This study was conducted to describe the trends and age-period-cohort effects on the incidence and mortality rate of cervical cancer in Korea.
Materials and MethodsThe incidence and mortality rate of cervical cancer among ≥ 20-year-old women from 1993 to 2012 were obtained from the Korea Central Cancer Registry and the Korean Statistical Information Service. Age-standardized rates were calculated and Joinpoint regression was used to evaluate the trends in the incidence and mortality rate. Age-period-cohort analysis was performed to investigate the independent effects of age, period and cohort.
ResultsThe incidence of cervical cancer decreased from 32.8 per 100,000 in 1993 to 15.9 per 100,000 in 2012 (annual percent change [APC], –3.9%; 95% confidence interval [CI], –4.2% to –3.6%). The mortality rate decreased from 5.2 per 100,000 in 1993 to 2.1 per 100,000 in 2012 (APC, –4.8%; 95% CI, –5.1% to –4.4%); however, the incidence and mortality rates among young women (< 30 years old) increased. An age-period-cohort model of the incidence and mortality rate showed decreasing period effects between 1993 and 2008 and decreasing cohort effects between 1928 and 1973, while birth cohorts after 1973 exhibited slight increases in the incidence and mortality rate of cervical cancer.
ConclusionRecent decreases in the incidence and mortality rate of cervical cancer were due to decreases in the period and cohort effects, which reflect the implementation of a cancer screening program and changes in lifestyle. However, our findings also highlighted an increase in cohort effects on the incidence and mortality rate among young women born after 1973.
Key words: Uterine cervical neoplasms, Incidence, Mortality, Age-period-cohort analysis, Trends
Introduction In Korea, the incidence and mortality rate of invasive cervical cancer have decreased since the introduction of a national cervical cancer screening program [1]. However, it has been suggested that the incidence of cervical cancer among young Korean women has increased during the last two decades [2]. Furthermore, changes in sexual behavior and the prevalence of human papillomavirus (HPV) infection among Korean women have also been reported [3,4]. Accordingly, age-period-cohort analysis could help separate the independent effects of age, period and cohort from trends in cancer rates [5]. Moreover, period effects reflect the factors that influence all age groups simultaneously, such as implementation of a screening program, while cohort effects reflect changes in lifestyle or external environmental exposures. To the best of our knowledge, only one Korean study has examined the trends and age-period-cohort effects on cervical cancer-related mortality [6], and that study did not evaluate incidence data and used mortality data that are now > 10 years old. Therefore, the present study was conducted to describe secular trends in the incidence and mortality rate of cervical cancer in Korea, and to use age-period-cohort analysis to evaluate these factors’ independent effects. Materials and Methods 1. Data sources Data regarding the incidence of cervical cancer between 1993 and 2012 were derived from the Korea National Cancer Incidence Database (KNCIDB) of the Korea Central Cancer Registry (KCCR). The KNCIDB was launched by the Korean Ministry of Health and Welfare in 1980, and is a nationwide hospital-based cancer registry. The KCCR collects data regarding approximately 80%-90% of cancer cases each year from > 180 training hospitals throughout the country [7]. Since 1999, the KCCR has covered the entire population under the population-based cancer registry program. The KNCIDB includes information regarding age, sex, diagnosis date, geographical region, histological type, primary site, and first treatment modality. Detailed information regarding the KNCIDB have been previously reported [8]. Data regarding cervical cancer-related mortality between 1993 and 2012 were obtained from Statistics Korea (http://kosis.kr). Patient records were anonymized and de-identified prior to analysis.Ethical approval for the research protocol was provided by the Institutional Review Board of the National Cancer Center, Goyang, Korea (NCC2015-0185).
