It has previously been hypothesised that tremor-onset with action in ET is due to disrupted cerebellar output (Buijink et al., 2015). The cerebellar nuclei transmit integrated sensorimotor signals to the wider motor network (e.g., Giuffrida et al., 1981; Armstrong and Edgley, 1984; Rowland and Jaeger, 2005; Becker and Person, 2019). Bilateral rhythmic optogenetic stimulation of the interpositus nucleus has been shown to induce a tremor in mice at the same frequency of the stimulation (Brown et al., 2020). This underscores the importance of increased cerebellar nuclear rhythmicity in generating and propagating tremorgenic rhythms. Neuropathological changes in ET may disrupt normal movement-related oscillations in the cerebellum, which in turn may disrupt cerebellar output from the cerebellar nuclei during movement (Pellerin and Lamarre, 1997; Hartmann and Bower, 1998; Courtemanche et al., 2002; Dugué et al., 2009; Baumel et al., 2021). Research to date, however, has not examined the impact of harmaline on cerebellar projections to ascending thalamo-cortical pathways, nor whether movement modulates activity in these pathways. Our aim was to examine harmaline’s effect on the cerebello-thalamo-cortical network in the awake behaving rat by recording local field potential (LFP) oscillations at the tremor frequency across the network and using coherence analysis to examine how these neural network interactions are modulated by movement. Cross-correlation analysis was carried out to examine the functional connectivity of oscillations between nodes of the tremor network. We observed that harmaline-induced tremor can be characterised as an action tremor associated with coherent tremor frequency oscillations across the cerebello-thalamo-cortical network, suggesting propagation of tremor oscillations across the network. Furthermore, harmaline induced prominent pathological oscillations in the cerebellum, and cerebellar coherence with tremor were observed when the animals were resting/immobile and moving. In contrast, thalamic coherence with tremor was modulated by movement. These findings provide evidence that harmaline-induced tremor involves comparable electrophysiological correlates to those reported in ET. Additionally, these findings suggest that the neural oscillations in the cerebellum and thalamus have different roles in modulating tremor, as thalamus but not cerebellar oscillations are influenced by movement and/or behavioural tremor amplitude in the harmaline model.
Materials and Methods AnimalsAll procedures were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and the University of Bristol Animal Welfare and Ethical Review Body. Experiments were performed on 14 adult male Lister Hooded rats (300–600 g). All animals were housed in groups under normal environmental conditions (~20°C and 45%–65% humidity), maintained on a 12/12 h light/dark reverse lighting cycle and provided with food and water ad libitum. Rats were handled daily for at least 1 week prior to surgery. Following surgery, the animals were housed separately, and their health was monitored closely with observational assessments and monitoring of weight.
SurgeryRats were anaesthetised with a combination of ketamine (50%) and medetomidine (30%) in saline (20%) delivered via i.p. injection at a dose of 1 ml/kg, and then placed in a stereotaxic frame and secured with atraumatic ear bars coated with local anaesthetic lidocaine (10% Xylocaine®). Occasionally an additional dose of 0.1 ml of the ketamine/medetomidine/saline solution was given to maintain surgical levels of anaesthesia, as evidenced by pedal and eye blink reflexes. Core body temperature was maintained at 36–37°C through a rectal thermometer and heat mat.
Previous research has shown the firing rate and rhythmic activity of cells within the medial cerebellar nucleus show a stronger response to harmaline than cells within the interpositus or lateral cerebellar nuclei (Batini et al., 1981; Lorden et al., 1992). Furthermore, the harmaline-induced rhythmic firing of IO neurons occurs mainly in the caudal medial accessory olive and caudal dorsal accessory olive, which provide climbing fibre projections to the vermal A and B zones which then project to the medial cerebellar nuclei and lateral vestibular nucleus (De Montigny and Lamarre, 1973; Llinás and Volkind, 1973; Batini et al., 1981). Therefore, the medial cerebellar nucleus was targeted in these experiments. Rats were implanted with microdrives targeting the medial cerebellar nuclei (1 mm lateral and 11.3 mm posterior from bregma, and 4.1 mm ventral from the surface of the cerebellum) and the ventral anterior and ventral lateral (VA/VL) complex of the thalamus (1.8 mm lateral, 2.28 mm posterior from bregma, and 5.1 mm ventral from the surface of the cerebral cortex), also known as the “motor thalamus” (Nakamura et al., 2014) with two-to-four tetrodes per brain site (impedance 80–400 kΩ at 1 kHz). Two EEG screws were implanted over either side of the motor cortex (3 mm lateral and 2 mm anterior from bregma), and two EEG screws were implanted over either side of the sensory cortex (3 mm lateral and 0.5 mm anterior to bregma).
For 7 out of 14 rats, one of the cerebellar tetrodes was cut ~2 mm shorter to simultaneously target the cerebellar cortex. All tetrodes were coated with fluorescent marker DiI (3% in absolute ethanol) before implantation. Pairs of flexible stainless-steel wires (Cooner wire, USA) were implanted and sutured into the neck EMG and either the triceps brachii or biceps femoris muscles to record EMG. A reference screw pre-soldered to the insulated silver wire was fixed into the right parietal skull plate, and a support screw was fixed into the left parietal skull plate. A ground screw soldered to an insulated silver wire was fixed over the right occipital skull plate. Following the completion of surgery, 1 ml dose of analgesic carprofen (5% in saline) was given subcutaneously followed by 0.1 ml of atipamezole (20% in saline) given via i.p. injection, to reverse the effects of the medetomidine.
Neurophysiological RecordingsAfter recovery from surgery, differential recordings were made using a tethered CerePlex μ Headstage and a Cereplex acquisition system (Blackrock Microsystems). Raw data were continuously recorded at a sampling rate of 30 kHz and all data were analysed off-line (see the section on “Data Processing”). Low pass filtered EEG and EMG data (<500 Hz) were sampled at 2 kHz. The Blackrock CerePlex μ Headstage also enabled 3-axis accelerometer recordings, which were low pass filtered (<500 Hz) and sampled at 2 kHz. Data were collected before (i.e., pre-harmaline) and after administration of harmaline hydrochloride (10 mg/kg, i.p.). Neural and EMG signals were sampled while rats were quietly at rest or moved freely around their home cage. Baseline data were collected across 2–5 days. After harmaline administration, data were continuously recorded until any visible deficits in motor function (e.g., tremor, ataxia) were completely recovered.
HistologyUpon completion of the experiment, animals were deeply anaesthetised with Euthatal (1 ml, i.p.) and an electrolytic lesion (3 μA for 30 s) made at the tetrode recording site with the greatest signal-to-noise ratio (SNR). Rats were then transcardially perfused with 0.9% saline, followed by 0.1 M phosphate buffer (PB) that contained 4% paraformaldehyde. Neural tissue was post-fixed in 4% paraformaldehyde for 24–72 h, and then transferred into 30% sucrose solution for 3–4 days before cutting and mounting sections. Cerebellar tissue was cut sagittally at a thickness of either 40 μm (n = 10 rats) or 100 μm (n = 6 rats). The thalamus was sectioned either in the coronal (n = 5 rats; 100 μm section thickness) or sagittal plane (n = 10 rats; 40 μm section thickness). Additional verification of tetrode placement within the cerebellum and thalamus was obtained by identifying histological tissue tracks of tetrodes marked with DiI. These were visualised with a fluorescent Axioskop 2 Plus microscope (Zeiss) fitted with a CoolLED pE-100 excitation system and images acquired using AxioVision software.
