Idiopathic Sudden Sensorineural Hearing Loss With Minimal Hearing Impairment
Abstract ObjectivesThe aim of the study was to determine the characteristics of patients who did not match the audiometric criteria of idiopathic sudden sensorineural hearing loss (SSNHL) but complained of acute hearing loss.
MethodsBy thorough medical chart reviews, historical cohort study was performed with consecutive data of 589 patients complaining of acute unilateral sensorineural hearing loss without identifiable causes between 2005 and 2013. Those patients demonstrating a hearing loss of at least 30 dB at three consecutive frequencies based on pure tone audiometry were classified as group I; the others were classified as group II. Patients' characteristics, final hearing, and hearing improvement rate (HIR) between the two groups were compared.
ResultsGroup II exhibited distinctive characteristics, including an early age of onset of the hearing loss (P<0.01), an absence of accompanying diabetes (P<0.01) and hypertension (P<0.01), and better unaffected hearing and final hearing compared with group I (P<0.001). However, the HIR of the patients in the two groups was not significantly different (P>0.05).
ConclusionPatients who did not meet the audiological criteria of SSNHL exhibited distinctive characteristics compared to SSNHL patients.
Keywords: Sudden Hearing Loss; Prognosis; Audiometry
INTRODUCTION Sudden hearing loss (SHL) is defined as a subjective hearing impairment in one or both ears that occurs with rapid onset over 3 days [12]. If the hearing loss is sensorineural in nature and greater than 30 dB over three sequential frequencies, it is referred to as sudden sensorineural hearing loss (SSHL). SSHL with no cause despite a thorough investigation is defined as idiopathic sudden sensorineural hearing loss (SSNHL) [12]. In contrast, patients who complained of SSNHL do not always meet the strict audiometric criteria and who occasionally have a smaller degree of hearing loss are included in SSNHL studies [3]. To our knowledge, there have been few studies concerning these patients, who complain of acute hearing loss, but do not meet the typical audiometric criteria of SSNHL.In this study, we aimed to compare SSNHL patients with those who did not meet the audiometric criteria to determine the difference of characteristics.
MATERIALS AND METHODS PatientsMedical records of patients who were diagnosed with unilateral SSNHL from 2005 to 2013 were retrospectively reviewed. All patients had undergone serological, audiological, and magnetic resonance imaging of the internal auditory canal to exclude other diseases. The age, sex, presence of diabetes or hypertension, presence of tinnitus and/or dizziness, and the interval between onset and treatment were documented. The criteria for exclusion were patients who are under 18 years, have concomitant meningitis, myelitis, vasculopathy, or neuropsychiatric disease, have a previous history or recurrence of SHL, have fluctuating symptoms including tinnitus, ear fullness, dizziness during study periods in order to exclude the possibility of Meniere's disease or another autoimmune disease, and patients who could not be observed for at least 3 months. The Institutional Review Board of Eulji University Hospital approved this study.
ClassificationWe classified the patients into two groups according to audiometric criteria: patients who demonstrated more than 30 dB of hearing loss in three sequential frequencies by initial pure tone audiometry were classified into group I, and the remainder of the patients who had an initial hearing loss below 30 dB and/or demonstrated more than 30 dB of hearing loss only in limited frequencies were included in group II.
TreatmentAll of the patients were hospitalized for 1 week, and oral methylprednisolone was used for 14 days (48 mg/day for 4 days, followed by a taper by 8 mg every 2 days). Moreover, for those patients who needed additional cointerventions, a continuous infusion of 10 µg per day of alprostadil for 7 days or an intravenous infusion of 88 mg of zinc sulfate hydrate supplementation daily for 7 days was applied.
Follow-up period and assessment of the hearing improvement rate Pure tone audiometry was performed during the initial visit and 3 months after the treatment was completed. The arithmetic mean of the hearing levels at 500, 1,000, 2,000, and 4,000 Hz was calculated for pure tone audiometry. Hearing improvement rates (HIRs) were calculated as the hearing gain divided by the initial hearing difference between the lesion side and the healthy side multiplied by 100 [4]. Audiogram patterns Initial audiogram patterns were classified into eight groups, including low-tone loss, mid tone loss, high tone loss, low-to-mid tone loss, mid-to-high tone loss, flat loss, total loss, and unclassified type. For this purpose, we used the previously reported seven pattern classifications for audiometric patterns [5]. In addition, we added an unclassified type for the patients who could not be classified among the other groups. Statistical analysisStudent t-test was performed to compare the differences between the quantitative independent values for the two groups. A paired t-test was utilized to compare the differences in values before and after treatment. Chi-square analysis was performed to compare different nominal scales. All of the statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA) with statistical significance set at a P-value of <0.05.
