What once seemed like science fiction is now becoming reality in health care. Artificial intelligence (AI) is a fast-moving technology that enables machines to perform tasks previously exclusive to humans.1 Advances in AI offer a glimpse of such health care benefits as decreasing postoperative complications, increasing quality of life, improving decision-making and decreasing the number of unnecessary procedures.2 When applied to the fields of medicine and dentistry, AI can play a crucial role in improving diagnosis accuracy and revolutionizing care. AI is currently used for a variety of purposes in dentistry: identification of normal and abnormal structures, diagnosis of diseases and prediction of treatment outcomes. Furthermore, AI is used extensively in dental laboratories and is playing a growing role in dental education. The following review describes current and future applications of AI in the clinical practice of dentistry.
What Is Artificial Intelligence?AI is a branch of computer science that aims to understand and build intelligent entities, often instantiated as software programs.3 It can be defined as a sequence of operations designed to perform a specific task.4 Historically, artificially intelligent systems applied hand-crafted rules to the specific tasks they were meant to solve. Each task required domain-specific knowledge, engineering and manual fine-tuning of the system by subject-matter experts. For instance, a system designed to detect lesions in medical imaging might look for abnormally coloured lumps of a given shape. The fine-tunable parts of the system might be a range of healthy tissue colours or minimum lengths and widths for a potential lump. Nowadays, medicine most commonly uses a branch of AI called machine learning5 and, more recently, deep learning.6
Machine learning (ML) is a branch of AI in which systems learn to perform intelligent tasks without a priori knowledge or hand-crafted rules. Instead, the systems identify patterns in examples from a large dataset, without human assistance. This is accomplished by defining an objective and optimizing the system’s tunable functions to reach it. In this process, known as training, an ML algorithm gains experience through exposure to random examples and gradual adjustments of the “tunables” toward the correct answer. As a result, the algorithm identifies patterns that it can then apply to new images. This technique is analogous to an adult showing several photos of cats to a child. The child eventually learns the patterns involved in recognizing a cat and identifying one in new images.
Deep learning (DL) is a sub-branch of ML wherein systems attempt to learn, not only a pattern, but also a hierarchy of composable patterns that build on each other. The combination and stacking of patterns create a “deep” system far more powerful than a plain, “shallow” one. For instance, a child does not recognize a cat in a single, indivisible step of pattern-matching; rather, the child first sees the edges of the object, a particular grouping of which defines a textured outline with simple shapes, such as eyes and ears. Among these components, larger groups such as heads and legs arise, and a particular grouping of these defines the whole cat.
An extremely popular class of DL algorithms is the artificial neural network (ANN), a structure composed of many small communicating units called neurons organized in layers. A neural network is composed of an input layer, an output layer and hidden layers in between.7 It is possible to have 1 or a few hidden layers (shallow neural network) or multiple/many hidden layers (deep neural network, DNN) (Figure 1, a and b). These layers are called hidden because their values are not pre-specified or visible to the outside. Their aim is to make it possible to build hierarchically on information retrieved from the visible input layer to compute the correct value of the visible output layer. The pattern of connections between neurons defines the particular neural network’s architecture, and the fine-tunable strengths of those connections are called the weights of the neural network.