Data ProcessingProcessing of raw data was performed offline using MATLAB (2018) version 9.4.0.813654 (R2018a) Natick, Massachusetts: The MathWorks Inc. Bipolar referencing configurations were used for all EMG, EEG, and tetrode LFP recordings. Where multiple tetrodes were inserted into a single brain region, the tetrode with the greatest SNR of multiunit activity was selected for group analysis. Data were band-pass filtered between 1 and 49 Hz, and then down-sampled to 1 kHz.
A principal component analysis was performed on tri-accelerometer data to combine measurements in three directions into one principal component or axis to capture the axis with maximal variance for subsequent spectral analysis.
To examine how neural network interactions are modulated by movement, electrophysiological data were divided into epochs where the rats were quietly resting vs. moving. This was achieved by applying a global movement-threshold value to a measure of total acceleration, which was the absolute magnitude of acceleration in any direction and was taken as a proxy of overall movement, to distinguish between any periods of resting vs. moving. Total acceleration, A, was calculated as:
Here, x, y, and z represent the three axes of the accelerometer. Total acceleration was then smoothed using a moving average filter with a window size of 100 samples. A global movement threshold of 1 m/s2 was then applied to the measure of total acceleration to distinguish periods of resting/immobility from movement (Pasquet et al., 2016; Meyer et al., 2018; Guitchounts et al., 2020). This threshold was verified against video recordings and performed well at identifying time points when rats begin to move around the cage or move to adjust their resting position. Electrophysiological data were then categorised into “resting” or “moving”, whereby rats were classified as either resting or moving if total acceleration, A, was below or above the threshold for the entire two-second non-overlapping epoch duration, respectively. Classified epochs were visually verified against the video recordings.
A trade-off between frequency resolution and the number of epochs was introduced when choosing epoch length. A shorter epoch size increased the number of total epochs, whereas a longer epoch size increased the low-frequency resolution of recorded signals but decreased the number of epochs in total. A 2-s-long epoch was chosen as it captured eight cycles of 4 Hz, which is the lowest tremor frequency recorded in patients, as well as providing a good number of epochs for analysis (average = 642 ± 330 epochs per condition per rat). Neural data were collated across baseline and harmaline conditions and collated data were z-score normalised to allow comparison of spectral amplitude across conditions and rats. Epochs containing data points larger than or equal to four standard deviations (SD) of the mean were rejected from further analysis to remove large amplitude artefacts (West et al., 2018).
Spectral AnalysisFor analysis of EMG, accelerometer, and LFP oscillations at the tremor frequency, fast Fourier transforms (FFT) were applied to each 2-s epoch (2,000 data points per epoch). Accelerometer amplitude ratio was defined as the signal amplitude within the tremor frequency range (9–15 Hz) divided by the total amplitude in the low-frequency range (0–15 Hz), calculated for each epoch as follows:
Amplitude ratio=∑Sxx (9−15Hz bins)∑Sxx (0−15Hz bins)(2)Here Sxx represents the absolute magnitude obtained from the FFT. Similar methods have been previously employed to examine changes in the distribution of power at tremor-specific frequencies (Iseri et al., 2011). Signal frequency coherence was computed by examining the magnitude-squared coherence (Cxy) for each epoch using the “mscohere” function in MATLAB:
Cxy=|Pxy (f)|Pxx (f) Pyy (f)(3)Here Cxy represents coherence, Pxy represents the cross-spectrum of the two signals and Pxx and Pyy represent the separate amplitude spectrum of the two signals.
In order to account for levels of coherence solely due to chance, we used a surrogate corrected version of the coherence. We focused on coherence in the tremor frequency band by averaging coherence values in the interval 9–15 Hz. Next, for each epoch, 99 surrogate datasets were generated using the iterative amplitude adjusted fast Fourier transform algorithm (IAAFFT) using MATLAB (Venema et al., 2016). Coherence was computed on each of the 99 surrogate epochs, and the statistical significance of coherence was examined by comparing mean coherence at the tremor frequency (9–15 Hz) for each recorded epoch against the mean coherence at 9–15 Hz for the 99 surrogate epochs. Finally, surrogate-corrected mean coherence values, C, were calculated as Rummel et al. (2010):
C=P−P (surr)1−P (surr)S(4)Here P represents the mean coherence coefficient across the tremor frequency coherence bins (9–15 Hz) of the real data, and P (surr) represents the mean coherence at the tremor frequency across 99 surrogate epochs. If P is greater than P (surr), then the null hypothesis that the two signals are independent can be rejected, and S takes a value of 1. Else, the null cannot be rejected, S takes the value of 0.
Cross-CorrelationsTime-lagged relationships between LFP recorded from each brain region, as well as with EMG, were calculated using cross-correlation analysis. Data were first band-pass filtered at the tremor frequency range (9–15 Hz) before dividing into epochs as described above. Epochs recorded during harmaline conditions when the rat was categorised as “moving” were selected for further analysis. Cross-correlations were calculated using the “xcorr” function in MATLAB. Time-lags showing maximum correlation coefficients were pooled across epochs, and the normalised probability of maximum cross-correlation at binned time-lags was calculated (±100 ms lags with a 2 ms bin width). Peak probability was calculated as the summed probability at the highest bin and the two adjacent probability bins (i.e., the sum at the peak). Peak probabilities that surpassed a threshold value of 0.1 were included within summary statistics examining time-lagged relationships between tremor signals recorded across the network.
Changes in Coherence Across the Neural NetworkTo examine changes in coherence across the recorded neural network, statistical analyses compared the area under the mean coherence curve at the tremor frequency (9–15 Hz) between the recorded and surrogate datasets using one-tailed paired t-tests. Holm-Bonferroni adjusted p-values were applied to control for multiple coherence comparisons per condition.
Multilevel Regression ModelsMultilevel regression models were fit using MLwiN (v3.05, Centre for Multilevel Modelling, University of Bristol, UK), to investigate the impact of harmaline on LFP amplitude at the tremor frequency and the impact of movement on LFP coherence at the tremor frequency in the harmaline treated rat. Models were specified with the amplitude ratio or surrogate-corrected mean coherence at 9–15 Hz as the response variable, with responses for each epoch (level 1) nested under each rat (level 2). A null model (no explanatory variables) was compared to a random intercepts model which specified a dichotomous explanatory variable for harmaline (harmaline vs. baseline) or movement (movement vs. resting). The random intercepts model was then compared to a random slopes model, which allows the relationship (i.e., slope) between the explanatory and response variables to vary across rats. The inclusion of this level 2 variation can provide a more accurate estimate of the standard error when random-slope variation is present (Bell et al., 2019).
Models are presented in equations 5–7 below, where yij is the response variable for rat j at sampled epoch i. The null model estimates the grand mean of the y, represented by β0, and the variability in the grand mean across rats, u0j, and sampled epochs eij. Model 2 and 3 additionally includes a regression coefficient β1 for the dummy coded explanatory variable, x, . The regression coefficient for the intercept β0ij represents the reference group, which is either the control condition when harmaline is included as the explanatory variable, x, or resting when movement is included as the explanatory variable, x. If the random intercepts models provided a significantly better fit than the null, the random intercepts model (model 2) was then statistically compared to the random slopes model (model 3).
