Gonorrhea is one of the most prevalent sexually transmitted infections. According to the World Health Organization, approximately 82 million cases of gonorrhea are reported globally each year (WHO, 2024a). In recent years, the incidence of gonorrhea has risen in countries such as China, the United States, and several European nations. An exceptional feature of the Neisseria gonorrhoeae pathogen is its rapid development of resistance to the antimicrobial drugs used in its treatment, which has repeatedly necessitated modifications to current treatment regimens and higher therapeutic doses (WHO, 2024b; Barbee and St Cyr, 2022). Particularly concerning is the emergence and widespread dissemination of multidrug-resistant N. gonorrhoeae clones, including strains resistant to current first-line treatments such as third-generation cephalosporins and macrolides. This increases the risk of the potential emergence of untreatable gonorrhea in the near future (Fifer et al., 2024; Ouk et al., 2024; Unemo et al., 2024).
In recent years, there has been a slight upward trend in the incidence of gonorrhea in the Russian Federation, with rates of 8.1 and 7.8 cases per 100,000 population in 2022 and 2023, respectively (Kubanov and Bogdanova, 2024; Shagabieva et al., 2023). Although there have been no reports of cephalosporin-resistant isolates to date, recent cross-border transmission has led to an increase in the proportion of macrolide-resistant isolates. This raises concerns about the future viability of combined antimicrobial therapy (ceftriaxone + azithromycin) in Russia, similar to the regimen recommended in the United States and several Western European countries (Kandinov et al., 2023a, 2023). Given the global spread of drug-resistant gonococcal clonal lineages, these trends underscore the importance of monitoring N. gonorrhoeae transmission and antimicrobial resistance within Russia.
Modern recommendations for the control of gonococcal infections include molecular genotyping protocols aimed at preventing transmission within populations and timely detection of drug-resistant genotypes (European Centre for Disease Prevention and Control, 2024). Currently in Russia, as in many other countries, N. gonorrhoeae isolates are monitored using genotyping systems such as MLST (Multilocus Sequence Typing) and NG-MAST (Neisseria gonorrhoeae Multi-Antigen Sequence Typing) (Shaskolskiy et al., 2020; Kandinov et al., 2023a). The whole-genome sequencing (WGS) and the cgMLST protocol offer the most comprehensive insights, including molecular genotyping and identification of genetic resistance determinants (Harrison et al., 2020). However, the relatively high cost of WGS and the personnel skill requirements for genome assembly and result processing remain barriers to the pervasive use of genome-wide sequencing for clinical isolates.
At the Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, hydrogel-based biological microarrays have been developed and successfully applied in clinical practice (Gryadunov et al., 2018; Shaskolskiy et al., 2021a). These microarrays provide rapid and efficient detection of selected nucleotide polymorphisms in DNA samples. Microarray-based assays have been designed to identify genetic determinants of resistance to key antimicrobial drugs for gonorrhea treatment, namely ceftriaxone and azithromycin (Shaskolskiy et al., 2021b; Kandinov et al., 2023a). Additionally, a microarray has been developed to detect 18 informative polymorphisms in genes used for MLST typing, providing relatively rapid and cost-effective genotyping of a large number of samples of N. gonorrhoeae clinical isolates (Kandinov et al., 2024).
Using these assays, clones of Russian isolates carrying the penA allele type 34.001, along with mutations in the ponA and porB genes, have been identified. These isolates exhibited reduced susceptibility to third-generation cephalosporins and belonged to the pandemically dangerous genogroup NG-MAST 1407 (Kandinov et al., 2020; Shaskolskiy et al., 2021b). More recently, due to the cross-border transmission of the European NG-MAST 12302 genogroup into Russia, there has been a sharp increase in the proportion of azithromycin-resistant isolates carrying the mosaic promoter (meningitidis-like promoter) of the mtrR gene and the mosaic sequence of the mtrD gene (Kandinov et al., 2023a).
However, the general trends in the evolution of the modern N. gonorrhoeae population in Russia remain unclear. Data on the current MLST genotypes of N. gonorrhoeae circulating in Russia are not fully available in the literature, nor is there sufficient information on the evolution of molecular types and genetic determinants of resistance. The lack of a comprehensive understanding of the Russian N. gonorrhoeae population may result in outdated clinical recommendations, potentially leading to an increase in gonococcal infections due to the ineffectiveness of current antimicrobial treatments.
The aim of this study was to characterize the N. gonorrhoeae population in Russia from 2015 to 2023, including data on antimicrobial susceptibility and genetic determinants of resistance to ceftriaxone and azithromycin. Additionally, the study aimed to assess biodiversity based on NG-MAST and MLST genotyping. The objectives also included forecasting the distribution of isolates with mosaic penA, mtrR, and mtrD genes to better understand the processes shaping the current N. gonorrhoeae population in Russia.
Materials and methodsCollection and characterization of Russian N. gonorrhoeae isolatesClinical isolates of N. gonorrhoeae collected in Russia were obtained from the State Scientific Centre of Dermatovenerology and Cosmetology of the Ministry of Health of the Russian Federation. Isolates were collected, analyzed, and stored as previously described (Kubanov et al., 2019; Shaskolskiy et al., 2019). Samples from the urethra in males and cervix/urethra in females (each sample from one patient) were seeded onto chocolate blood agar supplemented with 1% IsoVitaleX enrichment and 1% VCAT selective additive (vancomycin, colistin, amphotericin, and trimethoprim) (bioMérieux, Marcy l’Etoile, France). Primary identification of N. gonorrhoeae included Gram staining and rapid oxidase reaction. Complete identification of N. gonorrhoeae was based on the sugar utilization test using the NH identification card for the VITEK 2 Compact analyser (bioMérieux, Marcy l’Etoile, France) and MALDI-TOF MS using a MALDI Biotyper (Bruker Daltonics, Bremen, Germany).