In medicine and dentistry, one of the most commonly used subclasses of ANN is the convolutional neural network (CNN) (Figure 1c). A CNN uses a special neuron connection architecture and the mathematical operation, convolution, to process digital signals such as sound, image and video. CNNs use a sliding window to scan a small neighbourhood of inputs at a time, from left to right and top to bottom, to analyze a wider image or signal. They are extremely well adapted to the task of image classification and are the most-used algorithm for image recognition.7
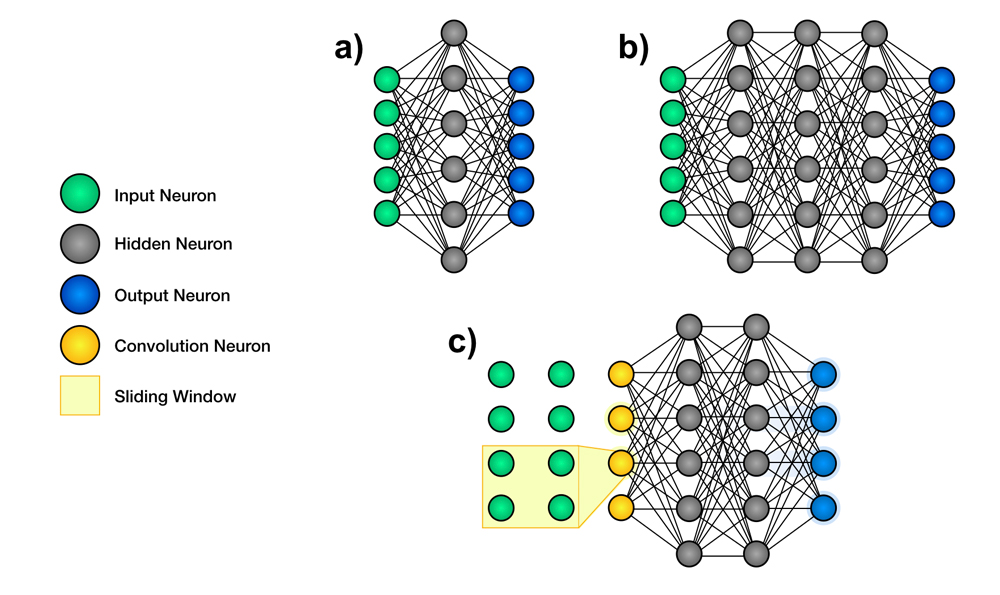
Figure 1: Schematic representation of the architecture of neural networks. Artificial neural networks are structures used in machine learning. They contain many small communicating units called neurons, which are organized in layers. a. Shallow neural networks are composed of an input layer, a few hidden layers and an output layer. b. Deep neural networks have an input layer, multiple hidden layers and an output layer. c. Convolutional neural networks use filters to scan a small neighbourhood of inputs.
Clinical Application of AI in DentistryRadiology
CNNs have shown promising ability to detect and identify anatomical structures. For example, some have been trained to identify and label teeth from periapical radiographs. CNNs have demonstrated a precision rate of 95.8–99.45% in detecting and identifying teeth, almost rivaling the work of clinical experts, whose precision rate was 99.98%.8,9
CNNs have also been used for the detection and diagnosis of dental caries.10 In 3000 periapical radiographs of posterior teeth, a deep CNN algorithm was able to detect carious lesions with an accuracy of 75.5–93.3% and a sensitivity of 74.5–97.1%. This is a considerable improvement over diagnosis by clinicians using radiographs alone, with sensitivity varying from 19% to 94%.11 Deep CNNs have great potential for improving the sensitivity of dental caries diagnosis and this, combined with their speed, makes them one of the most efficient tools used in this domain.
Orthodontics
ANNs have immense potential to aid in the clinical decision-making process. In orthodontic treatments, it is essential to plan treatments carefully to achieve predictable outcomes for patients. However, it is not uncommon to see teeth extractions included in the orthodontic treatment plan. Therefore, it is essential to ensure that the best clinical decision is made before initiating irreversible procedures. An ANN was used to help determine the need for tooth extraction before orthodontic therapy in patients with malocclusion.12,13 The four constructed ANNs, taking into consideration several clinical indices, showed an accuracy of 80–93% in determining whether extractions were needed to treat patients’ malocclusions.12,13
Periodontics
According to the 1999 American Academy of Periodontology classification of periodontal disease, 2 clinical types of periodontitis are recognized: aggressive (AgP) and chronic (CP) forms.14 Because of the complex pathogenesis of the disease, no single clinical, microbiological, histopathological or genetic test or combination of them can discriminate AgP from CP patients.15 Papantanopoulos and colleagues16 used an ANN to distinguish between AgP and CP in patients by using immunologic parameters, such as leukocytes, interleukins and IgG antibody titers. The one ANN was 90–98% accurate in classifying patients as AgP or CP. The best overall prediction was made by an ANN that included monocyte, eosinophil, neutrophil counts and CD4+/CD8+ T-cell ratio as inputs. The study concluded that ANNs can be employed for accurate diagnosis of AgP or CP using relatively simple and conveniently obtained parameters, such as leukocyte counts in peripheral blood.