2. Adjusting the cervical cancer-related mortality Although the incidence data has high quality and completeness, the majority of mortality data for cervical cancer has been recorded as unspecified uterine cancer deaths [6,9]. Therefore, it is necessary to redistribute unspecified uterine cancer deaths to cervical cancer deaths using a redistribution algorithm estimation of more accurate cervical cancer mortality trends. To accomplish this, we linked the incidence database with the mortality database, then extracted registered cases of malignant neoplasms of the cervix uteri (C53) among deaths from unspecified malignant neoplasms of the uterus (C55) based on the tenth edition of the International Classification of Diseases [10]. For the present study, we adjusted the numbers of cervical cancer-related deaths (C53) using the proportion of registered cases of cervical cancer (i.e., incidence, ‘C53’) to the number of deaths due to unspecified uterine cancer (C55) in each age group. The detailed redistribution algorithm has been documented in a previous report [6]. 3. Statistical analysis We excluded the incidence and mortality data from 11]. The Joinpoint regression analysis program was employed to estimate trends in the age-standardized incidences and mortality rates [12], and the results were expressed as the annual percent changes (APCs) and their 95% confidence intervals (CIs) for each period. Age-period-cohort effects were assessed using a log-linear model to describe trends in the incidence and mortality rate using the intrinsic estimator method [13]. The goodness of fit for each model was estimated using the likelihood ratio test and the Akaike information criterion (AIC). p-values of Results 1. Trends in incidence The age-standardized incidence of cervical cancer among ≥ 20-year-old women decreased from 32.8 per 100,000 in 1993 to 15.9 per 100,000 in 2012 (APC, –3.9%; 95% CI, –4.2% to –3.6%) (Table 1). The peak age for the incidence of cervical cancer steadily increased from 60-64 years during 1993-1997 to 75-79 years during 2008-2012 (Fig. 1A). The age-specific incidence of cervical cancer exhibited a decreasing trend for all age groups, with the exception of women who were Fig. 1B). The incidence of cervical cancer among 2. Trends in mortality rates The age-standardized mortality rate for cervical cancer among ≥ 20-year-old women decreased from 5.2 per 100,000 in 1993 to 2.1 per 100,000 in 2012 (APC, –4.8%; 95% CI, –5.1% to –4.4%) (Table 1). As shown in Fig. 2A, the mortality rate for cervical cancer increased with age, except among elderly people (≥ 70 years old) during 1993-1997. The age-specific mortality rate for cervical cancer exhibited a decreasing trend for all age groups, with the exception of women who were 20-24 years old and ≥ 75 years old (Fig. 2B). 3. Age-period-cohort models The age-period-cohort model of incidence had the lowest AIC value from the goodness of fit tests (Table 2). The age-period-cohort model of mortality had the lowest AIC value, and was selected as the model with the best fit based on the goodness of fit test results (Table 2). In the age-period-cohort models, the period effects for the incidence and mortality rate of cervical cancer both decreased from 1993 to 2008 (Fig. 3A and B). The risk ratio for cervical cancer incidence and mortality decreased among women who were born between 1928 and 1973 after we adjusted for age and period effects. However, the risk ratio for cervical cancer incidence and mortality increased among women who were born after 1973. Discussion Our findings revealed that the incidence and mortality rate of cervical cancer decreased from 1993 to 2012. The decreasing trends in the Korean incidence and mortality rate are consistent with a pattern of decreasing trends throughout developed countries [14]. Our age-period-cohort models also suggest that the decrease in the period and cohort effects significantly contributed to decreases in the incidence and mortality rate of cervical cancer. However, the incidence and mortality rate of cervical cancer gradually increased in the younger birth cohorts, and the recent birth cohort effects (after the 1973 birth year) also exhibited an increasing trend. 1. Cohort effect The decreasing cohort effects on the incidence and mortality rate among women who were born from 1928-1973 could be explained by improvements in their socioeconomic status, access to hospital-based treatment, and cervical cancer screening program [15]. Furthermore, the cohort effect that we observed was similar to that reported in Mumbai [16]. In this context, lower socioeconomic status is highly associated with the risk of HPV infection, which is a major risk factor for cervical cancer [17]. Thus, the economic growth in Korea may have resulted in improved access to hospital-based treatments and greater coverage for the cervical cancer screening program. However, the risk ratio for cervical cancer incidence and mortality exhibited an increasing trend among younger cohorts. There are some possible explanations for the increased cervical cancer incidence and mortality among young women in Korea. Specifically, sexual behavior