Cholestatic liver disease is a liver and bile system disorder caused by various reasons that result in the obstruction of bile production, secretion, and excretion, leading to the accumulation of bile in the liver and reflux into the blood, causing a series of organic damage, metabolic disorders, and functional disturbances (1, 2). With the advancement of genetic diagnostic technologies in recent years, the detection rate of genetically-related cholestatic liver diseases has been increasing (3). Previous studies have reported mutations in the ZFYVE19 gene associated with cholestatic liver disease in children (4–6), but there have been few reports in adult patients.
Here, we report a case of adult patient who developed liver cirrhosis due to familial cholestasis caused by ZFYVE19 gene mutation.
Case reportA 39-year-old male food delivery worker was referred to our hospital in June 2023 due to abnormal liver function for 11 years. During this period, he has repeatedly been found to have elevated transaminase levels during check-ups at other hospitals. Notably, aside from his parents’ consanguineous marriage, his younger brother died from esophagogastric bleeding caused by suspicious Wilson’s disease, but genetic testing was not carried out. The patient had no history of alcohol abuse or metabolic syndrome, and had not taken any other medications, herbal products, or dietary supplements.
The physical examination of the patient was normal. However, multiple liver function tests before admission showed a significant increase in gamma glutamyl transferase (GGT) levels, and mild increases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) levels, and total bile acids (see Table 1). Further laboratory tests for hepatitis B surface antigen, hepatitis C virus antibody, hepatitis E virus antibody, antinuclear antibody, antibody spectrum, and various autoantibodies (such as anti-smooth muscle autoantibody, antinuclear autoantibody, antimitochondrial autoantibody, and anti-liver kidney microsomal autoantibody) were negative. Because his younger brother died of Wilson’s disease, a serum ceruloplasmin test was performed, which showed a mild decrease. The copper-expelling test did not show abnormalities, and the ATP7B gene test had no pathogenic variations. Additional tests including iron, and ferritin also showed negative results. The upper abdominal magnetic resonance imaging (MRI) showed the presence of cirrhosis, a small amount of ascites, and splenomegaly (Figure 1). However, Upper gastrointestinal endoscopy did not reveal any gastroesophageal varices.
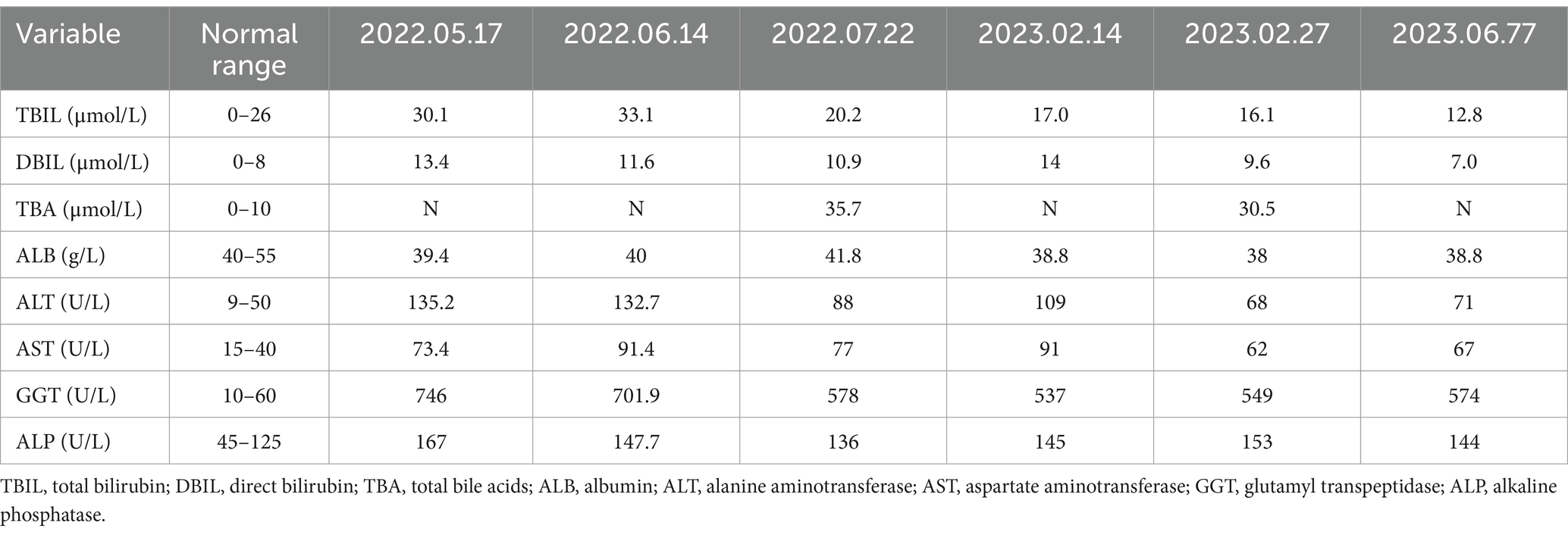
Table 1. The patient’s liver function test results before this hospital admission.
