
The success story of Pap smears in the dramatic decrease in the prevalence of cervical squamous cell carcinoma is further accelerated with advances in the understanding of Human papillomavirus (HPV) carcinogenesis of cervical cancers along with the efficacy of recently available HPV vaccines.[1] However, the glandular abnormalities reported in Pap smears have increased recently.[2]
Although Pap smears are less sensitive for the evaluation of epithelial cell abnormalities of glandular origin as compared to squamous lesions, they usually fall in the differential diagnoses of high-grade squamous cell lesions (HSILs) [Figure 1] and hyperchromatic crowded groups (HCGs). A high degree of suspicion, good clinical history, and methodical application of diagnostic criteria, especially in an algorithmic manner highlighted in this review would facilitate improved interpretation of cytologic features and facilitate methodical interpretation of glandular cell abnormalities [Figure 2].
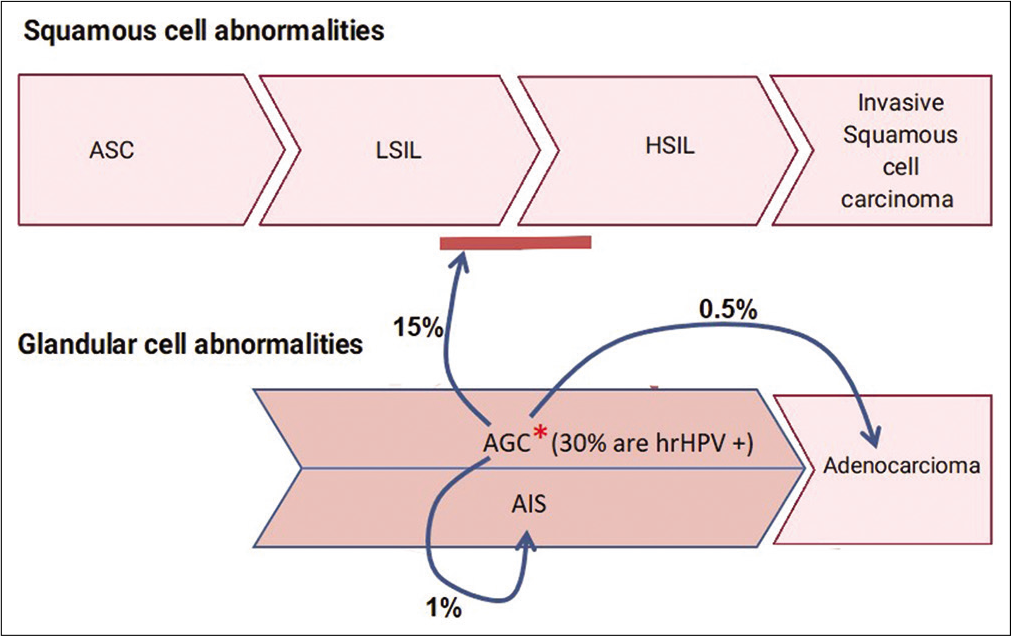
Figure 1:: Cyto-histo correlation of AGC: *Around 30% of AGC are High-risk HPV (hrHPV) positive2a. On cyto-histo correlation about 15% are associated with LSIL or HSIL. 1% are associated AIS and 0.5% with adenocarcinoma2.
Export to PPT
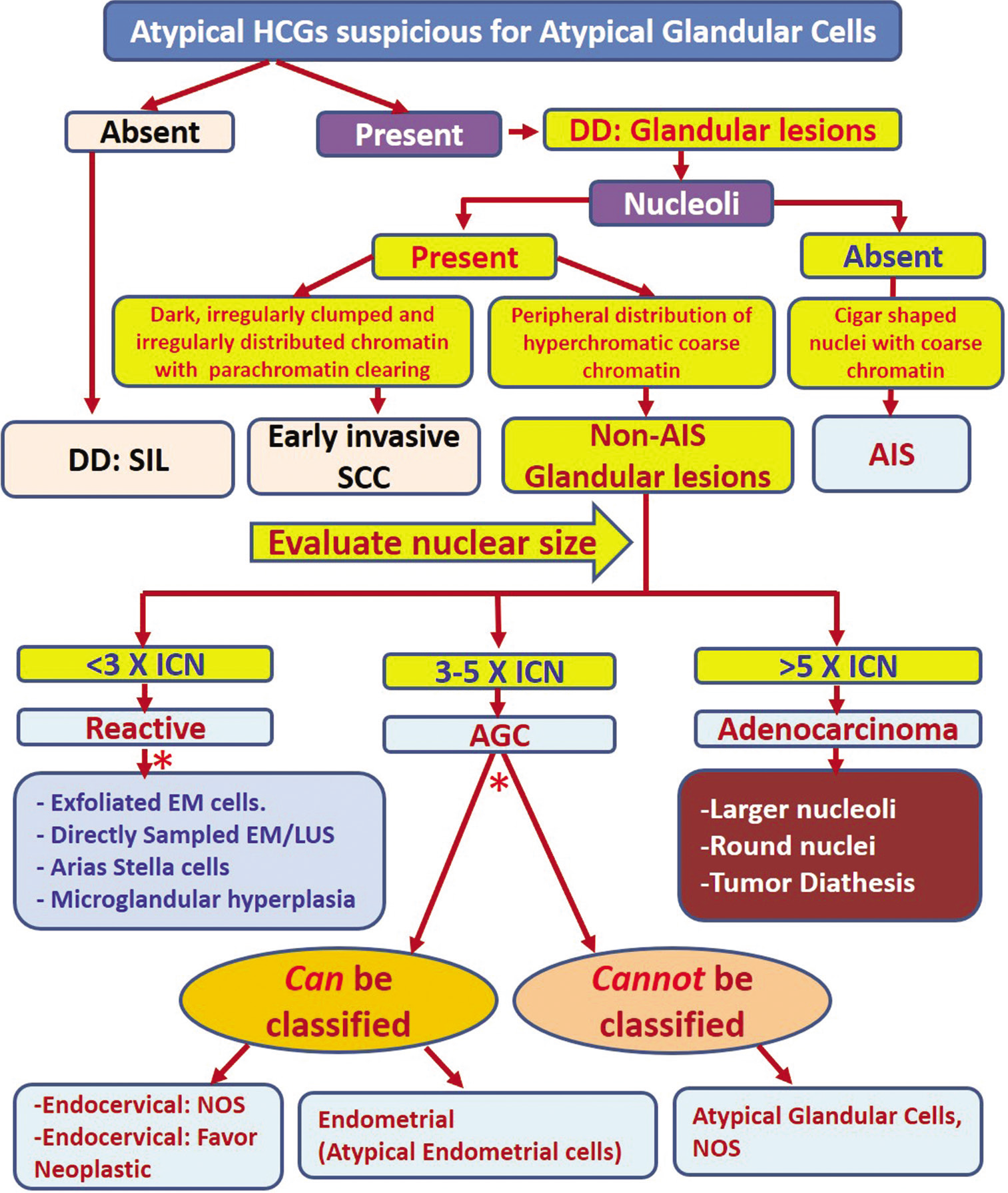
Figure 2:: Algorithmic approach for evaluating atypical HCGs. Apply the features described for specific category. DD: Differential diagnosis, EM: Endometrial cells, HCG: Hyperchromatic crowded group, AIS: Adenocarcinoma in situ, SCC: Squamous cell carcinoma, SIL: Squamous intraepithelial lesion, ICN: Intermediate cell nuclei, AGC: Atypical glandular cell, NOS: Not otherwise specified.
Export to PPT
The Bethesda System broadly categorizes glandular cell abnormalities into:
Endocervical adenocarcinoma in situ (AIS)
Atypical glandular cells (AGCs)
Endocervical cells:
a1 NOS or specify in comments;
Endometrial cells: NOS or specify in comments
Adenocarcinoma (AdCa)
Endocervical
Endometrial
Extrauterine
NOS
It was observed during the Bethesda Inter-observer Reproducibility Study (BIRST-2) in 2017 that the interpretation of AGC is challenging with lower interobserver reproducibility. The study showed that there was 1 / 3 (33%) concordance for AGC interpretations among participants (cytopathologists) and the Bethesda panel (experts).[3]
Even after many advances in cytological techniques, the interpretation of AGC remains elusive because of associated challenges in distinguishing them from other lesions such as HSIL and other HCGs with some cytomorphological overlaps [Figure 1]. This issue has enhanced significance in the light of recent increase in the incidence of cervical adenocarcinoma in situ (AIS) and invasive adenocarcinoma.[3,4] An algorithmic approach would help in improving the interpretation with precision, for more effective management [Figure 2].
The Society of Gynecologic Oncology recently recommended risk-based management of AIS in 2019 ASCCP consensus guidelines for glandular lesions. The guidelines do not specifically recommend cold knife conization over loop electrocautery excisional procedure (LEEP) but do state that excisional procedures should aim to remove an intact specimen for the evaluation of resection margins.[5] Performance of a LEEP followed by a “top hat” endocervical excision is less acceptable.[6]
The recurrence risk of AIS is only 2.6% with negative margins but increases to 19% with positive margins.[6,7] It is important to highlight that AIS is frequently associated with “skip lesions” with a propensity for non-contiguous multiple foci of AIS. Because of this, the risk of residual AIS on a second excisional specimen is 20% even with negative margins (compared to 53% if margins are positive). About 2% of these patients with negative margins may be associated with invasive cancer as compared to 6% if margins are positive.[8]
Ultimately for AIS, hysterectomy is preferred because it is often located within the endocervical canal and is difficult to recognize on colposcopy. However, fertility-sparing procedures are considered acceptable in select patients.