Model 1: NullLevel 1:yij=β0ijLevel 2:β0ij=β0+u0j+eij(5) Model 2: Random interceptsLevel 1:yij=β0ij+β1xijLevel 2:β0ij=β0+u0j+eij(6) Model 3: Random slopesLevel 1:yij=β0ij+β1jxijLevel 2:β0ij=β0+u0j+eijβ1j=β1+u1j(7)If diagnostic checks revealed a non-normal distribution of the residuals in the model, a generalised linear model with an approximate Poisson distribution of the response data was applied and the model was re-run (Leyland and Groenewegen, 2020). Wald chi-squared tests were applied to statistically examine the significance of the effects, testing the null hypothesis that the coefficient for the explanatory variable equals the coefficient for the baseline variable. This statistically compares the standardised regression coefficients for harmaline vs. control or for moving vs. resting.
Results Harmaline Induces a Tremor at 9–15 Hz in Awake RatsA whole-body tremor in response to systemic harmaline administration was observed within 5–15 min of intraperitoneal injection of harmaline and lasted for up to 3 h. The tremor could be readily identified in the raw EMG and accelerometer traces (Figures 1A,B). Harmaline induced a tremor at 9–15 Hz with a peak at 11 Hz, as shown in the EMG and accelerometer amplitude spectrum (shaded grey region; Figures 1C,D), where the peak tremor frequency remained relatively stable over time (Supplementary Figure 1). A Wald chi-square test showed the estimated coefficients for EMG and accelerometer amplitude ratio at 9–15 Hz were significantly greater for harmaline (EMG: mean = 0.36, 95% CI [0.33 0.39], Accelerometer: mean = 0.53, 95% CI [0.48 0.58]) than pre-harmaline conditions (EMG: mean = 0.24, 95% CI [0.21 0.26], χ2(1) = 4.385, p = 0.036, n = 7 rats, Accelerometer: mean = 0.30, 95% CI [0.28 0.32], χ2(1) = 75.633, p < 0.001, n = 12 rats; Figures 1E,F). These observations are in agreement with previous studies on harmaline-induced tremor in awake rodents (Pan et al., 2018, 2020).
FIGURE 1
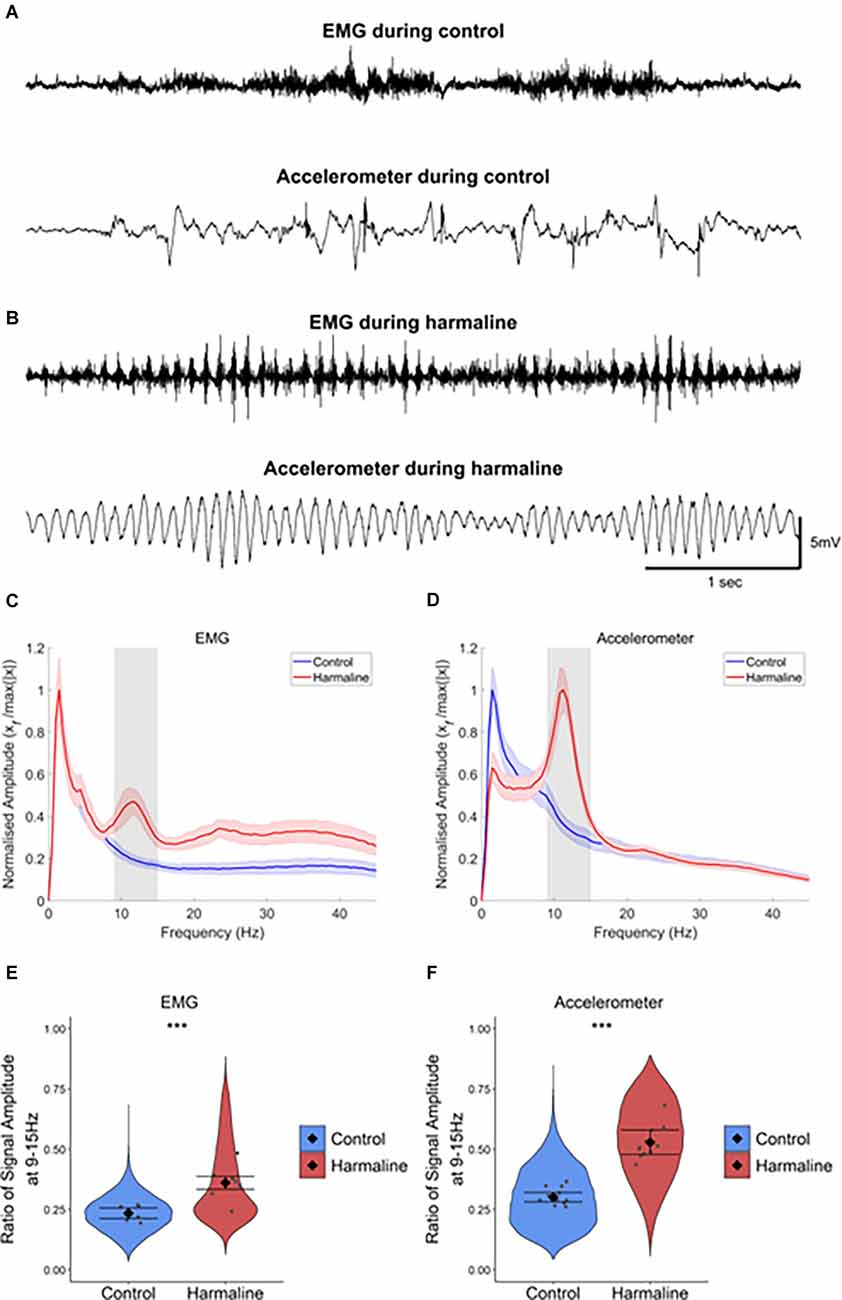
Figure 1. Harmaline induces a 9–15 Hz tremor. (A,B) Representative example EMG and accelerometer recording from a rat during (A) control and (B) harmaline. The upper trace is EMG, and the lower trace is accelerometer. (C,D) Mean (±SE) amplitude spectrum for (C) EMG (n = 7 rats) and (D) accelerometer (n = 12 rats) during control and harmaline. The solid line represents mean amplitude, and the coloured shaded areas represent SE. The grey area represents the tremor frequency (9–15 Hz). (E,F) Ratio of amplitude at the tremor peak for (E) EMG and (F) accelerometer, where the violin plots show the distribution of this ratio across all epochs (pooled across rats). Individual grey data points represent the mean per rat. Fixed effects parameter estimates (±CI) representing predicted mean estimates are shown by ♦ and corresponding error bars. *** indicates p < 0.001.
Harmaline Induces a Change in Neural Rhythms in Cerebellar CircuitsPost-mortem histology of the recording site location confirmed that from a total of 14 animals, five animals had cerebellar tetrode recording sites located in the medial cerebellar nucleus, five animals had tetrode recording sites located within the vermal cerebellar cortex and four animals had tetrode recording sites located in both the medial cerebellar nucleus and the vermal cerebellar cortex (Figures 2A–C). The LFP recorded from the cerebellar cortex and cerebellar nuclei across rats during pre-harmaline and harmaline conditions are shown in Figures 2D,E. An increase in rhythmic activity in the tremor frequency range (9–15 Hz; peak 11 Hz, shaded grey region) was seen in both the cerebellar cortex and medial cerebellar nucleus during harmaline compared to the pre-harmaline condition (Figures 2F,G). Smaller amplitude harmonic oscillations are also present at twice the tremor frequency (~23 Hz). Quantitatively, harmaline significantly increased the LFP amplitude ratio at 9–15 Hz in the cerebellar cortex and in the medial cerebellar nuclei (Figures 2H,I; cerebellar cortex: mean = 0.35, 95% CI [0.30 0.39], medial cerebellar nuclei: mean = 0.27, 95% CI [0.24 0.29]) compared to the control condition (cerebellar cortex: mean = 0.22, SE = 0.01, χ2(1) = 32.14, p < 0.01, n = 9 rats, medial cerebellar nuclei: mean = 0.23, 95% CI [0.22 0.24], χ2(1) = 8.32, p = 0.004, n = 8). One rat was excluded from the medial cerebellar nuclei analysis as the mean LFP amplitude for harmaline was greater than two standard deviations of the group mean. Taken together these results suggest that harmaline induces tremor frequency (9–15 Hz) oscillations in the vermal cortex and medial cerebellar nuclei.