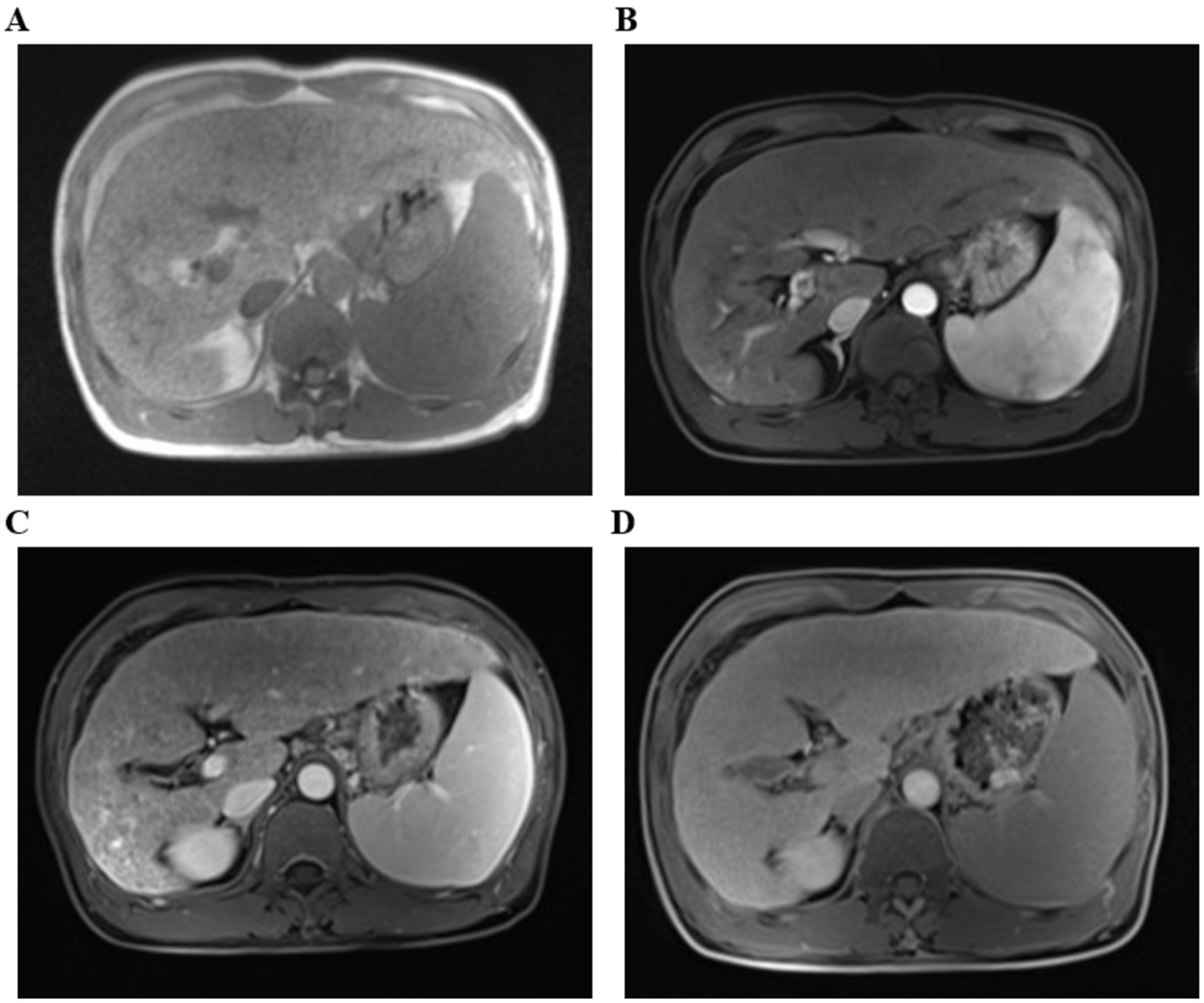
Figure 1. The upper abdominal magnetic resonance imaging (MRI) with T1-weighted sequences. (A) Flat phase. (B) Arterial phase. (C) Portal venous phase. (D) Delayed phase. (The arrow indicates that it suggest liver cirrhosis).