DIAGNOSTIC ALGORITHMWith aggressive surgical management at stake, the task of the cytopathologist is to accurately report abnormal glandular cells by excluding mimickers mostly HSIL and other HCGs. However, distinguishing these entities may not be straightforward, especially from squamous lesions involving endocervical glands.
The distinction between squamous and glandular lesions can be approached by examining subtle differences in nuclear and cytoplasmic features [Figure 3]. In general, nuclei of glandular cells show discernible nucleoli (except in AIS). The cytomorphological features are best examined at the periphery of the hyperchromatic crowded groups (HGs). The cytoplasm of glandular cells often displays a finely vacuolated or “textured” lacy pattern (in contrast to glassy/waxy relatively homogeneous cytoplasm in squamous epithelial cells). Nuclear size is usually larger than 5 times intermediate cell nuclei (ICN) (except in endometrioid adenocarcinoma) for distinguishing these cells from squamous epithelial lesions with overlapping features [Figure 2].[4]
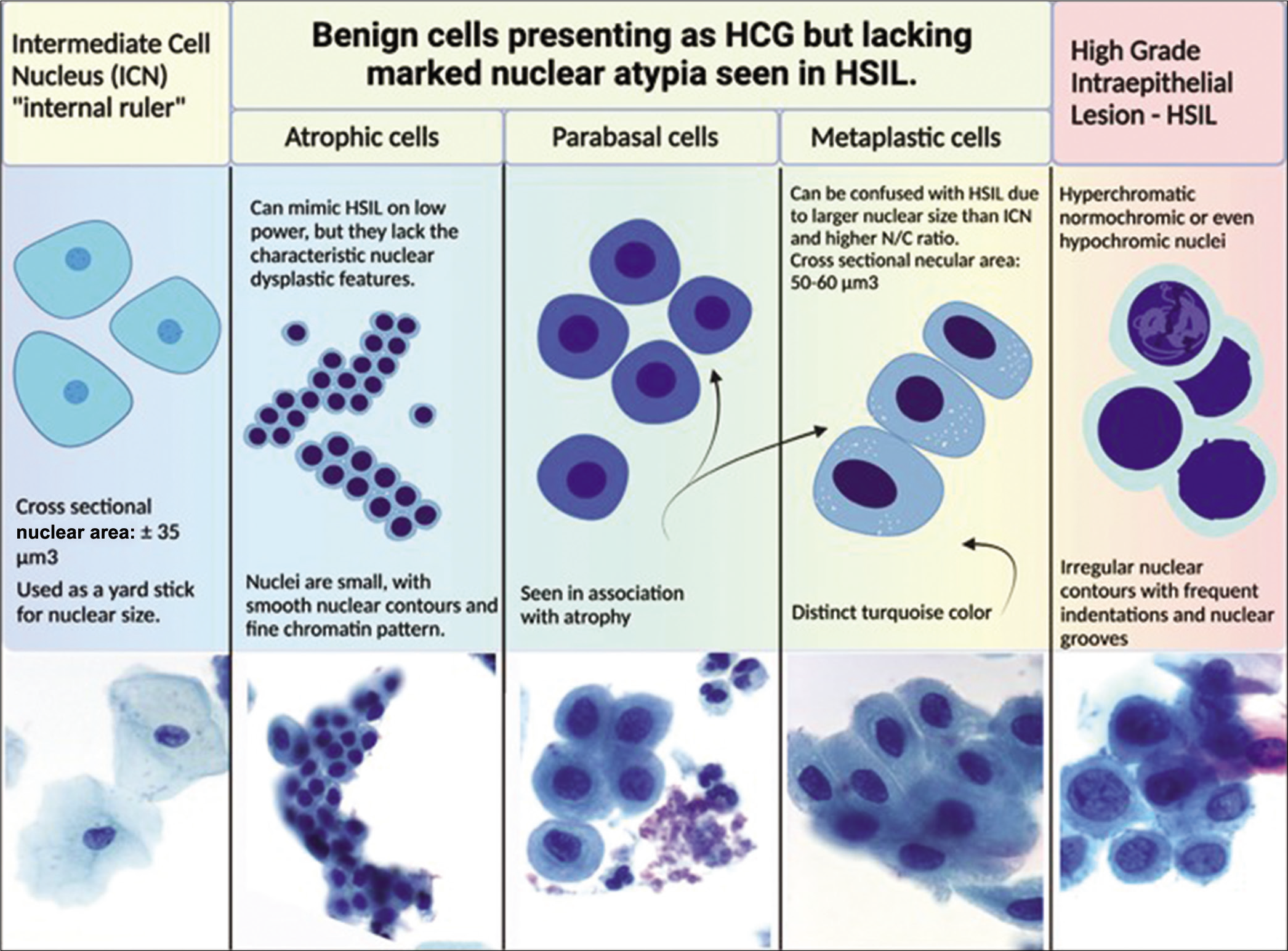
Figure 3:: Various non-neoplastic cellular components seen on Pap smear is preferable compared to AGC. The cell sizes and how they relate to each other are shown in the drawings (relative proportions are maintained). Actual images of superficial, intermediate, parabasal, metaplastic, and HSIL are shown below the drawings. (Reproduced under Open-Access charter from Ref #31).
Export to PPT
AIS demonstrates cigar-shaped, enlarged, hyperchromatic nuclei with coarse chromatin without nucleoli. In addition, AIS shows nuclear feathering at the periphery of the groups [Figure 4]. This feature is better seen in conventional direct smears than in liquid-based cytology preparations[3] As many of these features overlap with HSIL, AIS has to be usually distinguished from HSIL with or without endocervical gland involvement (rather than AGC).[9]
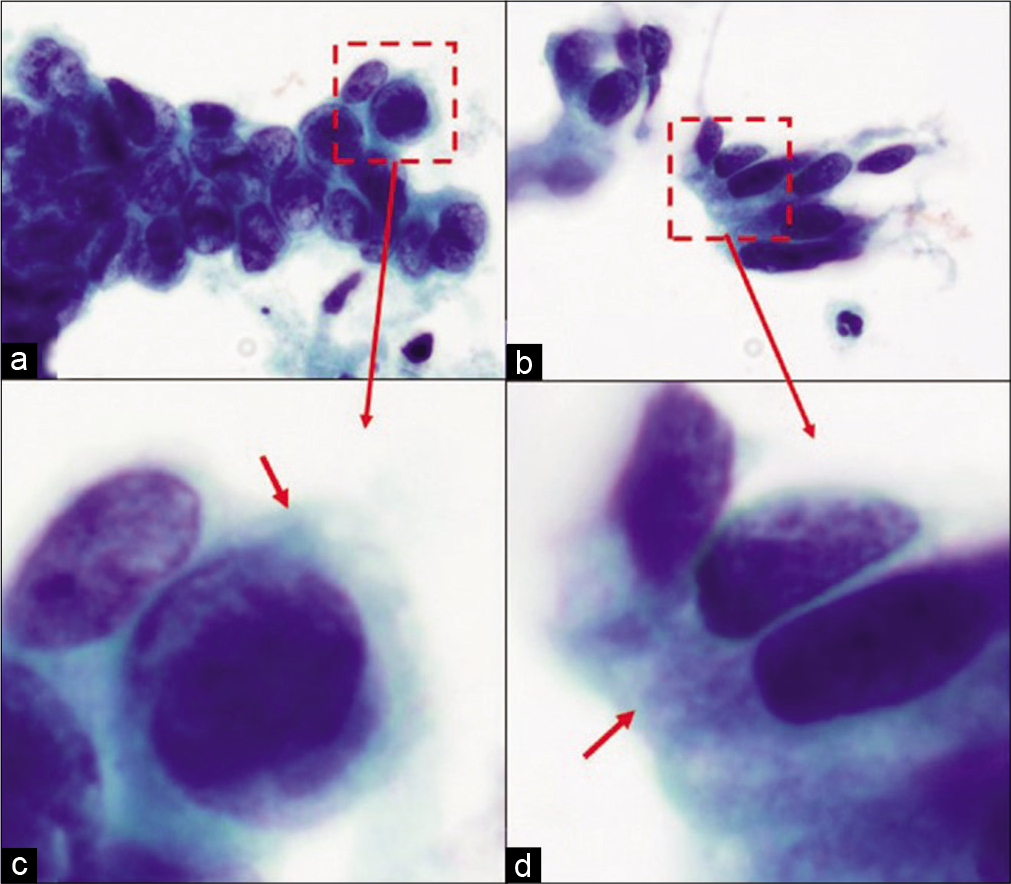
Figure 4:: HSIL versus AIS cytoplasm of HSIL versus AIS cells: (a and c) Groups of HSIL cells: The metaplastic cytoplasm of cells as seen in the cells at the periphery (arrow) is dense and homogeneous. (b and d) Groups of AIS cells: The cytoplasm shows lacy appearance (arrow).