FIGURE 2
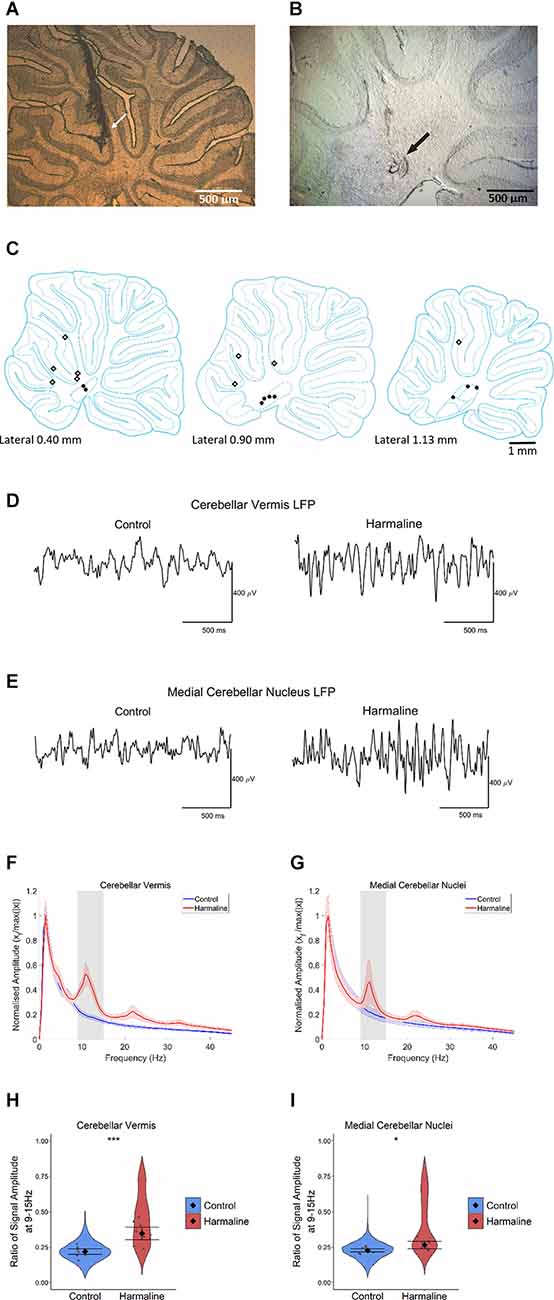
Figure 2. Harmalineincreases oscillatory activity in the cerebellum at the tremor frequency. (A,B) Examples of sagittal cerebellar sections with tetrode tracts in the (A) cerebellar vermis and (B) medial cerebellar nuclei from two different rats. Arrows indicate the approximate positions of the tetrode tips. (C) Summary diagram illustrating approximate electrode positions on sagittal sections of the cerebellum (adapted from Paxinos and Watson, 2005). Black dots represent approximate electrode positioning per rat in the medial cerebellar nuclei, and diamond symbols represent approximate electrode positions per rat in the cerebellar cortex. (D,E) Representative LFP traces from (D) cerebellar vermis and (E) medial cerebellar nuclei during pre-harmaline control and harmaline. (F,G) Mean (±SE) amplitude spectrum for (F) cerebellar vermis (n = 9) and (G) medial cerebellar nuclei (n = 9) during control and harmaline. The solid line represents mean amplitude, and the coloured shaded areas represent SE. The grey area represents the tremor frequency. (H,I) Ratio of amplitude at the tremor peak for (H) cerebellar vermis (n = 9) and (I) medial cerebellar nuclei (n = 8), where the violin plots show the distribution of this ratio across all epochs (pooled across rats). Individual grey points represent the mean per rat. Fixed effects parameter estimates (±CI) representing predicted mean estimates are shown by ♦ and corresponding error bars. *** indicates p < 0.001, and * indicates p < 0.05 (E,F).
Harmaline Was Not Found to Significantly Increase the Amplitude of Tremor Rhythms in Thalamocortical CircuitsTo examine the impact of harmaline on neural activity in thalamocortical circuits, the amplitude spectrum of LFP recorded from the motor region of the thalamus and subdural EEG (Figures 3C,D) recorded over the sensorimotor cortex were assessed. In a total of 10 animals, histological identification of tetrode tracks (Figures 3A,B) indicated that recordings were located within the VL/VA complex of the thalamus, also known as the “motor thalamus”, and we use this term to refer to the VL/VA thalamus complex. Prior to harmaline, a small peak in oscillatory activity at around 7 Hz was observed in both the motor thalamus and the EEG (vertical blue dotted line in Figures 3E,F). However, during harmaline, this 7 Hz oscillation was replaced by a smaller amplitude oscillation at 5 Hz (vertical red dotted line in Figures 3E,F). No distinct peaks in oscillatory activity were detected at the tremor frequency range (9–15 Hz, grey shaded regions in Figures 3E,F).
FIGURE 3
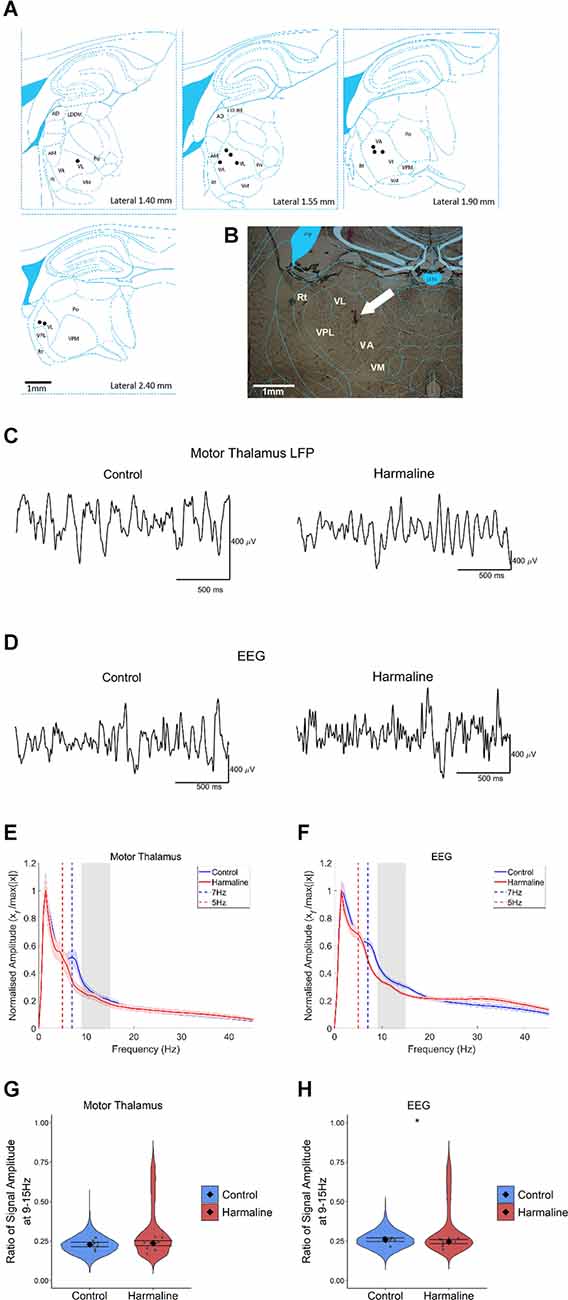
Figure 3. The amplitude of oscillations at the tremor frequency in the thalamocortical circuits. (A) Summary diagram illustrating approximate electrode positions on sagittal sections of the thalamus (adapted from Paxinos and Watson, 2005), where each black dot represents the approximate electrode positioning per rat and the VA/VL complex of the thalamus (i.e., motor thalamus). (B) Example coronal section from one rat with electrode approximately located in the VA/VL complex of the thalamus. The arrow indicates the approximate position of the tetrode tip. (C,D) Representative LFP traces from (C) motor thalamus and (E) EEG during pre-harmaline control and harmaline. (E,F) Mean (±SE) amplitude spectrum for (E) motor thalamus (n = 9 rats) and (F) EEG (n = 9 rats) during control and harmaline. The solid line represents mean amplitude, and the coloured shaded areas represent SE. The grey area represents the tremor frequency. (G,H) Ratio of amplitude at the tremor peak for (G) motor thalamus and (H) EEG, where the violin plots show the distribution of this ratio across all epochs (pooled across rats). Individual grey points represent the mean per rat. Fixed effects parameter estimates (±CI) representing predicted mean estimates are shown by ♦ and corresponding error bars. * indicates p < 0.05.