A percutaneous liver biopsy was also performed. Histological findings of liver biopsy specimens suggested liver cirrhosis with mild lobular inflammation, interfacial inflammation, and hepatocyte ballooning degeneration were encountered (Figure 2).
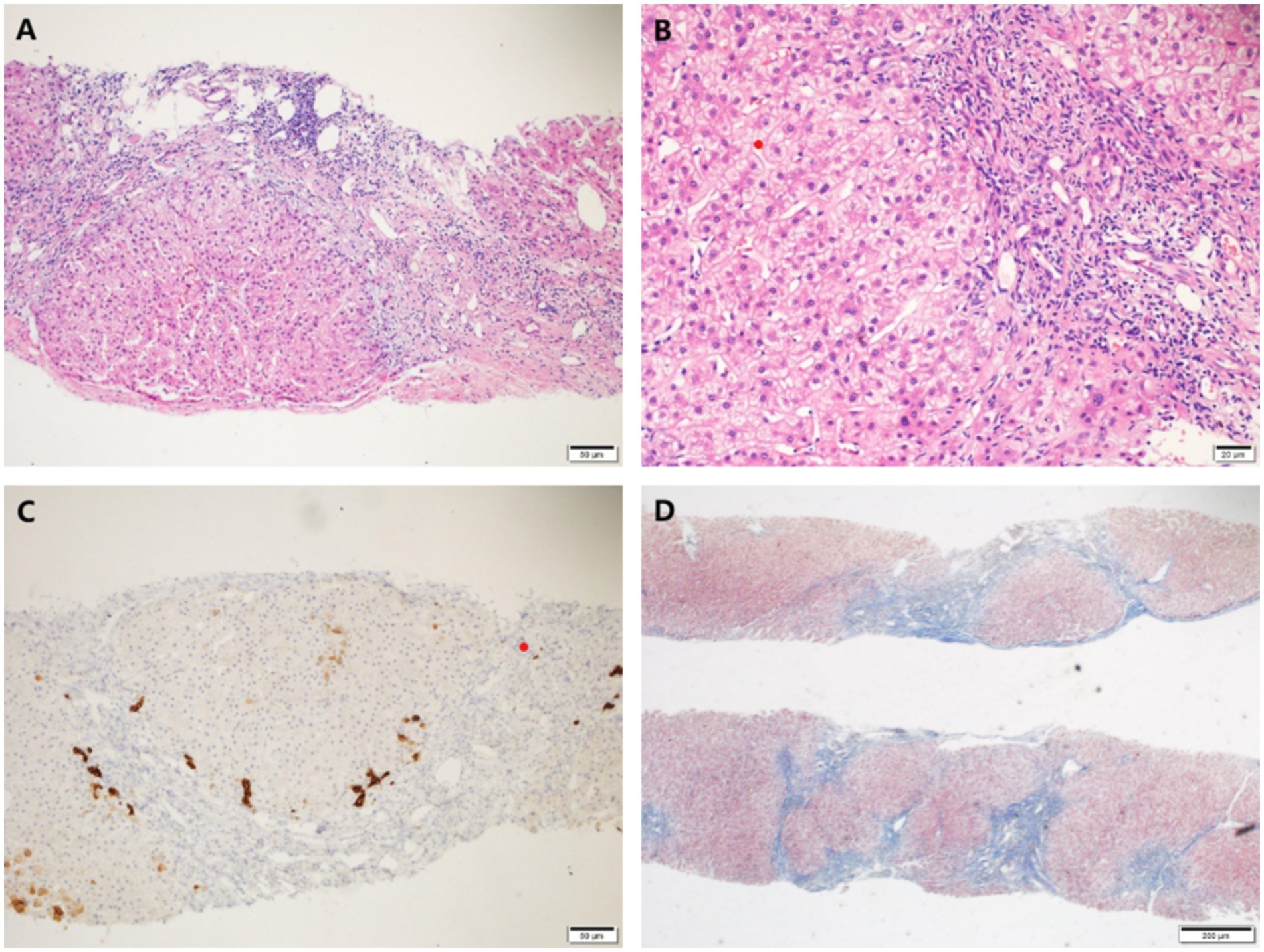
Figure 2. Histological findings of liver biopsy specimens. (A,B) H&E staining showed mild interface inflammation and lobular inflammation, as well as enlarged portal areas with fibrous tissue hyperplasia, formation of pseudo-lobules and ductal fibrosis. (C) CK7 staining showed partial atrophy of bile duct epithelium without loss. (D) Masson staining showed nodular proliferation of liver cells. Original magnification ×100 (A,C,D), ×200 (B).