RESULTS A total of 589 patients who complained of unilateral SHL were enrolled during the study period (Table 1). Four hundred twenty-eight patients, including 206 men (48.1%) and 222 women (51.9%), with a mean age of 49 years (range, 18 to 87 years) and mean days from onset to treatment of 3.44±2.62 days, belonged to the group I. Of the remaining patients, 161 patients comprising 65 men (40.4%) and 96 female (59.6%), aged between 18 and 80 years (mean age, 39 years), with a mean days from onset to treatment of 3.93±3.18 were classified in the group II.The mean ages of the patients in group I (48.78±14.77 years) and group II (39.06±12.22 years) were significantly different (P<0.01). Of the group I patients, 55 (12.9%) had diabetes and 100 (23.4%) had hypertension. On the other hand, 5 (3.1%) in group II had diabetes, and 17 (10.6%) had hypertension. These prevalence rates were significantly lower than those of group I (P<0.01).
With respect to the accompanying symptoms, 70.8% of the patients in group II had tinnitus, and 29.8% had dizziness. On the other hand, 68.5% of the patients in group I had tinnitus, and 22.9% had dizziness. There was no significant difference between the two groups.
A significant improvement in hearing at all of the tested frequencies was noted in both of the groups after treatment when we compared the pretreatment and posttreatment hearing levels (Fig. 1). Patients in group II showed better initial and final hearing compared with group I (PP=0.65). The initial audiogram patterns showed a difference according to group (PTable 2). In group I, the flat type (43.7%) was the most common, followed by low-to-mid tone hearing loss (21.5%), mid-to-high tone hearing loss (18.5%), and total hearing loss (11.2%). On the contrary, unclassified type (36.0%) was most commonly found in group II, followed by low-to-mid tone hearing loss (23.0%), high-tone hearing loss (19.3%), and low-tone hearing loss (13.0%). DISCUSSIONWe found clear evidence of differences between the characteristics of patients with SSNHL and those with acute hearing loss who do not meet the audiological criteria for SSNHL. The latter type of patients exhibited a tendency to be younger, lack diabetes and hypertension, have better initial and final hearing, and demonstrate different audiogram patterns, compared to SSNHL patients.
After a comprehensive review of recent studies, we found that vastly different audiometric inclusion criteria for SSNHL have been applied (Table 3) [6789101112]. We selected one of the most commonly used audiometric criteria, which measures a hearing loss of greater than 30 dB at three frequencies. Patients who do not meet these criteria tended to be discarded from most studies without any in-depth consideration. However, we found that these patients have their own distinctive characteristics that can be distinguished from typical SSNHL. First, the patients in group II may have corresponded to SSNHL patents with minimal hearing impairment. SSNHL occurs most frequently between the fifth and sixth decades and displays no gender bias. Accompanying tinnitus (41%-90%) and dizziness (29%-56%) have commonly been reported. Hypotheses regarding the pathogenesis of SSNHL include vascular compromise, cochlear membrane rupture, viral infection, and autoimmunity [13]. Although better initial and final hearing was shown in group II, HIR did not differ between the two groups (Table 1). This result might have arisen from our equivalent treatment of both types of patients. This approach was based on the assumption that both groups contained the same disease entity; if different treatment methods had been used, the results might have varied. Taken together, we assumed that young patients without underlying diseases that can cause microcirculation disorders, including diabetes and hypertension, could have demonstrated minimally affected SSNHL for a final classification into group II. Second, group II patients may be associated with acute low-tone hearing loss without vertigo (ALTHL). The generally used audiometric definition of ALTHL is that the sum of the hearing levels at 125, 250, and 500 Hz should be greater than 70 dB and that the sum of the hearing levels at 2, 4, and 8 kHz, 60 dB or less [14]. Fushiki et al. [14] reported that the mean age of patients with ALTHL was 37.8 years, which is younger than that of patients with low-tone SSNHL not belonging to the ALTHL group. Consistent with this finding, a younger age was associated with group II in this study (Table 1). In addition, aside from unclassified audiogram patterns, the low-to-mid tone hearing loss type was the most common in group II, suggesting that these ALTHL patients may be included in group II. However, a slightly higher prevalence of dizziness was distinctive in group II and differed from the characteristics of the ALTHL patients. Another possible diagnosis is Meniere disease. Although we excluded patients with Meniere disease at the beginning of this study and who had fluctuating symptoms during study periods, it is actually one of the most common diseases associated with such inner ear symptoms as hearing loss, tinnitus, ear fullness, and dizziness. Therefore, group II patients might be partially overlapped with early hydrops. Meniere disease is noted for the preponderance of females and accompanying dizziness, similar to the patients in this study [15]. However, Meniere disease manifests most frequently between the fourth and seventh decade of life, with a prevalence known to increase with age [16]. Therefore, the younger age of the affected members in group II may be distinguished as characteristic of Meniere disease.Next, it is possible that the patients in group II could have been affected by a specific novel disease entity due to their condition's distinguishing characteristics. However, we only performed a retrospective analysis based on audiological criteria; thus, a prospective study including more precise audio-vestibular tests and stricter inclusion/exclusion criteria will be helpful to confirm the possibility that these patients were afflicted by a separate disease entity.