Export to PPT
HCGs of atypical endometrial cell groups with nuclei larger than non-neoplastic endometrial cells show a high nuclear-cytoplasmic (N/C) ratios. In contrast to AIS, the nuclei with irregular nuclear outlines show nucleoli [Figure 5].[10]
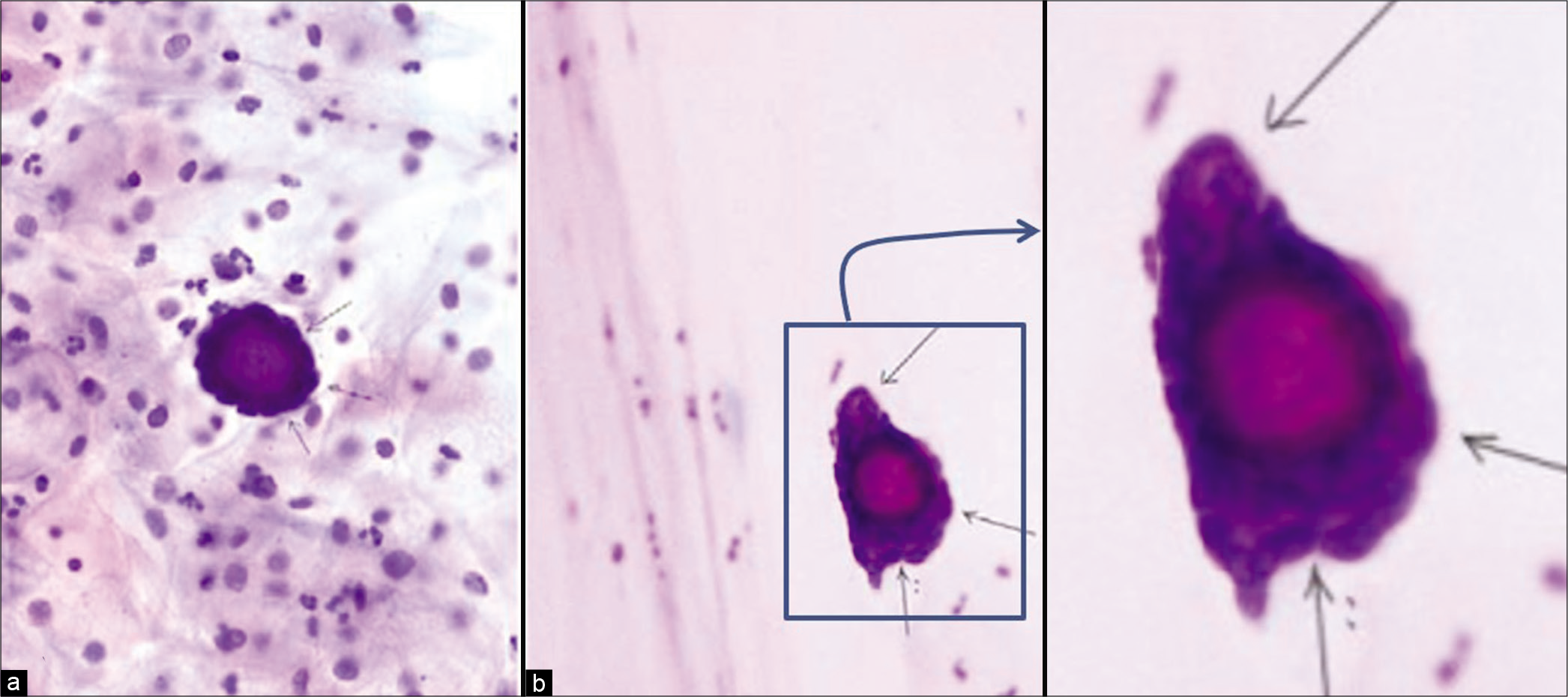
Figure 5:: Adenocarcinoma, other (a) psammoma body surrounded by atypical cells (thin arrows) in cervicovaginal smear (Papanicolaou stain, ×200); (b) psammoma body surrounded by atypical cells (thin arrows) in endometrial aspiration smear (Papanicolaou stain, ×200). Follow-up showed serous carcinoma of the ovary. (Reproduced under Open-Access charter from Ref #32).
Export to PPT
For other glandular lesions (other than AIS and endometrial adenocarcinoma), the questionable group with atypical cells can be interpreted as abnormal glandular cells based on size (usually more than 5 times ICN) of hyperchromatic nuclei with detectable nucleoli (chromocenters should not be confused for nucleoli). Larger-hyperchromatic nuclei with nucleoli favor AGC. Often, only the cytomorphological features of atypical cells may not be adequate. AGC is the option if the findings are not sufficient to render a definitive interpretation of adenocarcinoma.
HCGs are the most important differential with glandular lesions and are one of the significant diagnostic challenges in the cytopathology of Pap smears [Figures 6 and 7]. HCG is specifically defined as dark crowded cell groups of usually more than 15 cells identified at ×10 screening magnification. Studies have shown that the vast majority of HCGs are non-neoplastic. However, their presence should warrant more rigorous scrutiny, because HCGs in small proportion of cases may represent higher grade epithelial cell abnormalities including AGC.[10] AGC falls in “high-risk” category because of increased chances of AdCa, AIS, or HSIL (CINII/CINIII) on biopsy after AGC on Pap test. Based on various studies, 9–96% of AGC turn out to be HSIL and the majority have high-risk HPV (hrHPV).[2,3] However, if the nuclear features are interpreted strictly with the evaluation of nuclear details, especially with reference to nucleoli, this disparity should be minimal [Figure 2]. The chromocenters in nuclei of HSIL may be misinterpreted as nucleoli, leading to their misinterpretation as AGC instead of HSIL.
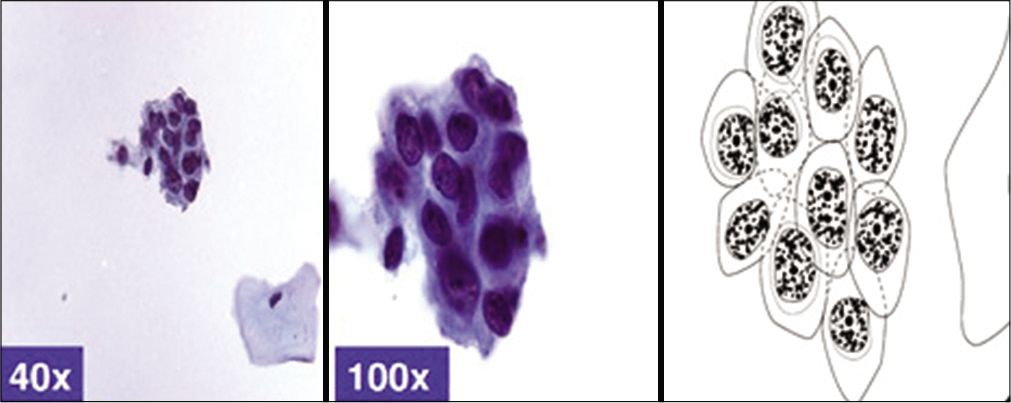
Figure 6:: Hyperchromatic crowded group of small parakeratotic cells showing cohesive groups of hyperchromatic cyanophilic small atypical parakeratotic (SAPK) cells with relatively straight cell borders with angulations better seen at periphery. N/C ratio is higher. Chromatin is smudgy. Some cells may show koilocytic space around nuclei. (Reproduced under Open-Access charter from Ref #10).
Export to PPT
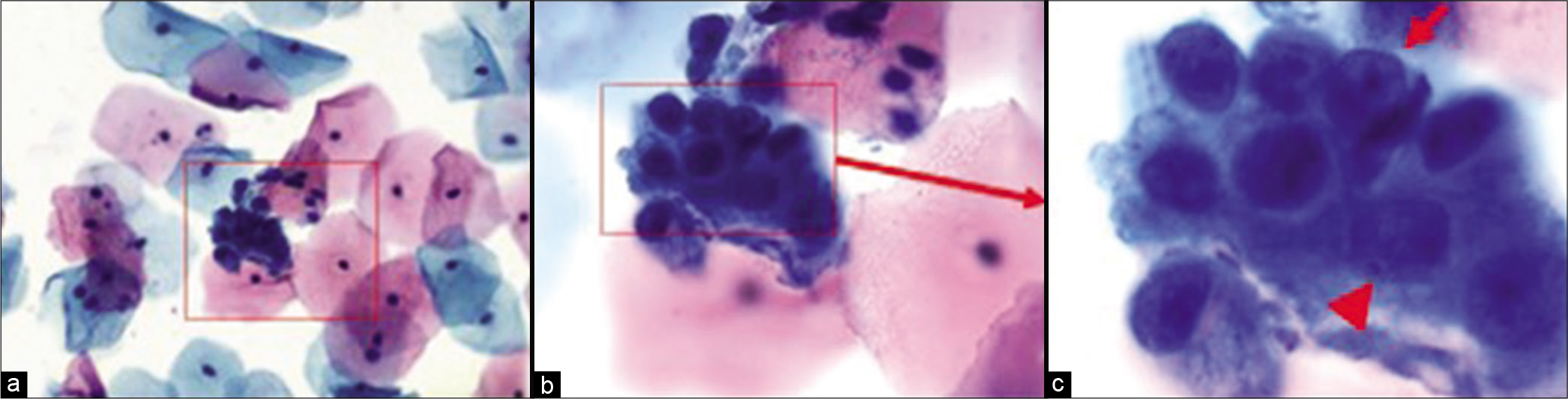
Figure 7:: HSIL with endocervical gland involvement: HSIL cells with endocervical gland involvement seen as (a) hyperchromatic crowded groups (HCG) showing (b) high N/C ratio, hyperchromatic nuclei evenly distributed coarse chromatin and prominent nucleoli at the periphery of HSIL group (arrow in c) as epigenetic feature. The dysplastic cells in the center (arrowhead in c) show features of HSIL without nucleoli.
Export to PPT
THE ROLE OF HPV TESTING AND SUBTYPINGMost cervical glandular lesions are associated with 16, 18, and 45 with higher likelihood of HPV positivity in younger women (<40 years, ≠ 90%) compared to older (>60 years, ≠ 40%).[11,12] Other hrHPV types include HPV 31, 33, 35, 39, 51, 52, 56, 58, 59, 66, and 68.