Various non-surgical and surgical methods have been devised for the treatment of periodontally compromised teeth (PCT) and supporting structures.17 Despite advances in treatment modalities, no significant improvement has been made in the method for diagnosing and predicting the prognosis of PCT. Clinical diagnostic and prognostic judgement depends heavily on empirical evidence.18 Lee and coworkers19 evaluated the potential utility and accuracy of deep CNN algorithms for diagnosing and predicting PCT. Using the CNN algorithm, the accuracy of PCT diagnosis proved to be 76.7–81.0%, while the accuracy of predicting the need for extraction was 73.4–82.8%. The noted difference in accuracy seemed to occur between different types of teeth, with premolars more accurately diagnosed as PCTs than molars (accuracies were 82.8% and 73.4%, respectively). This could be explained by the fact that premolars normally have a single root, whereas molars have 2 or 3 roots, thus exhibiting a more complex anatomy for a CNN to interpret.
Endodontics
Although mandibular molars tend to have similar root canal configurations, several atypical variations may occur.20 To minimize treatment failures related to morphological differences and to optimize the clinical outcomes of endodontic therapy, cone-beam computed tomography (CBCT) has become the gold standard. However, because of its higher dose of radiation compared with conventional radiographs,21 CBCT is not used systematically. To overcome such challenges, AI has been introduced to classify the given data using a CNN22 to determine whether the distal root of the first mandibular molar has 1 or more extra canals. Radiographs of 760 mandibular first molars taken with dental CBCT were analyzed. Once the presence or absence of the atypia was determined, image patches of the roots obtained from corresponding panoramic radiographs were processed by a deep-learning algorithm to classify morphology.
Although the CNN had a relatively high accuracy of 86.9%,20 several limitations exist regarding its clinical integration. The images must be segmented manually,23 which consumes a considerable amount of time. Furthermore, the obtained images must be of adequate size and should focus on a small region to allow the system to concentrate on the object being studied, while covering enough area to include pertinent information.24
Oral Pathology
Detection and diagnosis of oral lesions is of crucial importance in dental practices because early detection significantly improves prognosis. As some oral lesions can be precancerous or cancerous in nature, it is important to make an accurate diagnosis and prescribe appropriate treatment of the patient. CNN has been shown to be a promising aid throughout the process of diagnosis of head and neck cancer lesions. With specificity and accuracy at 78–81.8% and 80–83.3%, respectively (compared with those of specialists, which were 83.2% and 82.9% respectively), CNN shows great potential for detecting tumoural tissues in tissue samples or on radiographs.25,26
One study used a CNN algorithm to distinguish between 2 important maxillary tumours with similar radiologic appearance but different clinical properties: ameloblastomas and keratocystic odontogenic tumours.26 The specificity and the accuracy of diagnosis by the algorithm were 81.8% and 83.3%, respectively, comparable with those of clinical specialists at 81.1% and 83.2%. However, a more significant difference was observed in terms of diagnostic time: specialists took an average of 23.1 minutes to reach a diagnosis, while the CNN achieved similar results in 38 s.26
Challenges of AIThe management and sharing of clinical data are major challenges in the implementation of AI systems in health care. Personal data from patients are necessary for initial training of AI algorithms, as well as ongoing training, validation and improvement. Furthermore, the development of AI will prompt data sharing among different institutions and, in some cases, across national boundaries. To integrate AI into clinical operations, systems must be adapted to protect patient confidentiality and privacy.27 Thus, before considering broader distribution, personal data will have to be anonymized.28 Even with the ability to take these precautions, there is skepticism in the health care community about secure data sharing.
AI systems are also associated with safety issues. Mechanisms must be created to control the quality of the algorithms used in AI. To remedy this situation, the United States Food and Drug Administration has created a new drug category, “Software as Medical Device,” through which it regulates safe innovation and patient safety.29 Ambiguous accountability in the use of AI systems is another concern. Who will be held responsible for a patient who faces unintentional consequences resulting from an error or adverse event caused by the AI technology? Is it the professional’s fault, or is it the fault of the developer who built the algorithm? Given that our legal system is based on the fundamental assumption that fault and crime are ultimately attributable to humans, substituting humans with autonomous agents raises numerous questions of legal and ethical order. These issues will continue to represent a considerable challenge to our legal system for the foreseeable future.