A Wald test revealed no significant difference in motor thalamus LFP amplitude ratio at 9–15 Hz for harmaline (mean = 0.24, 95% CI [0.22 0.25]) vs. control (mean = 0.23, 95% CI [0.21 0.24], χ2(1) = 0.84, p = 0.359, n = 9; Figure 3G). However, there was a small but significant decrease in EEG amplitude ratio at 9–15 Hz during harmaline (mean = 0.25, 95% CI [0.23 0.26]) compared to the pre-harmaline condition (mean = 0.26, 95% CI [0.25 0.27]), (χ2(1) = 3.85, p = 0.05, n = 9; Figure 3H). These findings illustrate a change in the rhythmicity of thalamocortical activity during harmaline vs. control conditions, where there is a shift from 7 Hz to 5 Hz.
Harmaline-Induced Tremor Amplitude Is Modulated by MovementAs harmaline tremor is reported to be an action tremor that resembles ET (Pan et al., 2018, 2020), it is of interest to examine how activity and interactions within the tremor-related neural network are modulated by movement. To examine whether harmaline-induced tremor changes in severity during movement, accelerometer activity was compared during periods of movement vs. resting, which was defined by total acceleration falling above or below a threshold of 1 m/s2, respectively, for the entire epoch duration (Figure 4A). In both behavioural states, the accelerometer spectrum has a distinct peak at the tremor frequency range (9–15 Hz, grey banded section of each panel (Figure 4B). A Wald test showed that total accelerometer amplitude at the tremor frequency was significantly greater during movement (mean = 2.35, 95% CI [2.07 2.62]) than during rest (mean = 0.51, 95% CI [0.39 0.63], χ2(1) = 166.42, p < 0.001, n = 12; Figure 4C).
FIGURE 4
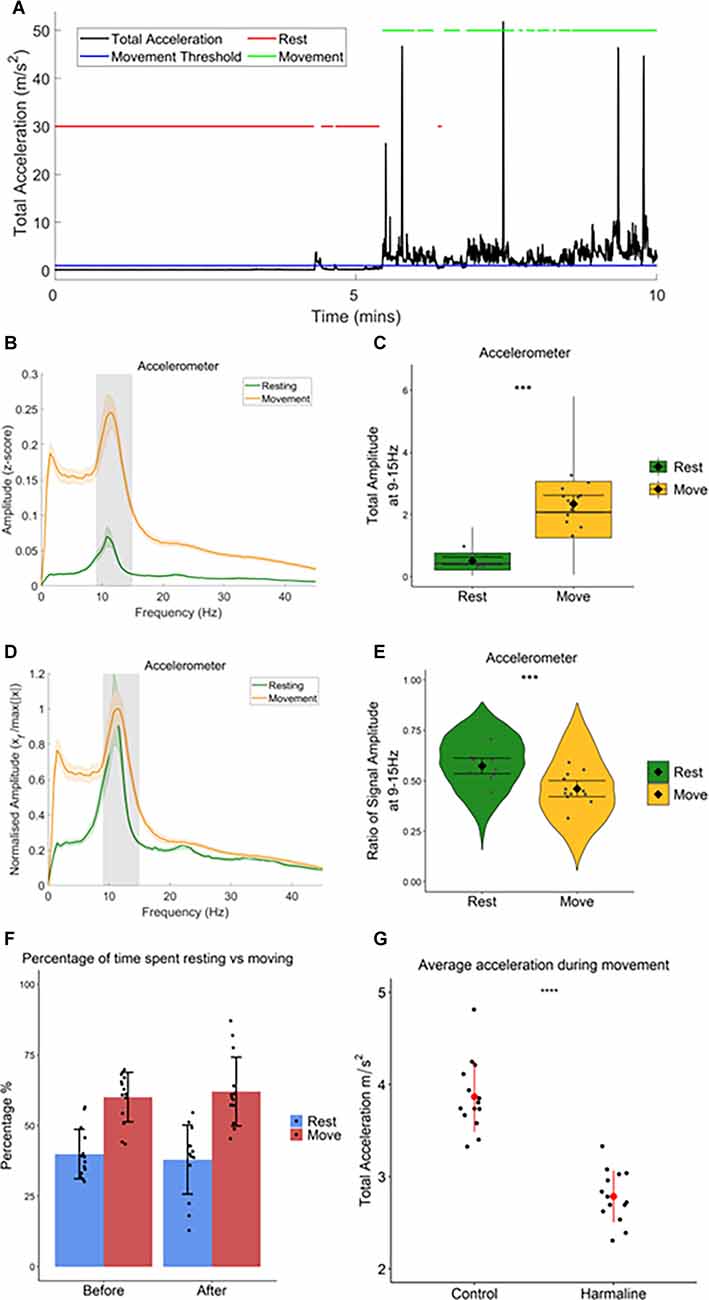
Figure 4. Totalamplitude of harmaline-induced tremor increases withmovement. (A) Movement threshold (1 ms−2; bluehorizontal line) applied to total acceleration data (black) todistinguish periods of rest and movement. Red lines demonstrate timepoints categorised as “rest”, and green lines demonstrate timepoints categorised as “movement”. (B) Total (±SE) and(D) relative (±SE) amplitude accelerometer spectrum (n = 12 rats) during rest and movement. The solid line represents the mean, and the coloured shaded areas represent SE. The grey area represents the tremor frequency. (C) Total accelerometer amplitude and (E) ratio of accelerometer amplitude at the tremor peak, across all epochs (pooled across rats). Individual grey points represent the mean per rat. Fixed effects parameter estimates (±CI) representing predicted mean estimates are shown by ♦ and corresponding error bars. *** indicates p < 0.001. (F) Percentage of time spent resting and moving during baseline (before) and harmaline conditions. Each dot represents one rat. Error bars represent ±SD. (G) Average total acceleration during movement before (control) and after harmaline treatment. Each dot represents one rat. Error bars represent ± SD. *** indicates p < 0.001.