Some adenocarcinoma (AdCa) negative for hrHPV and can be encountered in cervical Pap smears. The HPV-negative AdCa in Pap smears include the majority of endometrial carcinomas, clear cell carcinoma, high-grade serous carcinoma, mesonephric AdCa, and adenocarcinomas of other sites[13]
At present, guidelines about testing for hrHPV in Pap smears with an AGC interpretation are not available. For example, HPV is more likely to be positive in the AGC group with concomitant squamous lesions compared to lower HPV positivity in cases with atypical endometrial cells. Therefore, positive HPV status in AGC group is associated with higher detection rate of clinically significant lesions compared to HPV-negative cases with negative predictive value of 99.2%.
The source of glandular cells in cervical cytology preparations could be from a variety of anatomic sites along the tract from the ovary – fallopian tube – uterus – endocervix tract – to the vagina. However, the most common source of glandular cells is endocervix and/or endometrium. Although the endocervical canal anatomically runs from the external os to the internal os, the endocervical glandular epithelium is not limited by anatomic borders. Rather the transformation zone, (a line that marks the transition from the glandular lining of endocervix to the squamous epithelium of ectocervix), is dynamic and constantly changing depending on multiple factors including age, parity, and hormonal changes, especially during the reproductive years. Because, the cytomorphology of endocervical glandular cells can be diverse, familiarity with these features cannot be overemphasized [Figures 8 and 9].
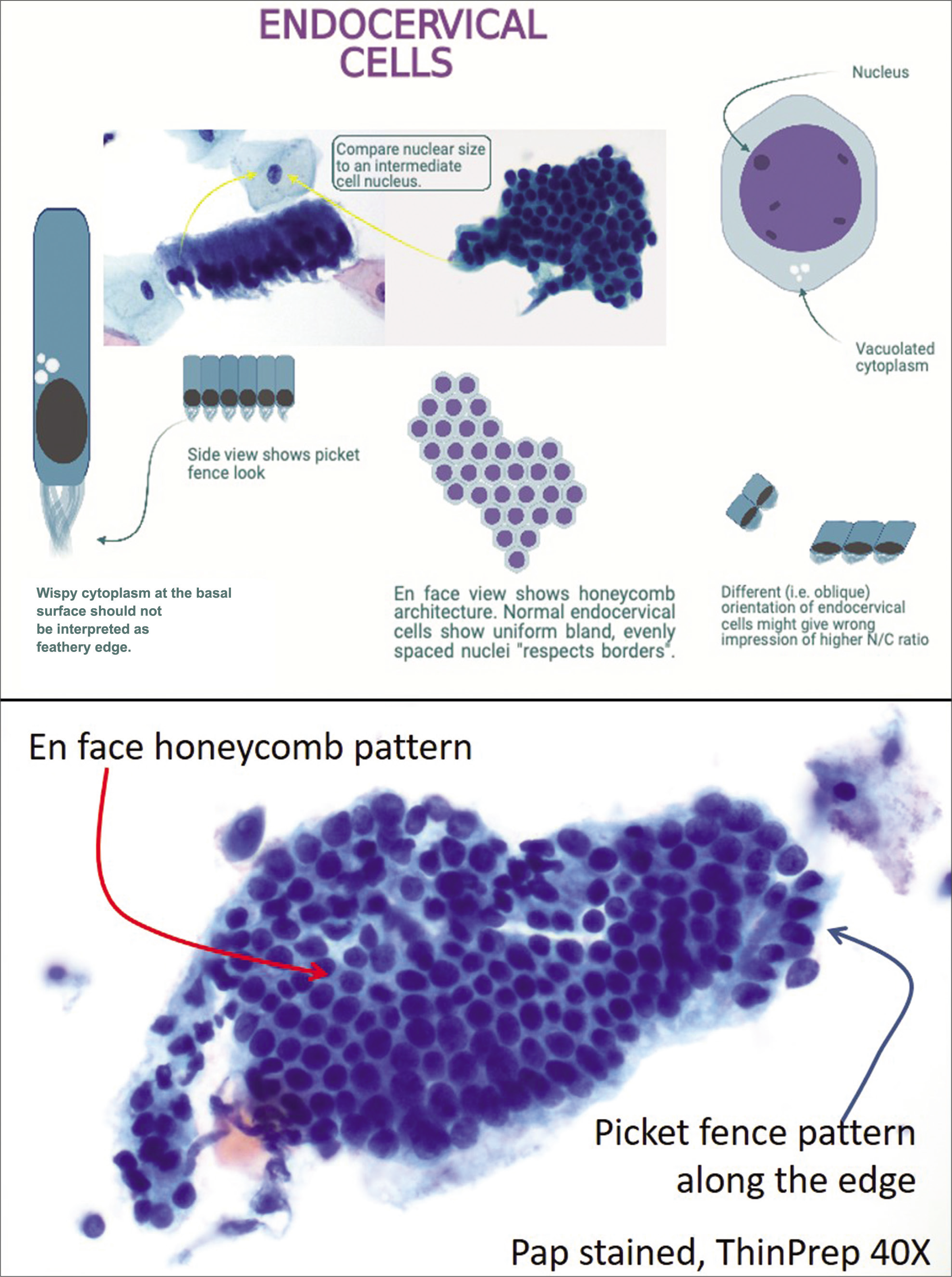
Figure 8:: Cytomorphology of endocervical glandular cells.
Export to PPT
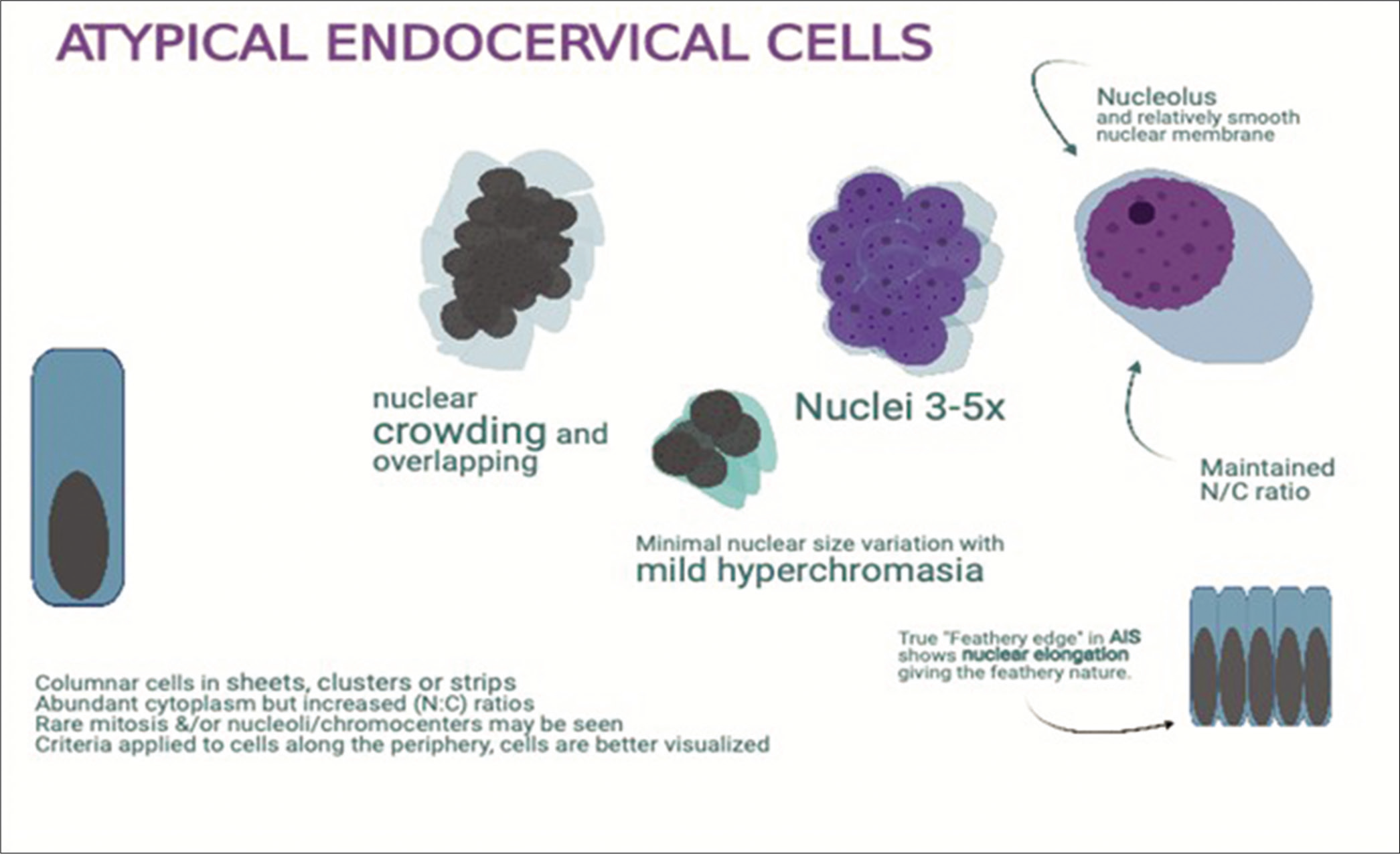
Figure 9:: Atypical endocervical cells: enlarged nuclei (3 to 5X ICN) with prominent nucleoli with minimal architectural disorganization with architectural atypia.
Export to PPT
GLANDULAR CELL ABNORMALITIES Endocervical AISThe typical endocervical AIS is a non-invasive high-grade endocervical glandular lesion that is characterized by palisades of cells with cigar-shaped, hyperchromatic nuclei with coarse chromatin, nuclear enlargement with high N/C ratios, pseudostratification, and mitotic activity usually with apoptotic bodies.