The cause of liver cirrhosis was still unclear, so genetic testing was performed on both the patient and their family members. Using Sanger technology, the c.314C> site of the ZFYVE19 gene in the patient was verified, revealing a homozygous mutation of ZFYVE19: c.314C>G (p.S105X) (Figure 3). The final diagnosis was liver cirrhosis caused by familial cholestatic liver disease related to ZFYVE19 gene mutation.

Figure 3. Genetic analysis and familial verification of ZFYVE19. The patient (A) carried a homozygous mutation at the c.314C>G site of the ZFYVE19 gene, while his parents (B,C) and son (D) carried heterozygous mutations.
The patient regularly received ursodeoxycholic acid (UDCA, 13-15 mg/kg/day) treatment, and his liver function gradually improved during the four-month follow-up. Liver function test results on October 30, 2023 showed a decrease in GGT levels to 181 U/L, with ALT, AST, and ALP levels approximately normal.
DiscussionThe zinc finger FYVE-type containing 19 (ZFYVE19) gene is located on chromosome 15 (15q15.1), coding for the protein ANCHR (6, 7). According to related studies (7), the ANCHR protein contains a FYVE-type zinc finger structure and two type 1 MIT-interacting motifs (MIMs), which can prevent cell division by binding to vacuolar protein sorting 4 (VPS4). VPS4 plays a key role in the transition from the G2 phase of DNA synthesis to metaphase in the cell division cycle. The pathogenesis of liver disease due to mutations in ZFYVE19 genes remains unknown. Luan et al. (6) and Mandato et al. (4) believe that VPS4 is involved in the occurrence of cilia (8, 9). Abnormal binding of ZFYVE19 to VPS4 during mutations may lead to abnormal chromosome segregation and DNA damage, resulting in disruptions to ciliary regulatory pathways, dysplasia of primary cilia, and malfunction of posterior bile duct cells. The pathology presents as portal vein dysplasia, fibrosis of portal veins, hyperplasia, and malformation of bile duct cells.
The main targets of liver diseases caused by ZFYVE19 gene mutations are located in the portal vein and cholangiocytes, thus, cholestasis and portal hypertension are the main clinical manifestations, such as jaundice, pruritus, acute upper gastrointestinal bleeding, and ascites (4, 6, 10). Previous case reports have predominantly involved children and rarely adults (3, 4, 11). However, this case occurred in an adult male whose parents were cousins, and his younger brother died of esophagogastric bleeding caused by suspicious Wilson’s disease. The reason for his delayed onset might be the variation of the gene site. Subsequent diagnostic tests, including liver function analysis, abdominal CT/MRI, gastroscopy, and liver tissue pathology, failed to confirm the pathogen. The final diagnosis was made through whole exome sequencing (WES), confirming that the patient had developed cirrhosis due to a ZFYVE19 gene mutation-related hereditary cholestatic liver disease.
For the treatment of this disease, ursodeoxycholic acid (UDCA, 15–20 mg/kg/day) can improve cholestasis and thus improve liver function (1, 2, 9). Despite UDCA response in patients, some of them still have undergone liver transplantation (LT) for recurrent upper gastrointestinal bleeding, and no obvious complications have been observed in follow-up. Therefore, liver transplantation is considered an effective therapy (4, 6, 12). Furthermore, odevixibat, an inhibitor of the ileal bile acid transporter, has been proven to be effective in treating pruritus (5). In the case described here, the patient is regularly receiving treatment with UDCA, resulting in a significant decrease in GGT and ALP levels compared to baseline. Luan W et al. have demonstrated that ZFYVE19 can greatly improve liver fibrosis and abnormalities in the biliary system at the optimal expression level using a Zfyve19−/− mouse model with adeno-associated virus vector. Gene therapy is expected to be an effective treatment for this disease (13).
In conclusion, we report a special case of cholestasis-ZFYVE19 gene mutation, which broadened the spectrum of genetically related liver diseases. This also confirms that the use of WES may enhance the diagnostic accuracy in cases of undiagnosed cholestasis.
Data availability statementThe datasets presented in this article are not readily available due to patient privacy concerns. Requests to access the datasets should be directed to the corresponding authors.