Figure 4B also illustrates that across the entire spectrum (<45 Hz) accelerometer amplitude was greater during movement than during rest. To ensure that the difference in tremor frequency amplitude between resting and moving was not related to the generalised increase in accelerometer amplitude during movement, the amplitude ratio at the tremor frequency was also examined (see “Methods” Section) to compare the relative amplitude of oscillations at 9–15 Hz (Figure 4D). This revealed a significant increase in the ratio of accelerometer tremor frequency amplitude for rest (mean = 0.57, 95% CI [0.054 0.61]) compared to movement (mean = 0.46, 95% CI [0.42 0.50], χ2(1) = 31.66, p < 0.001, n = 12; Figure 4E), indicating that the increase in total accelerometer amplitude during movement was not specific to the tremor frequency range.
In summary, these results, therefore, suggest that harmaline-induced tremor is present when rats are at rest and also during movement, but the amplitude of rhythmic activity across the frequency spectrum studied (0–45 Hz) increases significantly with movement. This corresponds with the visual inspection of tremor, where a low amplitude tremor was visible during resting, but the tremor became much more pronounced when the rat moved around the cage.
In addition to harmaline producing a significant action tremor in rats, general ataxia was also observed 5–15 min following the intraperitoneal injection and could last for up to 3 h. This included a loss of coordination, an unsteady gait, and an increased spreading of the paws and foot slips during walking. There was also an absence of rearing and an increase in occurrences where the rat was lying down or leaning on the side of the cage. However, when the percentage of time rats spent actively moving vs. being quietly at rest was compared there was no statistically significant difference between pre-harmaline (median = 62.0%) and harmaline (median = 59.6%, z = −0.408, p = 0.683, r = −0.109, n = 14 rats). This suggests that harmaline has little or no effect on overall activity levels in rats (Figure 4F). However, rats’ movements were, significantly slower (on average by 28%) during harmaline [mean = 2.79 m/s2, SD = 0.28] in comparison to control conditions [mean = 3.87 m/s2, SD = 0.38, t(13) = 9.883, p < 0.001, n = 14 rats; Figure 4G].
Harmaline-Induced Changes in Coherence During Movement and RestTo examine the degree to which neural oscillations across the cerebello-thalamo-cortical network correlate with harmaline-induced behavioural tremor, coherence between the neural activity recorded from each of the three brain regions under investigation and tremor activity measured via the accelerometer were assessed during rest and movement following harmaline administration.
During rest and movement, a peak in coherence between the accelerometer and the cerebellar vermis LFP (Figure 5A), and the accelerometer and medial cerebellar nuclear LFP (Figure 5B) was evident at the tremor frequency (9–15 Hz, grey shaded area). A harmonic peak in coherence at double the tremor frequency (~23 Hz) is also evident, which is likely due to a non-sinusoidal neural oscillation at the tremor rhythm. Surrogate analysis revealed that 76.6% of epochs showed significant cerebellar vermis-kinematic coherence at 9–15 Hz in comparison to the surrogate dataset (n = 8,142 out of 10,632 epochs, which included 76.2% of epochs classified as “resting”, and 77.0% of epochs classified as “moving”). The analysis also revealed that 65.0% of epochs also showed significant medial cerebellar nuclear-kinematic coherence at 9–15 Hz (n = 6,478 out of 9,963, this included 64.7% of epochs classified as “resting”, and 65.0% of epochs classified as “moving”). Overall, this shows average coherence at 9–15 Hz was greater for the recorded data (Figures 5A,B; green and yellow lines) compared to surrogate datasets (Figures 5A,B, blue and purple lines).
FIGURE 5
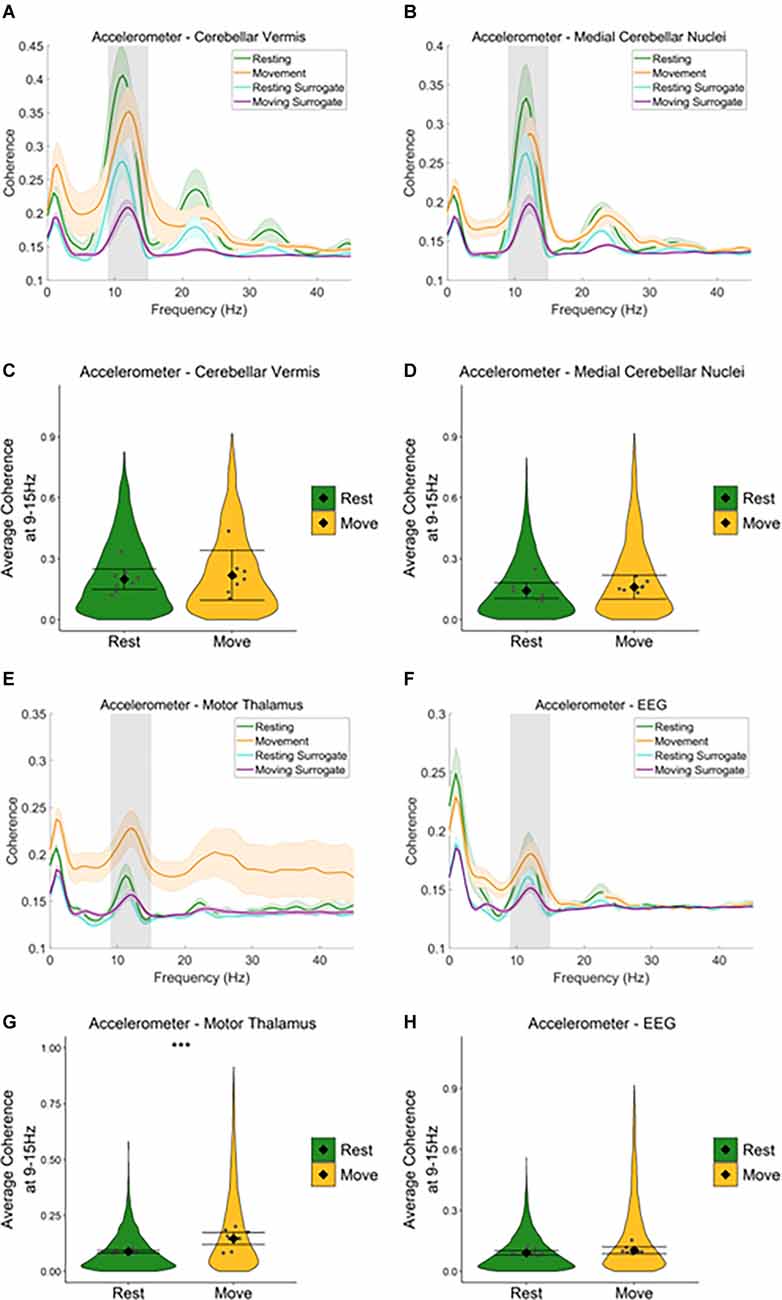
Figure 5. Neural oscillations across the cerebello-thalamo-cortical network correlate with harmaline-induced behavioural tremor. (A,B) Mean (±SE) (A) cerebellarcortex-kinematic (n = 8 rats), (B) cerebellar nuclei-kinematic (n = 7 rats) during resting and movement for real and surrogate datasets. The solid line represents meancoherence, and the coloured shaded areas represent SE. The grey area represents the tremor frequency. (C,D) Average surrogate-corrected (C) cerebellarcortex-kinematic, (D) cerebellar nuclei-kinematic coherence at the tremor peak across all epochs with significant coherence (pooled across rats). Individual greypoints represent the mean per rat. Fixed effects parameter estimates (±CI) representing predicted mean estimates are shown by ♦ and corresponding error bars. ***indicates p < 0.001 (E) thalamic-kinematic ((textitn = 8 rats), and (F) cortico-kinematic (textitn = 6 rats) coherence during resting and movement for real and surrogate datasets. The solid line represents mean coherence, and the coloured shaded areas represent SE. The grey area represents the tremor frequency. (G,H) Averagesurrogate-corrected (G) thalamic-kinematic and (H) cortico-kinematic coherence at the tremor peak across all epochs with significant coherence (pooled acrossrats). Individual grey points represent the mean per rat. Fixed effects parameter estimates (±CI) representing predicted mean estimates are shown by ♦ andcorresponding error bars. *** indicates p < 0.001.