Histological variants, that include conventional, endometrioid, tubal, intestinal, and stratified mucinous, are diagnostically challenging. This is true even on biopsies and LEEP specimens.[14,15] However, a few features such as intestinal differentiation are always suspicious for significant lesion because non-neoplastic intestinal metaplasia is exceedingly rare in endocervix. Most of these variants of AIS may have to be interpreted as AGC-NOS or AGC endocervical-NOS.
Diagnostic criteriaThe cell groups show palisading nuclear arrangement with elongated oval- to cigar-shaped nuclei, which protrude out along the periphery because of scant cytoplasm related to high N/C ratio. This is typically referred to as nuclear feathering. This feature is better seen in conventional direct Pap smears as compared to liquid-based cytology (LBC) preparations [Figure 10]
The groups present as sheets, clusters, pseudostratified strips, and rosettes with nuclear crowding and overlap without well-defined honeycomb pattern
High N/C ratios
Variation in nuclear size
Nucleoli are usually inconspicuous (chromocenters should not be confused for nucleoli)
Mitoses and apoptotic bodies may be present
Background is typically clean without diathesis, although inflammatory debris may be present
Atypical squamous cells may be present if there is a coexisting squamous lesion [Figures 4 and 11].
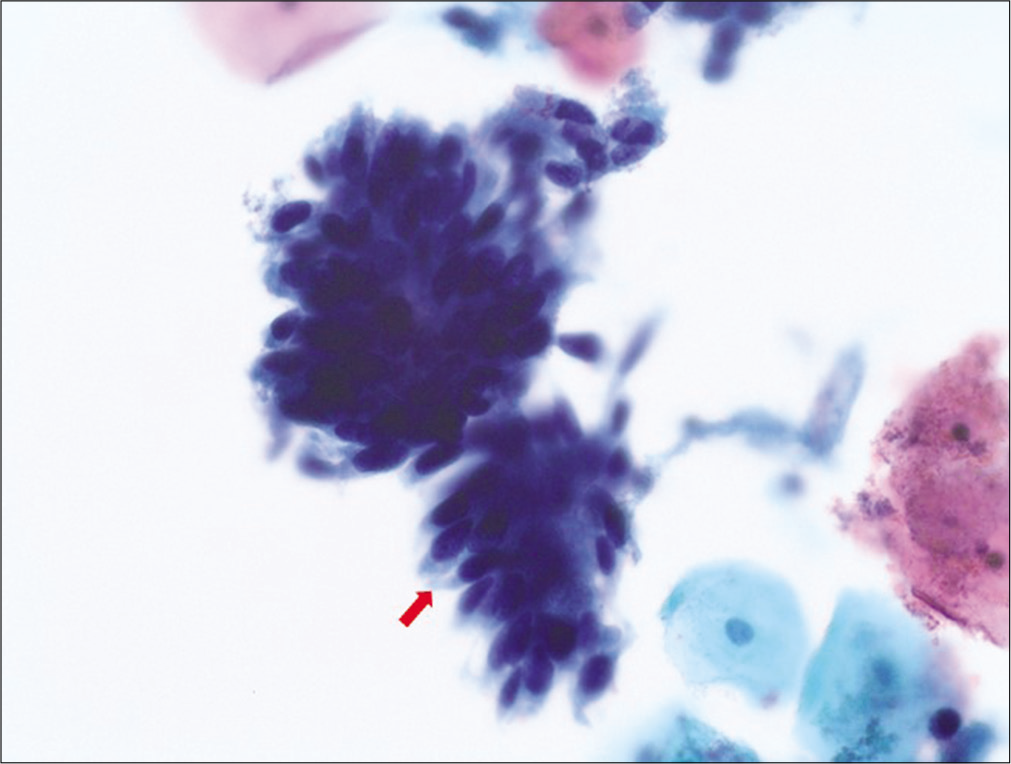
Figure 10:: Adenocarcinoma in situ (AIS): hyperchromatic crowded groups (HCGs) of cells exhibiting high N/C ratio with elongated (cigar shaped), hyperchromatic nuclei without nucleoli with some variation in nuclear sizes and lacy cytoplasm. Note the “feathering” of the protruding nuclei at the periphery of the HCG.
Export to PPT
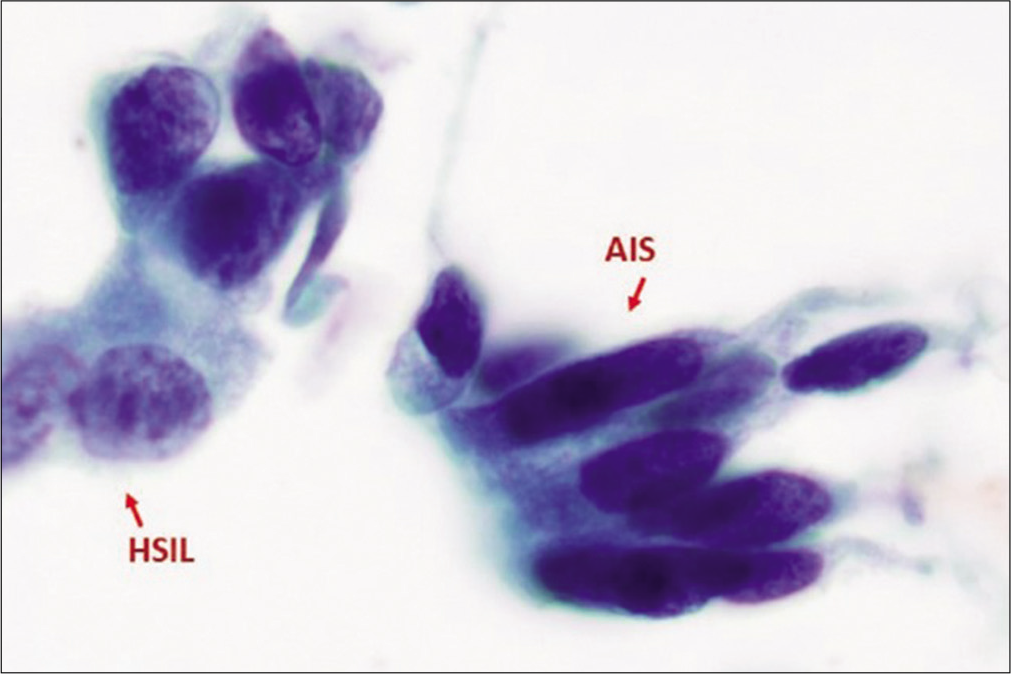
Figure 11:: Adenocarcinoma in situ (AIS) along with HSIL. AIS cells exhibits nuclear atypia characterized by hyperchromasia without nucleoli similar to that seen in HSIL, but with cigar-shaped elongated nuclei with lacy cytoplasm (as compared to dense, metaplastic cytoplasm in HSIL cells). (Reproduced under Open-Access charter from Ref #31).
Export to PPT
Differential diagnosisNon-neoplastic HCGs [Figure 3]
HCG of endometrial cells: Small tight spherical structures of small cells, mixed gland/stromal fragments with spindle cell stromal fragments (may have blood vessels) with hyperchromasia. The spherical groups usually show epithelial cells along the periphery with stromal component in the center. The cells show high N/C ratio with nuclear contour irregularities without significant nuclear atypia. Nucleoli are usually not prominent
HCG of tubal metaplastic cells: Group of columnar cells with cilia and/or terminal bars round to oval dark nuclei with high N/C ratio with pseudostratification. Goblet cells may be seen. Pleomorphism may be present. Lack of apoptotic bodies, coarse chromatin, uneven chromatin distribution with parachromatin clearing.
HCG of parabasal cells: Groups of cells with higher N/C ratio and overlapping relatively bland oval to round nuclei with tiny nucleoli. Usually seen in the background of atrophic cellular changes.
High-grade squamous intraepithelial lesion (HSIL)
HSIL cell groups with enlarged hyperchromatic nuclei with coarse chromatin (also without nucleoli similar to AIS) may resemble AIS. However, HSIL nuclei are mostly round to oval (in contrast to elongated, cigar-shaped nuclei in AIS) without palisading and without nuclear feathering in syncytial groups with nuclear overlap
Degree of nuclear enlargement is more variable. Nuclear contours may be irregular.
Nuclei are generally hyperchromatic but may be normochromatic or rarely even hypochromatic.
Chromatin may be fine or coarsely granular and is evenly distributed.
Nucleoli are generally absent, but may occasionally be seen along the periphery of groups, particularly when HSIL involves endocervical glands.
Appearance of the cytoplasm is usually metaplastic, but variable (“immature,” lacy, and delicate or densely metaplastic, occasionally, “mature,” and densely keratinized).
Atypical glandular cells (AGCs) AGC endocervical, NOSThis category includes AGCs with a morphology that is short of adenocarcinoma but overlap with reactive endocervical cells and cells with reparative changes. Other factors limiting the definitive interpretation of adenocarcinoma may be quantitative and/or other qualitative issues including poor preservation and degenerative changes.
The qualifier “favor reactive” is potentially misleading and, therefore, is not included in the current Bethesda terminology. Instead, “Not Otherwise Specified” (NOS) is recommendation to be used with appropriate comment(s). If the index of suspicion is high, but still not meeting criteria for AIS or AdCa, the designation “favor neoplastic” (FN) may be used (See next category below). The significant morphologic feature is that it shows nuclear atypia that exceeds an obvious reactive or reparative change. The key for proper designation of atypical endocervical cells lies in the ability to exclude reactive conditions that include repair, tubal metaplasia, radiation, and intrauterine device (IUD) effect [Figures 12 and 13].