Ethics statementThe studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University [No. (2015)084-2]. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from patient’s previous hospitalizations. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributionsM-YX: Writing – original draft, Writing – review & editing. L-LH: Writing – original draft, Writing – review & editing. QZ: Funding acquisition, Supervision, Validation, Writing – review & editing. Y-YZ: Writing – review & editing.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Clinical Research Center for Liver and Intestinal Diseases of Fujian Province (No. 2021Y2006); Natural Science Foundation of Fujian Province, China (No. 2022Y0011).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References1. European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. (2009) 51:237–67. doi: 10.1016/j.jhep.2009.04.009
Crossref Full Text | Google Scholar
2. Chinese Medical Association and Chinese Society of Hepatology. Guideline on the management of cholestasis liver disease. J Clin Hepatol. (2022) 38:62–9. doi: 10.3969/j.issn.1001-5256.2022.01.010
Crossref Full Text | Google Scholar
3. Chen, CB, Hsu, JS, Chen, PL, Wu, JF, Li, HY, Liou, BY, et al. Combining panel-based next-generation sequencing and exome sequencing for genetic liver diseases. J Pediatr. (2023) 258:113408. doi: 10.1016/j.jpeds.2023.113408
Crossref Full Text | Google Scholar
4. Mandato, C, Siano, MA, Nazzaro, L, Gelzo, M, Francalanci, P, Rizzo, F, et al. A ZFYVE19 gene mutation associated with neonatal cholestasis and cilia dysfunction: case report with a novel pathogenic variant. Orphanet J Rare Dis. (2021) 16:179. doi: 10.1186/s13023-021-01775-8
PubMed Abstract | Crossref Full Text | Google Scholar
5. Pepe, A, Colucci, A, Carucci, M, Nazzaro, L, Bucci, C, Ranucci, G, et al. Case report: Add-on treatment with odevixibat in a new subtype of progressive familial intrahepatic cholestasis broadens the therapeutic horizon of genetic cholestasis. Front Pediatr. (2023) 11:1061535. doi: 10.3389/fped.2023.1061535
Crossref Full Text | Google Scholar
6. Luan, W, Hao, C, Li, J, Wei, Q, Gong, J, Qiu, Y, et al. Biallelic loss-of-function ZFYVE19 mutations are associated with congenital hepatic fibrosis, sclerosing cholangiopathy and high-GGT cholestasis. J Med Genet. (2021) 58:514–25. doi: 10.1136/jmedgenet-2019-106706
Crossref Full Text | Google Scholar
7. Thoresen, SB, Campsteijn, C, Vietri, M, Schink, KO, Liestøl, K, Andersen, JS, et al. ANCHR mediates Aurora-B-dependent abscission checkpoint control through retention of VPS4. Nat Cell Biol. (2014) 16:547–57. doi: 10.1038/ncb2959
PubMed Abstract | Crossref Full Text | Google Scholar
9. Xie, S, Wei, S, Ma, X, Wang, R, He, T, Zhang, Z, et al. Genetic alterations and molecular mechanisms underlying hereditary intrahepatic cholestasis. Front Pharmacol. (2023) 14:1173542. doi: 10.3389/fphar.2023.1173542
PubMed Abstract | Crossref Full Text | Google Scholar
10. Gan, W, Liao, B, and Li, X. A rare cause of liver fibrosis in adulthood. Gastroenterology. (2022) 162:e1–3. doi: 10.1053/j.gastro.2021.08.048
Crossref Full Text | Google Scholar
11. Zheng, Y, Guo, H, Chen, L, Cheng, W, Yan, K, Zhang, Z, et al. Diagnostic yield and novel candidate genes by next generation sequencing in 166 children with intrahepatic cholestasis. Hepatol Int. (2023) 18:661–72. doi: 10.1007/s12072-023-10553-6
PubMed Abstract | Crossref Full Text | Google Scholar
12. Kavallar, AM, Mayerhofer, C, Aldrian, D, Okamoto, T, Muller, T, and Vogel, GF. Management and outcomes after liver transplantation for progressive familial intrahepatic cholestasis: a systematic review and meta-analysis. Hepatol Commun. (2023) 7:e0286. doi: 10.1097/HC9.0000000000000286
PubMed Abstract | Crossref Full Text | Google Scholar
13. Zhang, Y, Tang, D, Wang, L, Yang, J, Wu, X, Xiao, X, et al. Prevention of portal-tract fibrosis in Zfyve19−/− mouse model with adeno-associated virus vector delivering ZFYVE19. Hum Gene Ther. (2023) 34:1219–29. doi: 10.1089/hum.2023.041
留言 (0)