in younger cohorts has changed compared with older cohorts and the age at first intercourse has become earlier [3]. These changes in sexual behavior may have led to the increased prevalence of HPV infection in more recent Korean birth cohorts [4]. Additionally, there have been changes in the nature of cervical cancer in young women. For example, in situ adenocarcinoma or adenocarcinoma has increased among young women in western countries [18-20]. In Korea, adenocarcinoma increased from 1993 to 2001 among young women aged 20-29 years old [1]. Adenocarcinoma is usually located in the cervical canal; therefore, it was relatively hard to detect in cervical cancer screening compared to squamous cell carcinoma [21]. Indeed, it was reported that the proportion of the lymph node metastasis and tumor size have increased among young women aged 22]. Finally, young women have a relatively low screening rate for cervical cancer and this rate showed slight decreasing trends among women aged 30-39 years old [23]. Taken together, these factors have likely contributed to the rise in incidence and mortality of cervical cancer among young women in Korea. 2. Period effect Cervical cancer screening programs are effective and powerful tools that can reduce the risk of cervical cancer and prevent cervical cancer-related deaths. For example, the implementation of cervical cancer screening programs has decreased the incidence and mortality rate of cervical cancer in Europe and North America [14,24], as well as in Asia [25,26]. Based on the age-period-cohort analysis results, period effects imply that this screening has beneficial effects [14]. However, using period effects as a surrogate marker for the screening effects assumes that the cervical cancer screening was implemented at the same time in all age groups. Although the Korea screening program was implemented in 1996, not all age groups received free access to the program at the same time [1]. In addition, the participation rate varies according to age group [6,23]. Thus, differences in the participation rates may have resulted in the cohorts affecting the cervical cancer incidence and mortality rather than the periods, which were considered a surrogate marker for the screening effects [14]. 3. Strengths and limitations Our results were internally consistent with the incidence and mortality rate of cervical cancer. In addition, our study population represents the entire population of Korea and our results are biologically plausible based on the results from other studies [16,27]. Nevertheless, this study has several limitations that warrant consideration. First, the completeness of the incidence data from before 1999 may be lower than that from after 1999, and this discrepancy may have introduced bias into our estimated trends and age-period-cohort effects. However, the incidence data from after 1993 were collected by the Gynecologic Oncology Committee of Korea, which launched a gynecological cancer registry in 1991, and it is likely that there were very few discrepancies between the data from 1993-1998 and the data from 1999-2012. Second, the early mortality data are less reliable, and the validity of the cervical cancer-related mortality data would be expected to increase over time. Therefore, we adjusted these data by matching the mortality data with the cancer registry data using a redistribution algorithm. Nevertheless, it is possible that residual errors were included in the cervical cancer-related mortality data, despite the use of the redistribution algorithm. Third, age-period-cohort models always have an internal limitation that is related to identifiability. However, the intrinsic estimator method is a variant of the principal components regression method that provides an unbiased estimation and has a smaller variance than constrained models [28]. ConclusionThe present study revealed that the incidence and mortality rate of cervical cancer in Korea have decreased since 1993. This recent decrease in the incidence and mortality rate of cervical cancer may be due to decreases in the period and cohort effects, which likely reflect the implementation of a Korean cancer screening program and changes in lifestyle. However, the risks of cervical cancer incidence and mortality tended to increase in the younger cohorts. These findings suggest that a more effective cervical cancer prevention program is required for younger Korean women, such as HPV vaccination, which may be a useful tool for preventing cervical cancer among this population.
Conflicts of InterestConflict of interest relevant to this article was not reported.
AcknowledgmentsThis work was supported by a research grant from the National Cancer Center, Republic of Korea (No. 1610201-1).
Fig. 1.Incidence rates of cervical cancer. (A) The age-specific incidences rates of cervical cancer according to time period; each line connects the age-specific incidence for a 5-year period. (B) The birth cohort–specific incidence rates of cervical cancer according to age group; each line connects the birth cohort–specific incidence for a 5-year age group.
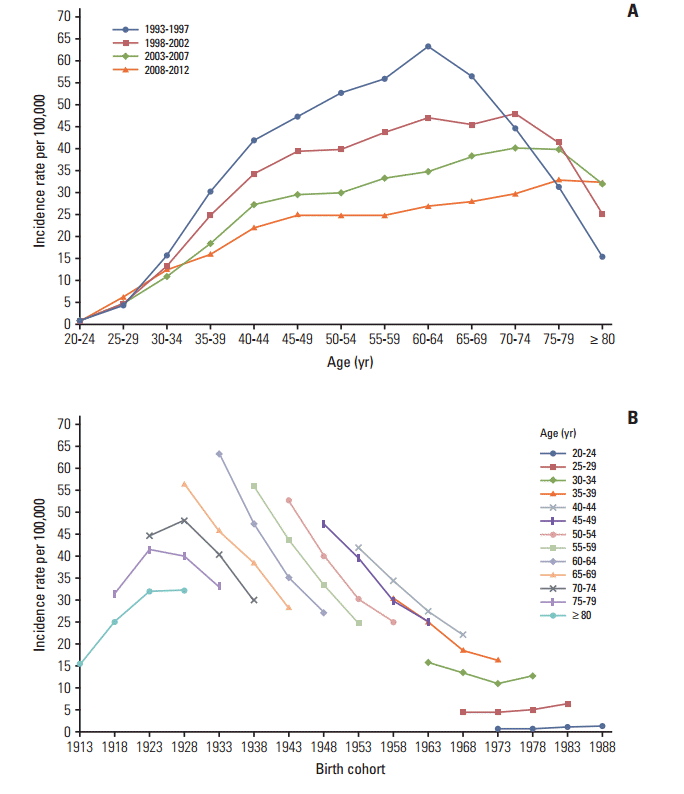 Fig. 2.