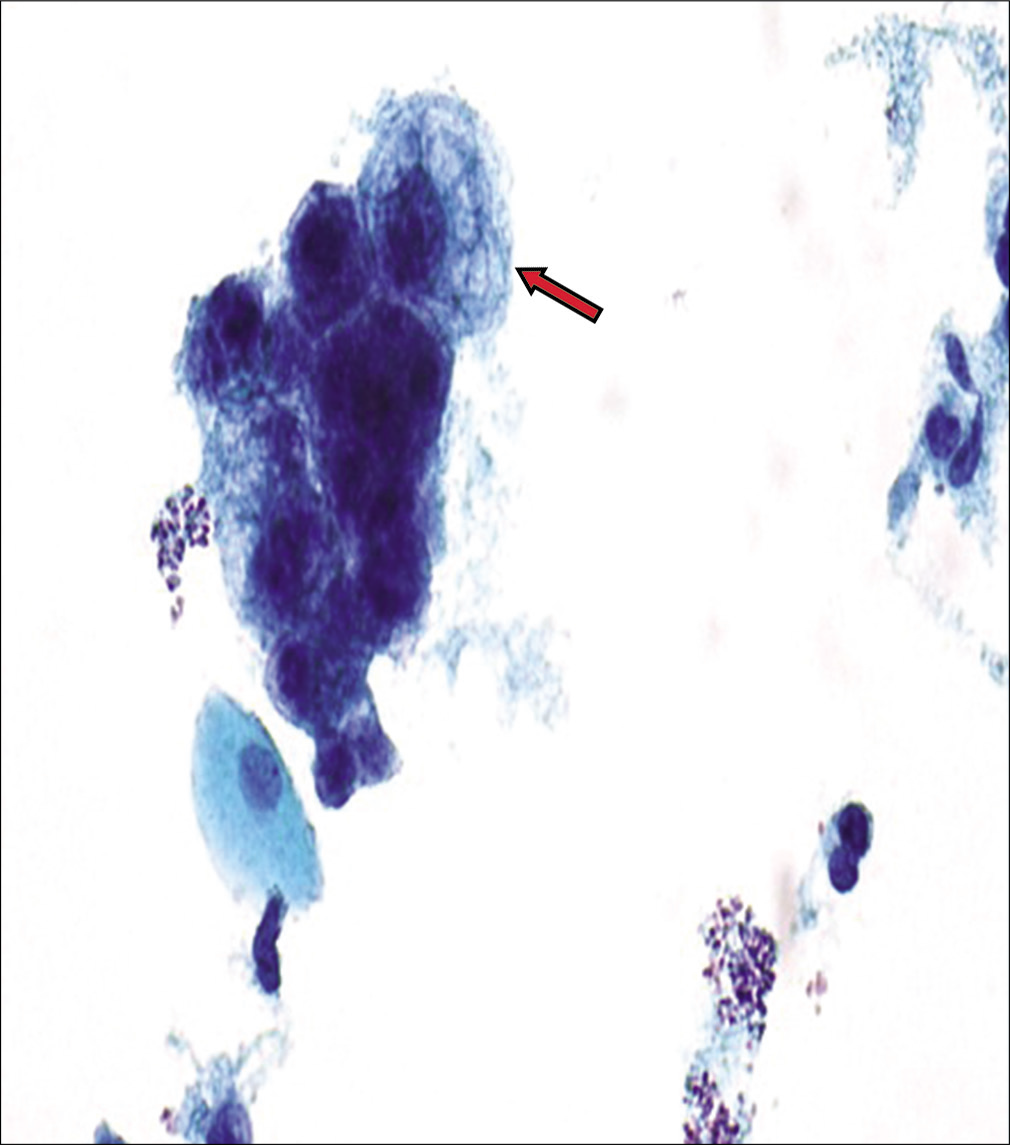
Figure 12:: AGC, Endocervical, NOS (specify in comment – radiation effect). This hyperchromatic crowded group shows a relatively small crowded group of cells (arrow) with cytoplasmic vacuoles (both degenerative and mucin vacuoles). The nuclei in cells with cytomegaly are around 3 times the size of intermediate cell nuclei and show minimal nuclear atypia with nuclear enlargement (but without significant change in N/C ratio), smudgy chromatin, and some variation in nuclear size with prominent nucleoli.
Export to PPT
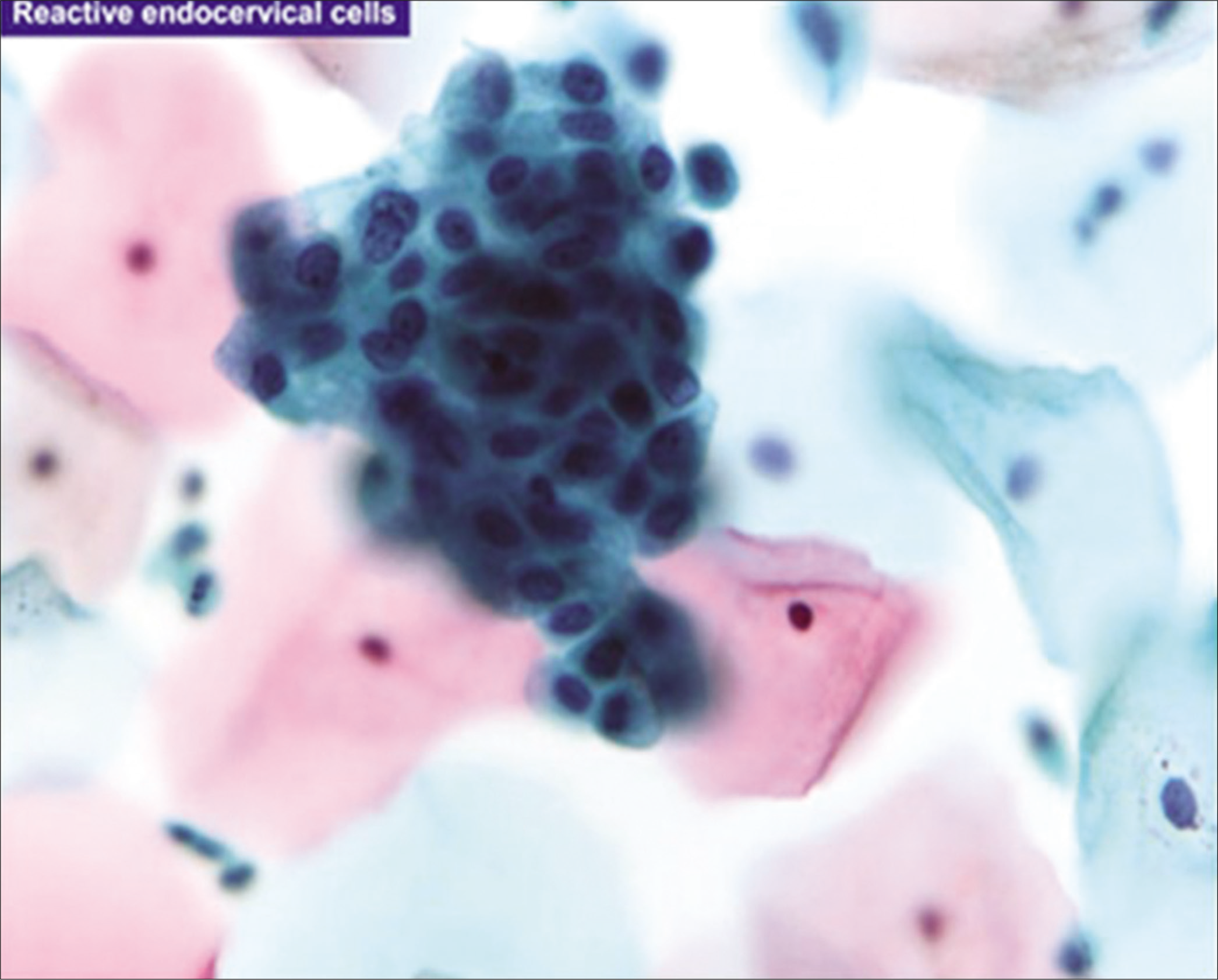
Figure 13:: Reactive endocervical cells with relatively prominent nucleoli and some nuclear crowding (×100). (Reproduced under Open-Access charter from Ref #35).
Export to PPT
Diagnostic criteriaEndocervical cells with architectural atypia: Columnar cells arranged in strips and sheets with nuclear crowding, overlap, and/or pseudostratification
Nuclei are enlarged 3 to 5X size of intermediate cell nuclei (ICN)
Minimal variation in chromatin pattern and nuclear size
Higher nuclear to cytoplasmic ratios
Cell borders may be ill-defined.
AGC endocervical, favor neoplasticThis category includes AGCs with a morphology that is suggestive of endocervical adenocarcinoma but either quantitatively or qualitatively falls short of an interpretation of endocervical adenocarcinoma.