Model coefficients for surrogate-corrected mean coherence were compared across resting and movement (Figures 5C,D). This revealed no difference in surrogate-corrected mean coherence at the tremor frequency across resting and movement, for either cerebellar vermis-kinematic coherence (Rest: mean = 0.20, 95% CI [0.15 0.25], Movement: mean = 0.22, 95% CI [0.10 0.34], χ2(1) = 0.28, p = 0.599, n = 8) nor medial cerebellar-nuclei-kinematic coherence (Rest: log mean = −1.93, 95% CI [−1.19 −1.68], mean = 0.14, 95% CI [0.10, 0.18] Movement: log mean = −1.84, 95% CI [−1.91, −1.58], mean = 0.16, 95% CI [0.10, 0.22], χ2(1) = 0.16, p = 0.684, n = 7).
Together, the surrogate analysis, therefore suggests that tremor-frequency oscillations in the cerebellar vermis and medial cerebellar nuclei are significantly correlated with kinematic measures of tremor in harmaline-treated rats for the majority of epochs (76.2% for the cerebellar cortex, 65.0% for the cerebellar nuclei). Furthermore, epochs showing statistically significant coherence are distributed equally across resting and movement, and the strength of coherence is not modulated by movement.
Equivalent analysis of the motor thalamus and EEG data also revealed a peak in coherence between the accelerometer and motor thalamic LFP (Figure 5E), and the accelerometer and EEG (Figure 5F) at the tremor frequency (9–15 Hz, grey shaded area) during rest and movement. However, the peak coherence was lower than that found for the cerebellar vermis and medial cerebellar nuclei. Broader peaks in coherence were also observed at double the tremor frequency (~24 Hz). Surrogate analysis revealed that 54.5% of epochs showed significant motor thalamo-kinematic coherence at 9–15 Hz in comparison to the surrogate dataset (n = 5,597 out of 10,267 epochs, 49.0% of “resting” epochs, 61.8% of “moving” epochs), where average motor thalamo-kinematic coherence was greater for recorded data compared to surrogate datasets (Figure 5E). Comparison of surrogate-corrected mean coherence coefficients also revealed a significant increase in tremor frequency coherence for movement (log mean = −1.93, 95% CI [−2.25, −1.29], mean = 0.15, 95% CI [0.11, 0.28]) compared to resting (log mean = −2.41, 95% CI [−2.53, −2.30], mean = 0.09, 95% CI [0.08, 0.10], χ2(1) = 28.07, p < 0.001, n = 8; Figure 5G). Surrogate analysis also showed 52.3% of epochs showed significant EEG-kinematic coherence at 9–15 Hz in comparison to the surrogate dataset (n = 6,402 out of 12,249 epochs, 50.82% of “resting” epochs, 54.0% of “moving” epochs). However, there was no significant difference in surrogate-corrected mean coherence coefficients at the tremor frequency for movement (log mean = −2.26, 95% CI [−2.29, −1.97], mean = 0.10, 95% CI [0.09, 0.12]) vs. resting epochs (log mean = −2.382, 95% CI [−2.52, −2.24], mean = 0.09, 95% CI [0.08, 0.10]), χ2(1) = 2.47, p = 0.116, n = 9; Figure 5H).