Finally, the transparency of AI algorithms and data is a substantial issue. The quality of predictions performed by AI systems relies heavily on the accuracy of annotations and labeling of the dataset used in training. Poorly labeled data can lead to poor results.30 Clinic-labeled datasets may be of inconsistent quality, thus limiting the efficacy of the resultant AI systems. Furthermore, health care professionals should possess a full understanding of the decisions and predictions made by an AI system, as well as the capability to defend them.31 Interpretability of AI technology is a known problem, and major advances are required before certain classes of algorithms, such as neural networks, can make clinical diagnoses or treatment recommendations with full transparency.29
ConclusionAlthough multiple studies have shown potential applications of AI in dentistry, these systems are far from being able to replace dental professionals. Rather, the use of AI should be viewed as a complementary asset, to assist dentists and specialists. It is crucial to ensure that AI is integrated in a safe and controlled manner to assure that humans retain the ability to direct treatment and make informed decisions in dentistry.
The road to successful integration of AI into dentistry will necessitate training in dental and continuing education, a challenge that most institutions are not currently prepared for. In addition, AI plays a critical role in virtual reality (VR) and augmented reality (AR). A new term, mixed reality, incorporates aspects of generative AI, VR and AR into computer-superimposed information overlays to enhance learning and surgical planning.32 As various AI systems for diverse dental disciplines are being developed and have produced encouraging preliminary results, a future for AI in the health care system cannot be discounted. AI systems show promise as a great aid to oral health professionals.
THE AUTHORS

Dr. Nguyen is an Assistant Professor, Faculty of Dentistry, McGill University, Montreal, Quebec.

Ms. Larrivée is a 4th year dental student, Faculty of Dental Medicine, Université de Montréal, Montreal, Quebec.

Ms. Lee is a 4th year dental student, Faculty of Dental Medicine, Université de Montréal, Montreal, Quebec.

Mr. Bilaniuk is a Research Software Developer, Mila – Quebec AI Institute, Montreal, Quebec.

Dr. Durand is an Associate Professor, Faculty of Dental Medicine, Université de Montréal, Montreal, Quebec.
Corresponding author: Dr. Thomas T. Nguyen, Assistant Professor, Division of Periodontics, McGill Faculty of Dentistry, 2001 McGill College Avenue, Montreal, QC, H3A 1G1. Email: thomas.nguyen@mcgill.ca
Acknowledgements: The authors thank Dr. John Syrbu and Dr. Borys Bilaniuk for their expertise and contribution to this review.
The authors have no declared financial interests.
This article has been peer reviewed.
References Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2(10):719-31. Topol EJ. Deep medicine: how artificial intelligence can make healthcare human again. 1st ed. New York: Basic Books; 2019. Russell SJ, Norvig P. Artificial intelligence: a modern approach. 3rd ed. Hoboken, N.J.: Prentice Hall; 2010. Muller J, Massaron L. Artificial intelligence for dummies. Hoboken, N.J.: John Wiley & Sons; 2018. James G, Witten D, Hastie T, Tibshirani R. An introduction to statistical learning with applications in R. New York: Springer; 2013. Goodfellow I, Bengio Y, Courville A. Deep learning. 1st ed. Cambridge, Mass.: MIT Press; 2016. Nielsen MA. Neural networks and deep learning. Determination Press; 2015. Available: http://neuralnetworksanddeeplearning.com/ (accessed 2021 April 16). Zhang K, Wu J, Chen H, Lyu P. An effective teeth recognition method using label tree with cascade network structure. Comput Med Imaging Graph. 2018;68:61-70. Tuzoff DV, Tuzova LN, Bornstein MM, Krasnov AS, Kharchenko MA, Nikolenko SI, et al. Tooth detection and numbering in panoramic radiographs using convolutional neural networks. Dentomaxillofac Radiol. 2019;48(4):20180051. Lee JH, Kim DH, Jeong SN, Choi SH. Detection and diagnosis of dental caries using a deep learning-based convolutional neural network algorithm. J Dent. 2018;77:106-11. Bader JD, Shugars DA, Bonito AJ. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 2001;65(10):960-8. Xie X, Wang L, Wang A. Artificial neural network modeling for deciding if extractions are necessary prior to orthodontic treatment. Angle Orthod. 2010;80(2):262-6. Jung SK, Kim TW. New approach for the diagnosis of extractions with neural network machine learning. Am J Orthod Dentofacial Orthop. 2016;149(1):127-33. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1-6. Armitage GC. Learned and unlearned concepts in periodontal diagnostics: a 50-year perspective. Periodontol 2000. 2013;62(1):20-36. Papantonopoulos G, Takahashi K, Bountis T, Loos BG. Artificial neural networks for the diagnosis of aggressive periodontitis trained by immunologic parameters. PLoS One. 2014;9(3):e89757. Papantonopoulos G, Takahashi K, Bountis T, Loos BG. Using cellular automata experiments to model periodontitis: a first step towards understanding the nonlinear dynamics of the disease. Int J Bifurcation Chaos. 2013;23(3):1350056. Papantonopoulos G, Takahashi K, Bountis T, Loos BG. Mathematical modeling suggests periodontitis behaves as a non-linear chaotic dynamical process. J Periodontol. 2013;84(10):e29-39. Lee JH, Kim DH, Jegon SN, Choi SH. Diagnosis and prediction of periodontally compromised teeth using a deep learning-based convolutional neural network algorithm. J Periodontal Implant Sci. 2018;48(2):of114-23. Hiraiwa T, Ariji Y, Fukuda M, Kise Y, Nakata K, Katsumata A, et al. A deep-learning artificial intelligence system for assessment of root morphology of the mandibular first molar on panoramic radiography. Dentomaxillofac Radiol. 2019;48(3):20180218. Zhang X, Xiong S, Ma Y, Han T, Chen X, Wan F, et al. A cone-beam computed tomographic study on mandibular first molars in a Chinese subpopulation. PLoS One. 2015;10(8):e0134919. Xue Y, Zhang R, Deng Y, Chen K, Jiang T. A preliminary examination of the diagnostic value of deep learning in hip osteoarthritis. PLoS One. 2017;12(6):e0178992. Wang X, Yang W, Weinreb J, Han J, Li Q, Kong X, et al. Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep. 2017;7(1):15415. Trebeschi S, van Griethuysen JJM, Lambregts DMJ, Lahaye MJ, Parmar C, Bakers FCH, et al. Deep learning for fully-automated localization and segmentation of rectal cancer on multiparametric MR. Sci Rep. 2017;7(1):5301. Halicek M, Lu G, Little JV, Wang X, Patel M, Griffith CC, et al. Deep convolutional neural networks for classifying head and neck cancer using hyperspectral imaging. J Biomed Opt. 2017;22(6):60503. Poedjiastoeti W, Suebnukarn S. Application of convolutional neural network in the diagnosis of jaw tumors. Healthc Inform Res. 2018;24(3):236-41. Char DS, Shah NH, Magnus D. Implementing machine learning in healthcare — addressing ethical challenges. N Eng J Med. 2018;378(11):981-3. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25(1):30-6. Software as medical device (SaMD). Maryland: United States Food & Drug Administration; 2018. Available: https://www.fda.gov/medical-devices/digital-health/software-medical-device-samd (accessed 2019 June 7). Redman TC. If your data is bad, your machine learning tools are useless. Harv Bus Rev. 2018;2 April. Available: https://hbr.org/2018/04/if-your-data-is-bad-your-machine-learning-tools-are-useless (accessed 2019 June 12). Murphy KP. Machine learning: a probabilistic perspective. Cambridge, Mass.: MIT Press, 2012. Ferro AS, Nicholson K, Koka S. Innovative trends in implant dentistry training and education: a narrative review. J Clin Med. 2019;8(10):1618.
留言 (0)