Fig. 2.
Mortality rates for cervical cancer. (A) The age-specific mortality rates of cervical cancer according to time period; each line connects the age-specific mortality rate for a 5-year period. (B) The birth cohort–specific mortality rates for cervical cancer according to age group; each line connects the birth cohort–specific mortality rate for a 5-year age group.
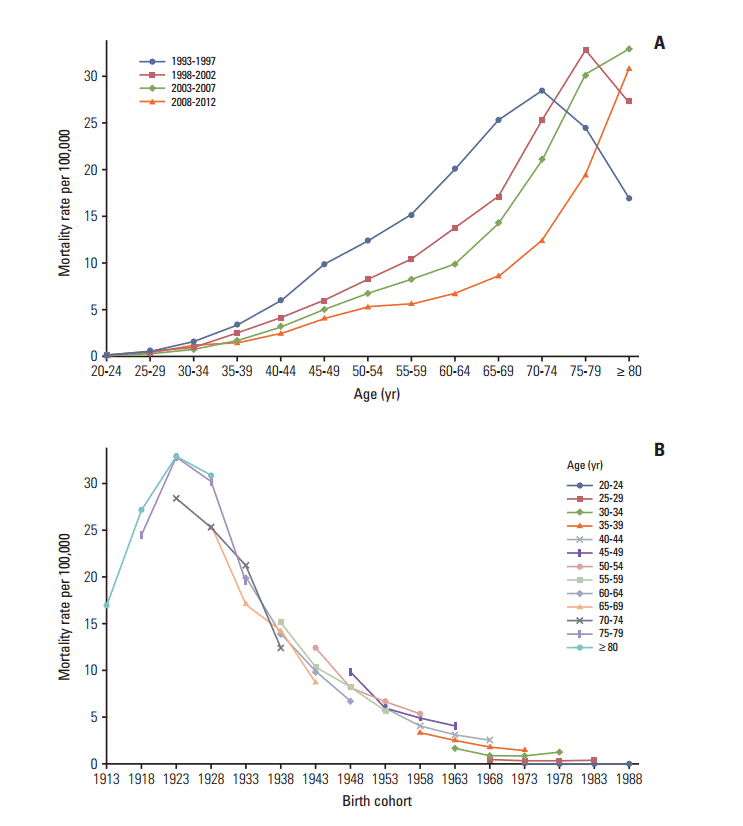 Fig. 3.
Fig. 3.
Age-period-cohort analysis of cervical cancer. (A) The incidences of cervical cancer in Korea. The blue line indicates the age effect and 95% confidence interval, the red line indicates the period effect and 95% confidence interval, and the green line indicates the cohort effect and corresponding 95% confidence interval. (B) The mortality rates for cervical cancer in Korea. The blue line indicates the age effect and 95% confidence interval, the red line indicates the period effect and 95% confidence interval, and the green line indicates the cohort effect and corresponding 95% confidence interval.
 Table 1.
Table 1.
Joinpoint regression analysis of trends in the incidence and mortality rate of cervical cancer
Category ASR APC (95% CI) 1993 2012 Incidence 32.8 15.9 –3.9 (–4.2 to –3.6)* Mortality 5.2 2.1 –4.8 (–5.1 to –4.4)* Table 2.Goodness of fit for the age-period-cohort models of cervical cancer incidence and mortality in Korea
Model Incidence Mortality df Log-likelihood AIC p-valuea) df Log-likelihood AIC p-valuea) Age 39 –2,888.1 111.6 < 0.01 39 –1,294.2 50.3 < 0.01 Period 48 –20,333.4 782.2 < 0.01 48 –13,631.6 524.4 < 0.01 Cohort 36 –4,478.8 172.9 < 0.01 36 –650.0 25.6 < 0.01 Age+period 36 –927.1 36.3 < 0.01 36 –608.0 24.0 < 0.01 Age+cohort 24 –257.8 11.0 0.02 24 –224.2 9.7 < 0.01 Period+cohort 33 –4,407.7 170.3 < 0.01 33 –612.8 24.3 < 0.01 Age+period+cohort (intrinsic estimator) 22 –253.9 10.9 22 –218.2 9.5 References 1. Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Jun JK, et al. Trends in the incidence of in situ and invasive cervical cancer by age group and histological type in Korea from 1993 to 2009. PLoS One. 2013;8:e72012








 Share:
Share:  METRICS
METRICS 
留言 (0)