A similar pattern was seen when examining average coherence values at the tremor frequency for medial cerebellar nuclei-thalamic and motor thalamus-EEG coherence (Figures 6 and Figure 7). A peak in medial cerebellar nucleo-motor thalamic coherence was observed at the tremor frequency (Figure 6A), with coherence at this frequency greater for recorded data compared to surrogate datasets during movement only, and not during rest (Figures 6C,D). A total of 47.3% of epochs showed significant medial cerebellar nucleo-motor thalamic coherence at 9–15 Hz in comparison to the surrogate dataset (n = 3,635 out of 7,681 epochs, 44.7% of “resting” epochs, 49.2% of “moving” epochs). There was also a significant increase in surrogate-corrected medial cerebellar nucleo-motor thalamic coherence for movement (log mean = −2.31, 95% CI [−2.36, −1.74], mean = 0.10, 95% CI [0.08, 0.13]) compared to resting (log mean = −2.58, 95% CI [−2.77, −2.39], mean = 0.08, 95% CI [0.07, 0.08], χ2(1) = 5.79, p = 0.016, n = 6; Figure 6B). In addition to the peak in medial cerebellar nucleo-motor thalamic coherence at the tremor frequency, a small peak was also observed at approximately half the tremor frequency during movement (grey and green dotted lines, Figure 6D). During the control condition, a peak in medial cerebellar nucleo-motor thalamic coherence was observed at ~8 Hz, during movement only (green vertical dotted line; Figure 6F) and not during rest (Figure 6E), which may reflect an intrinsic movement-related oscillation.
FIGURE 6
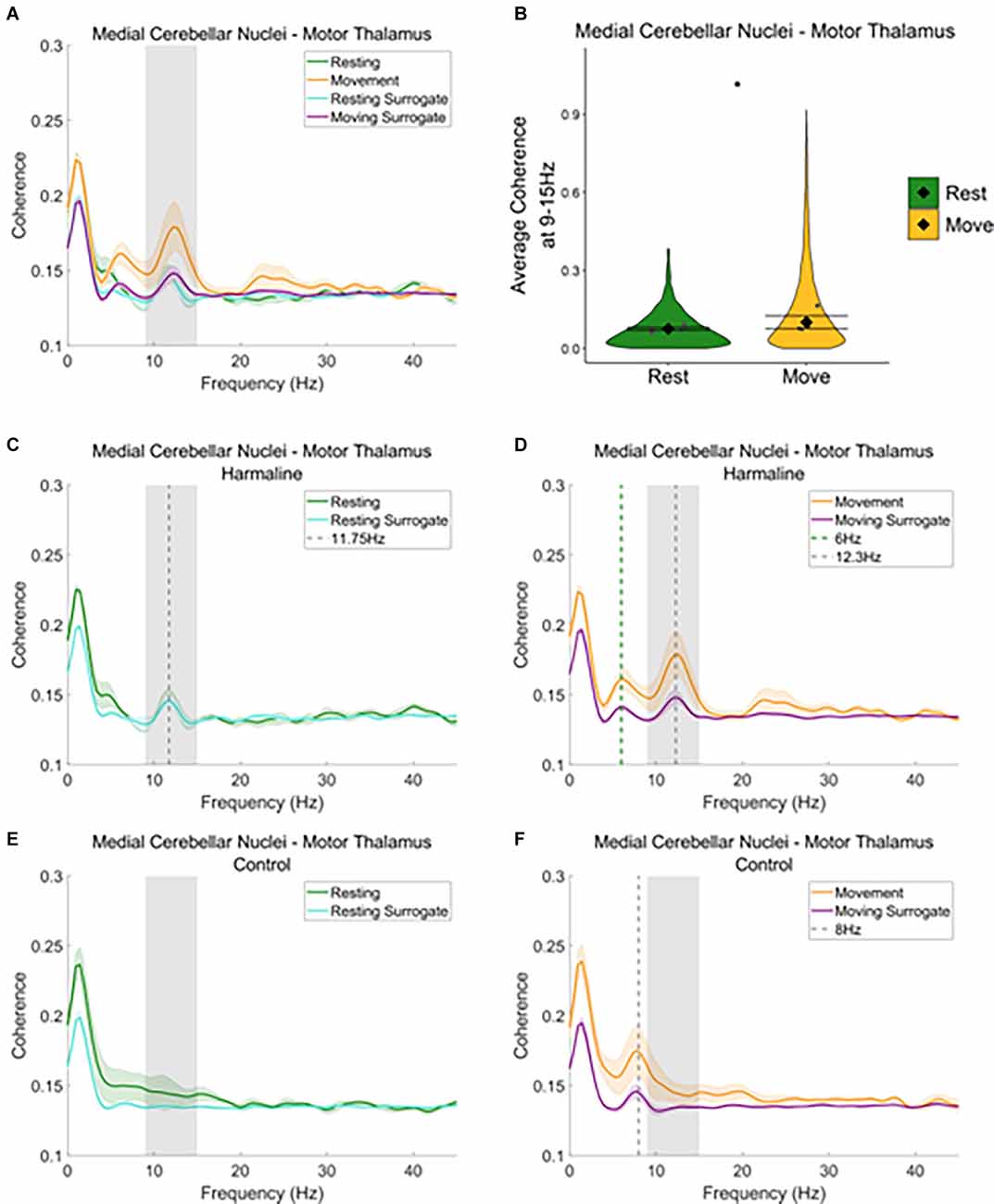
Figure 6. Cerebello-thalamiccoherence in the harmaline model. (A) Mean (±SE)(A) cerebellar nuclei-thalamic (n = 6) coherenceduring resting and movement for real and surrogate datasets. Thesolid line represents mean coherence, and the coloured shaded areasrepresent SE. The grey area represents the tremor frequency.(B) Average surrogate-corrected cerebellar nuclei-thalamiccoherence at the tremor peak across all epochs with significantcoherence (pooled across rats). Individual grey points represent themean per rat. Fixed effects parameter estimates (±CI)representing predicted mean estimates are shown by ♦ and corresponding error bars. * indicates p < 0.05. (C–F) Mean (±SE) cerebellar nuclei-thalamic (n = 6); coherence in harmaline model (C,D) and control (E,F) during resting (C,E) and movement (D,F) for real and surrogate datasets. The solid line represents mean coherence, and the coloured shaded areas represent SE. The grey area represents the tremor frequency.
FIGURE 7
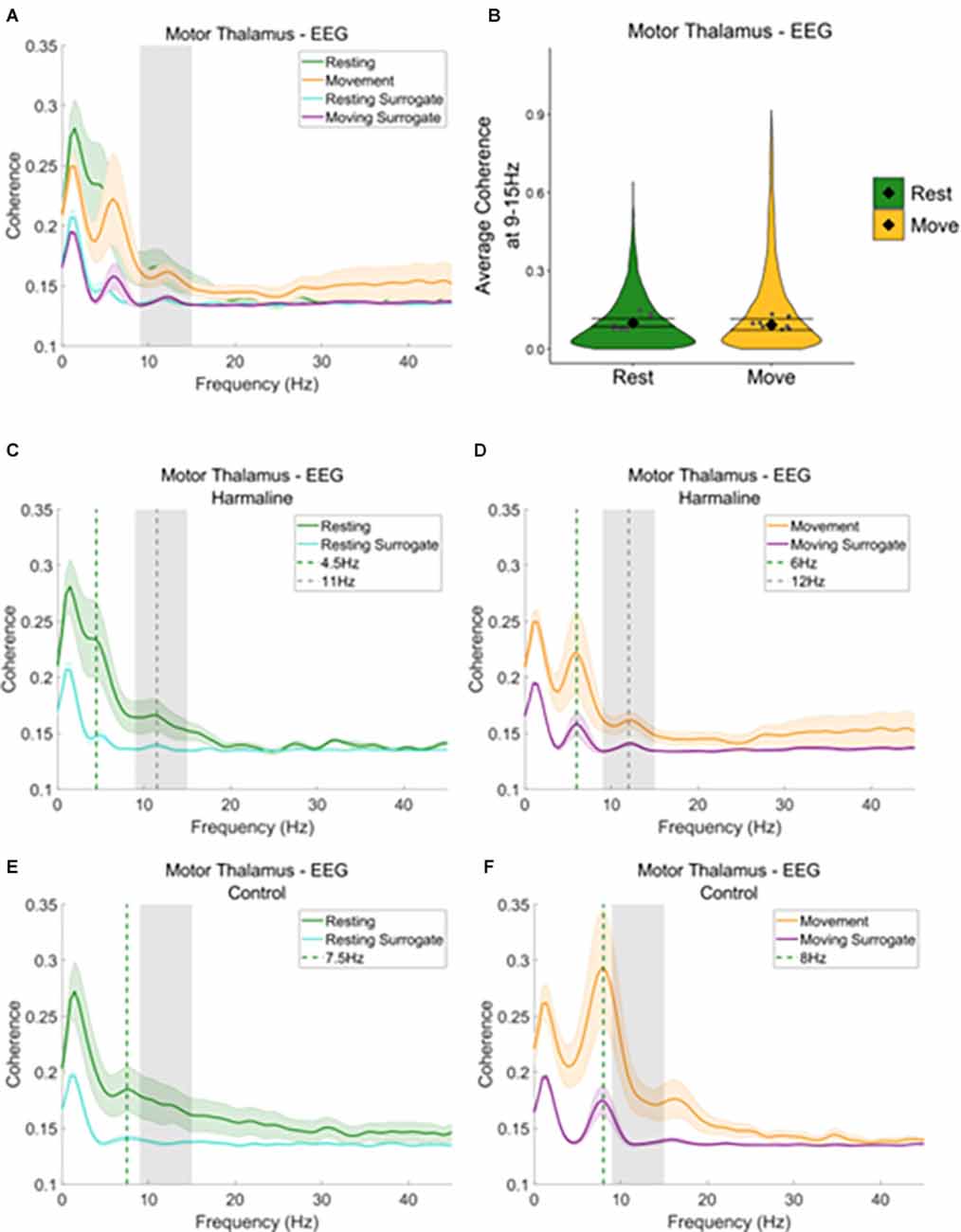
Figure 7. Thalamocorticalcoherence in the harmaline model. (A) Mean (±SE) thalamocortical coherence (n = 8) during resting and movement for real and surrogate datasets. The solid line represents mean coherence, and the coloured shaded areas represent SE. The grey area represents the tremor frequency. (B) Average surrogate-corrected thalamocortical coherence at the tremor peak across all epochs with significant coherence (pooled across rats). Individual grey points represent mean per rat. Fixed effects parameter estimates (±CI) representing predicted mean estimates are shown by ♦ and corresponding error bars. (C–F) Mean (±SE) thalamocortical coherence (n = 8); coherence in harmaline model (C,D) and control (E,F) during resting (C,E) and movement (D,F) for real and surrogate datasets. The solid line represents mean coherence, and the coloured shaded areas represent SE. The grey area represents the tremor frequency.
When examining motor thalamus-EEG coherence, a small peak in coherence was also found at the tremor frequency range (9–15 Hz; Figure 7A, grey dotted line Figures 7C,D), where coherence was greater for recorded data compared to surrogate datasets. A total of 52.6% of epochs showed significant motor thalamus-EEG coherence at 9–15 Hz in comparison to the surrogate da
留言 (0)