Diagnostic criteria: [Figures 14 and 15]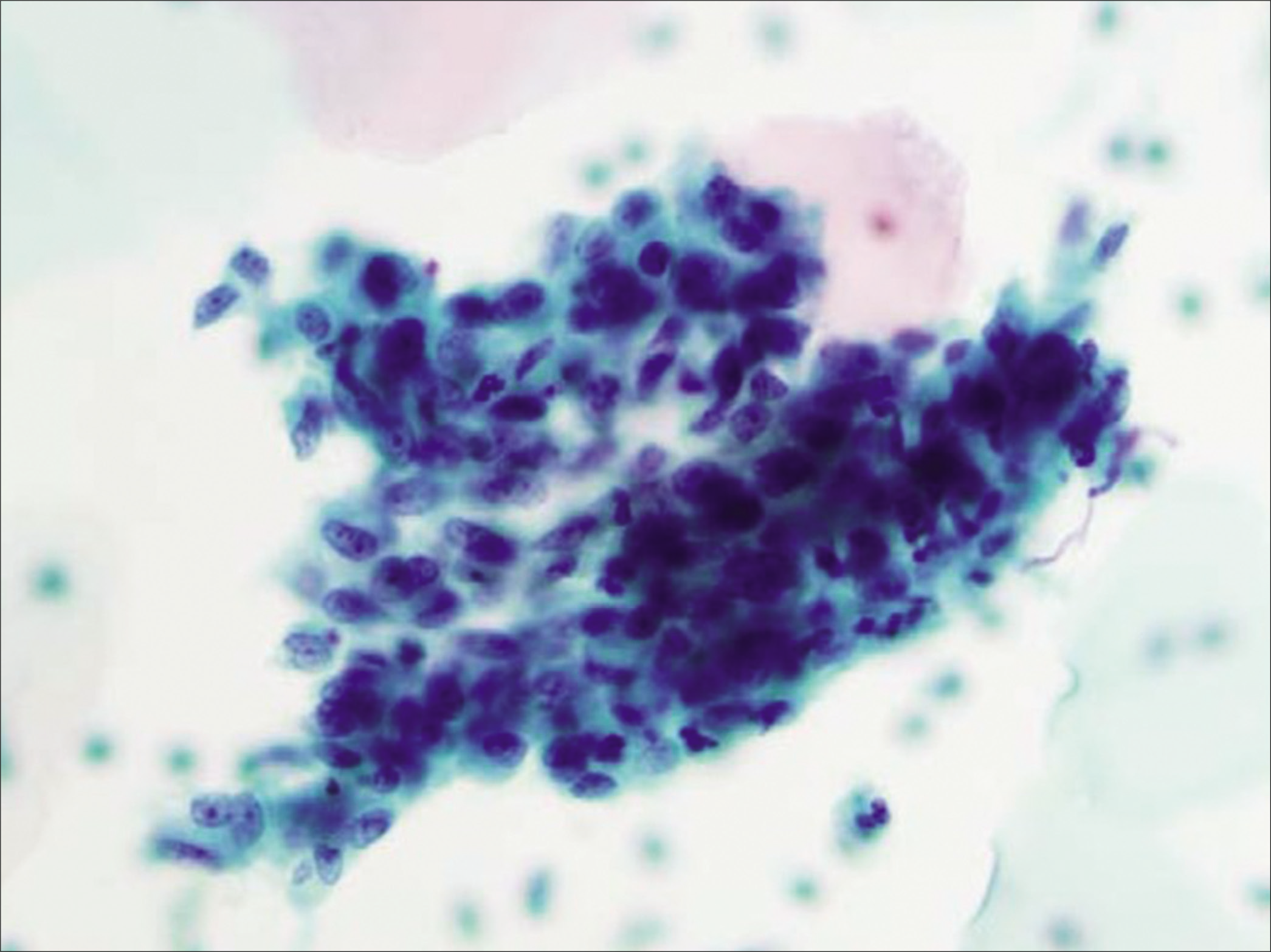
Figure 14:: Atypical glandular cells (AGCs), endocervical, favor neoplastic: three-dimensional groups of endocervical cells with enlarged nuclei (5X ICN) with nucleoli and some nuclear overlap of the randomly oriented nuclei (×40). (Reproduced under Open-Access charter from Ref #35).
Export to PPT
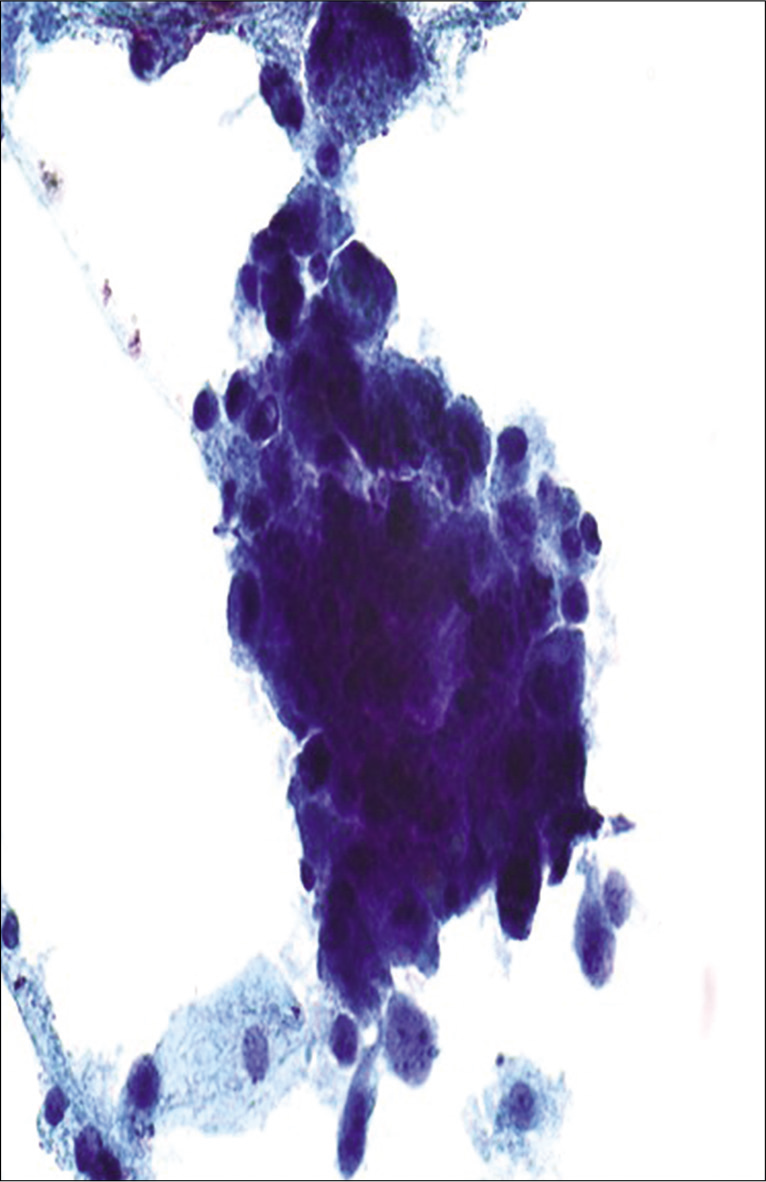
Figure 15:: Atypical glandular cells (AGCs), endocervical, favor neoplastic: poorly preserved hyperchromatic crowded group of cells with high N/C ratio and enlarged nuclei (more than 5X ICN) with nuclear overlap. Cytoplasm is finely vacuolated (definitive interpretation is limited due to poor preservation).
Export to PPT
Endocervical cells with architectural atypia: Columnar cells arranged in strips and sheets with nuclear crowding, overlap, and/or pseudostratification
Rare cell groups with high N/C ratio and rosettes (gland formations)
Large nuclei (3 to 5X size of ICN) hyperchromatic nuclei with coarse chromatin with heterogeneity
Occasional mitoses with or without apoptotic bodies.
Approach to diagnosisOn a low-power screening, multiple three-dimensional HCGs or two-dimensional sheets or strips of hyperchromatic cells can be identified with variable degree of overlapping.[16,17]
On higher magnification, the two most important criteria are nuclear size and chromatin pattern. Enlarged nuclei (3 to 5X ICN) with nucleoli and increased nuclear to cytoplasmic ratio are best assessed at the periphery of a given group. The characteristics features of atypia; hyperchromasia; and slightly coarse chromatin are present with a slightly irregular nuclear membrane. Occasionally, mitotic figures and apoptotic debris can be seen. However, inability to evaluate nuclear details of the atypical cells in the central area of such HCGs of HSIL with glandular involvement may be misinterpreted as AGC.
AGCs with ill-defined nuclear feathering may be interpreted as AGC, favor neoplastic. Feathering, characterized by nuclear protrusion from the periphery of the group, is a feature that can be identified easily on conventional Pap smear. It is not distinctly appreciated in a liquid-based preparation.[9] Overall, category of “endocervical glands, favor neoplastic,” may be selected when the atypical features are not convincing enough for definitive interpretation as adenocarcinoma.
AGC endometrial (AEM)This category includes AGC with cytomorphological features of abnormal endometrial cells. Due to the lack of poor reproducibility, this category is not further designated as favor neoplastic or NOS. Endometrial cells with atypical features represent a wide spectrum of conditions, and it is not possible to predict malignancy based on these features.
The distinction of cytologically atypical endometrial cells is based primarily on the criterion of increased nuclear size with occasional nucleoli. In addition to endometrial hyperplasia and endometrioid carcinoma, AGC endometrial may be associated with endometrial polyps, chronic endometritis, and IUD.