For audiometric patterns, seven-pattern classification introduced by Chang et al. [5] that we used in this study had been designed to distinguish patients with mid frequency hearing loss from those with low-tone or high-tone hearing loss in traditional Sheehy classification, which consisted of low-tone loss, high-tone loss, flat type hearing loss, and total hearing loss [517]. Seven-pattern classification holds advantage over Sheehy classification in terms of a detailed analysis, however, is not yet widely used because of complexity.In conclusion, patients who do not meet the audiological criteria of SSNHL but do qualify for SSHL exhibited distinctive characteristics, including a predominance of a younger age, absence of diabetes and hypertension, better initial and final hearing, and different audiogram patterns, compared to SSNHL patients.
CONFLICT OF INTERESTCONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References 1. Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012 3;146(3 Suppl):S1–S35. PMID: 22383545.Pretreatment and posttreatment hearing levels. (A) Group I. (B) Group II. Group I, ≥30 dB of hearing loss in three sequential frequencies; Group II, <30 dB and/or ≥30 dB of hearing loss only in limited frequencies.
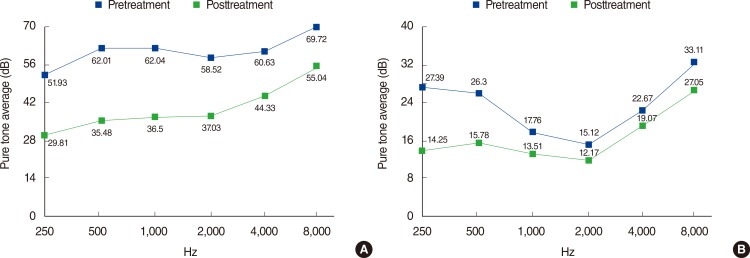 Table 1.
Table 1.
Baseline clinical characteristics
Characteristic Total population P-value Group I (n=428) Group II (n=161) Age (year) 48.78±14.77 39.06±12.22 <0.01 Male sex 206 (48.1) 65 (40.4) 0.09 Diabetes mellitus 55 (12.9) 5 (3.1) <0.01 Hypertension 100 (23.4) 17 (10.6) 0.01 Days from onset to treatment 3.44±2.62 3.93±3.18 0.83 Right side 219 (51.2) 96 (59.6) 0.07 Dizziness 98 (22.9) 48 (29.8) 0.08 Tinnitus 293 (68.5) 114 (70.8) 0.85 Intratympanic injection 5 (1.2) 4 (2.5) 0.75 Alprostadil injection 348 (81.3) 143 (88.8) 0.03 Zinc injection 32 (7.5) 99 (61.5) 0.42 Initial hearing (dB) 60.80±22.26 20.47±7.49 <0.01 Initial contralateral hearing (dB) 25.85±21.11 16.53±18.18 <0.01 Final hearing (dB) 38.33±25.55 15.13±12.05 <0.01 HIR (%) 72.17±252.08 65.56±145.65 0.76 Table 2.Patterns of initial hearing loss
Audiogram pattern Frequency ears, n (%) Group I (n=428) Group II (n=161) Low-tone loss 9 (2.1) 21 (13.0) Mid-tone loss 1 (0.2) 4 (2.5) High-tone loss 6 (1.4) 31 (19.3) Low-to-mid tone loss 92 (21.5) 37 (23.0) Mid-to-high tone loss 79 (18.5) 7 (4.3) Flat loss 187 (43.7) 3 (1.9) Total loss 48 (11.2) 0 Unclassified 6 (1.4) 58 (36.0) Table 3.Summary of the criteria used in recent studies
Source Audiometric criteria Age (year) Days to treatment Affected ear Unaffected ear Sano et al. [6] (2013) None Average (500–2,000 Hz) ≤30 dB ≥20 ≤30 Ciccone et al. [7] (2012) Typical* None - - Stachler et al. [1] (2012) Typical None - - Kim et al. [8] (2012) Typical None ≥15 ≤7 Suzuki et al. [4, 9] (2011, 2012) Average (250–4,000 Hz) ≥40 dB None All ≤30 Wu et al. [10] (2011) Typical Better ear ≤30 dB ≥18 ≤7 Arslan et al. [11] (2011) ≥20 dB in 3 consecutive frequencies None ≥18 ≤30 Hikita-Watanabe et al. [18] (2010) Typical None All ≤7 Hiraumi et al. [12] (2010) Average (250–4,000 Hz) ≥40 dB None ≥18 ≤14 TOOLS








 Share:
Share:  METRICS
METRICS 
留言 (0)