Diagnostic criteria: [Figures 16 and 17]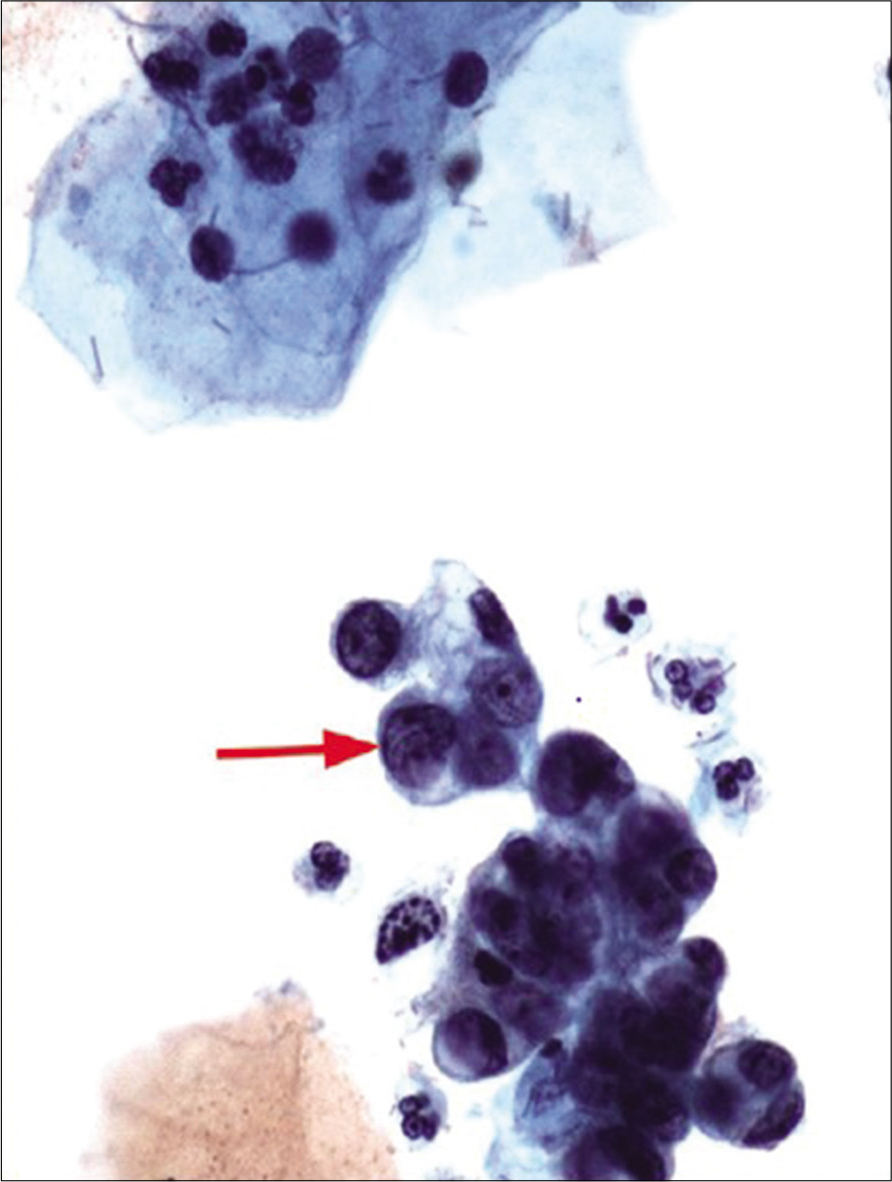
Figure 16:: Atypical glandular cells (AGCs)-endometrial cells (NOS) hyperchromatic crowded group of cells with nuclear size larger than normal endometrial cells (twice that of an intermediate cell nucleus) (red arrow). The nuclei show nucleoli. The histologic follow-up is well-differentiated endometrioid adenocarcinoma, FIGO Grade 1. Pap stain, ×40 (Reproduced under Open-Access charter from Ref #27).
Export to PPT
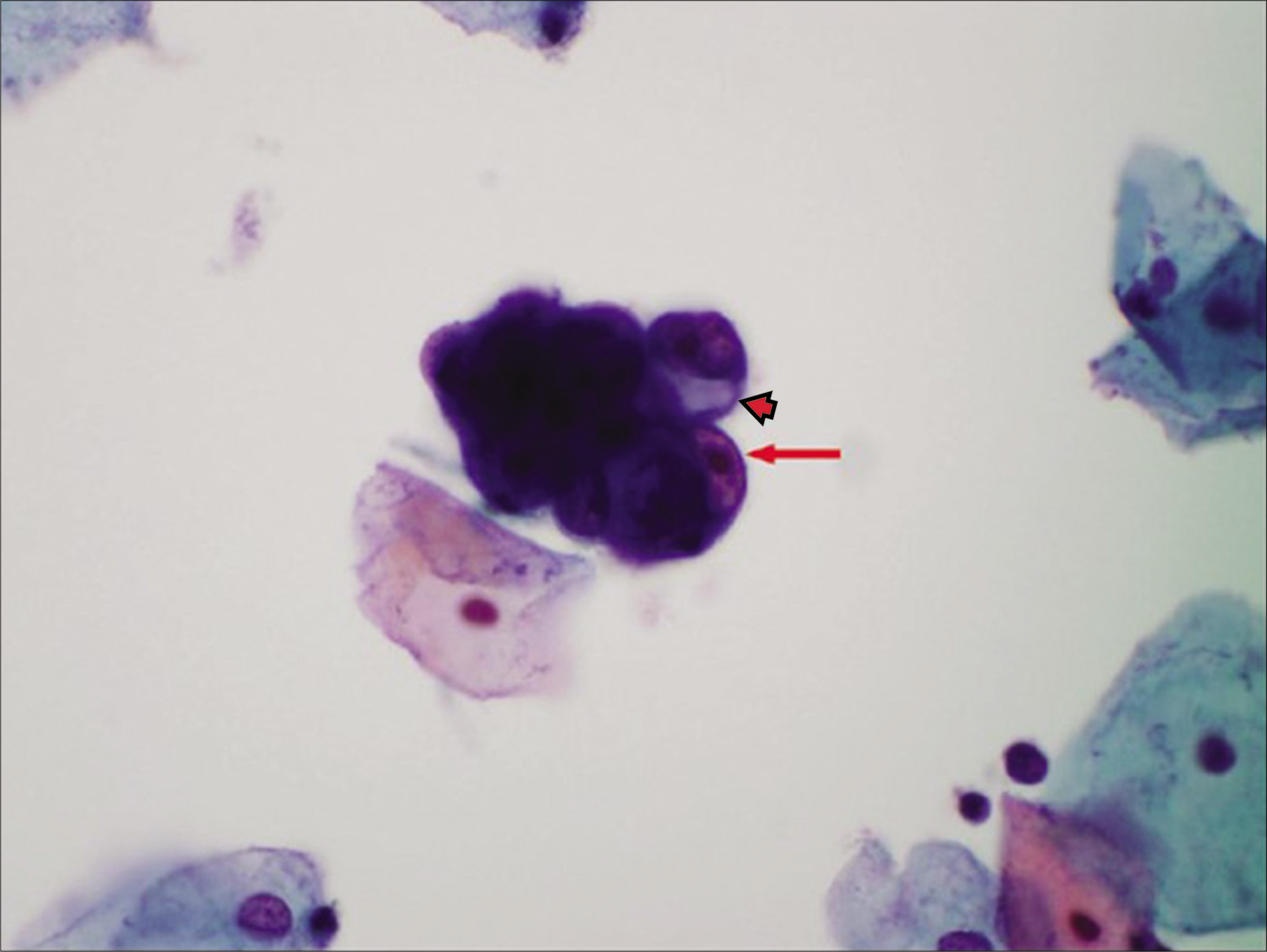
Figure 17:: Atypical glandular cells (AGCs)-endometrial cells (NOS) atypical endometrial cells as a small hyperchromatic crowded group of glandular cells with visible nucleoli (red arrow) with cytoplasmic vacuole (arrowhead). The histologic follow-up is endometrial intraepithelial neoplasia. (Pap stain) (Reproduced under Open-Access charter from Ref #27).
Export to PPT
Small cohesive HCGs of endometrial cells with usually 5–10 cells per group
Nuclei are slightly enlarged compared to normal endometrial cells
Mild hyperchromasia
Chromatin heterogeneity
Occasional small nucleoli
Scant cytoplasm occasionally shows vacuole (degenerative)
Cell borders are ill defined.
Approach to diagnosisUnder low-power HCGs are seen as spheres of small groups of cells and may resemble exodus of three-dimensional cluster of endometrial cells with high N/C ratio.[18] The individual nuclei appear larger than the normal endometrial nuclei. On high power, the nuclei are hyperchromatic, with occasional small nucleoli. The cytoplasm is usually scant, with occasional cytoplasmic vacuolation (degeneration related) can be seen with ill-defined cell borders.
Differential diagnoses/mimickers of AGCThe nuclei of HSIL cells do not show easily detectable nucleoli (chromocenters may be present but should not be confused with nucleoli) unless associated with endocervical cells in cases with HSIL with endocervical gland involvement
The nuclei of HSIL cells although larger than ICN are smaller than AGC nuclei
Nuclear-to-cytoplasmic ratio is higher in HSIL compared to AGC
HSIL nuclear contours are usually irregular as compared to AGC
HSIL cell cytoplasm is variable but usually “immature” dense, homogeneous cyanophilic (with or without vacuoles) but may be “mature” densely keratinized. Compared to lacy delicate or vacuolated cytoplasm in AGC cells.
Small-cell carcinoma and other high-grade tumors [Figure 19]
Small-cell neuroendocrine carcinoma is an uncommon and highly aggressive carcinoma of the cervix. Like small-cell carcinoma of other locations, it is composed of relatively uniform small cells with scant cytoplasm. On low power, the cells are seen in loosely cohesive hyperchromatic groups. Under high power, the cells show hyperchromatic nuclei without nucleolar prominence with salt-and-pepper chromatin and scant cyanophilic cytoplasm. Some groups may show crush artifact
Other differential diagnosis in this category includes small-cell primitive neuroectodermal tumor, melanoma, and undifferentiated carcinoma
Tubal metaplasia[
留言 (0)