During the period from 2015 to 2023, samples were received each year from at least 18 participating regions geographically distributed across Russia. In total, 996 samples were collected and analyzed during the study period: 2015 – 123, 2016 – 253, 2017 – 127, 2018 – 150, 2019 – 119, 2020 – 116, 2021 – 46, and 2022–2023 – 62. The isolates collected in 2022–2023 were pooled to maintain the sampling balance, which had been disrupted by the COVID-19 pandemic. The N. gonorrhoeae isolates were stored at -70°C and used for DNA isolation and phenotypic susceptibility analyses.
N. gonorrhoeae antimicrobial susceptibility testingCeftriaxone and azithromycin susceptibility testing of N. gonorrhoeae isolates and determination of the MIC were carried out using the agar dilution method. The obtained MIC values were compared with breakpoints, including ECOFF parameters from The European Committee on Antimicrobial Susceptibility Testing (EUCAST), 2024. For ceftriaxone, isolates with an MICcro of ≤ 0.125 mg/L were considered susceptible, those with MICcro ≤ 0.06 mg/L were considered decreased susceptible, and isolates with MICcro > 0.125 mg/L were considered resistant. For azithromycin, isolates with MICazi ≤ 1 mg/L were considered susceptible, and those with MICazi > 1 mg/L were considered resistant.
Identification of genetic determinants of N. gonorrhoeae resistance to ceftriaxone and azithromycin and genotyping using microarray technologyClinical isolates of N. gonorrhoeae were analyzed using previously designed hydrogel-based microarrays. The microarrays were produced by copolymerization immobilization technique according to a procedure developed earlier (Shaskolskiy et al., 2021a). Mutations in the penA, ponA, and porB genes were detected using a microarray developed for analyzing genetic determinants of resistance to third-generation cephalosporins (ceftriaxone) (Shaskolskiy et al., 2021a). The analysis targeted the following gene codons: penA (311, 312, 316, 345-346, 501, 512, 542, 545, 551), ponA (421), and porB (120 and 121).
Mutations in the promoter region of the mtrR gene (positions -35 and -10), mutations in the mtrD gene (codons 821 and 823), and mutations in the 23S rRNA gene of all four copies (position 2611) were detected using a previously developed microarray designed to analyze genetic determinants of resistance to macrolides (azithromycin) (Kandinov et al., 2023a). For the initial mass identification of MLST genotypes, we used another microarray along with the miniMLST typing scheme (Kandinov et al., 2024); results were subsequently verified using classical genotyping methods, including Sanger sequencing.
Genotyping of Russian N. gonorrhoeae isolates using NG-MAST and MLST schemesMolecular typing of N. gonorrhoeae isolates was performed using standard NG-MAST and MLST protocols. The internal regions of the porB and tbpB genes (NG-MAST) and the abcZ, adk, aroE, fumC, gdh, pdhC, and pgm genes (MLST) were subjected to PCR amplification. The resulting products were purified and sequenced. Allele numbers for the two NG-MAST sequences and seven MLST sequences were assigned according to the pubMLST database (https://pubmlst.org). NG-MAST and MLST sequence type numbers were obtained for each isolate. A complete list of all isolates and their corresponding sequence type numbers is presented in Supplementary Table S1.
Phylogenetically related genogroups were identified for all the NG-MAST types. Genogroups were defined as described previously (Shaskolskiy et al., 2020) as a set of porB and tbpB alleles (variable internal regions), where the concatenated sequence of both alleles (880 bp) differed by no more than 5 nucleotides from the concatenated sequence of both alleles of the major sequence type (ST) in the genogroup. Allele similarity was assessed using the MEGA X software. Major genogroups were defined as those containing at least 1% of the total sample of isolates (996 isolates).
Constructing a phylogenetic tree (phylogram)A multiple sequence alignment of nine concatenated loci from the abcZ, adk, aroE, fumC, gdh, pdhC, pgm, porB, and tbpB genes used for MLST and NG-MAST genotyping was generated, resulting in 376 unique sequences after removal of duplicates. A maximum likelihood phylogenetic tree was constructed using RAxML software (version 8.2.4; https://usegalaxy.eu), with 1000 rapid bootstrap inferences. The phylogenetic tree was visualized using FigTree v1.4.4 software (http://tree.bio.ed.ac.uk).
A statistical analysis and forecasting of the frequencies of isolates with mosaic penA, mtrR, and mtrD genesThe frequencies of mosaic genes were treated as time series. To analyze the time series and make predictions using ARIMA models, the guide by Hyndman and Athanasopoulos (2018) was used. Calculations were performed in R using the following libraries: fpp2 (version 2.5), tseries (version 0.10-58), and forecast (version 8.23).
Stationarity of the series was assessed using the KPSS test and the Augmented Dickey-Fuller test. If necessary, the lambda parameter for the Box-Cox transformation was calculated. Differentiation was carried out based on the required number of iterations, and model parameters were selected using the AICc criterion. For the resulting model, residuals were examined by plotting the autocorrelation function (ACF) of the residuals and performing a portmanteau test of the residuals.
ResultsCeftriaxone and azithromycin susceptibility testingA total of 996 N. gonorrhoeae isolates were sequentially collected and tested for MICs of ceftriaxone and azithromycin (Supplementary Table S1). The MIC range for ceftriaxone was 0.002–0.12 mg/L, while the MICs for azithromycin ranged from 0.02 mg/L to 8 mg/L. The proportions of isolates with reduced susceptibility to ceftriaxone and resistance to azithromycin detected in Russia during the period from 2015 to 2023 are shown in Figure 1.
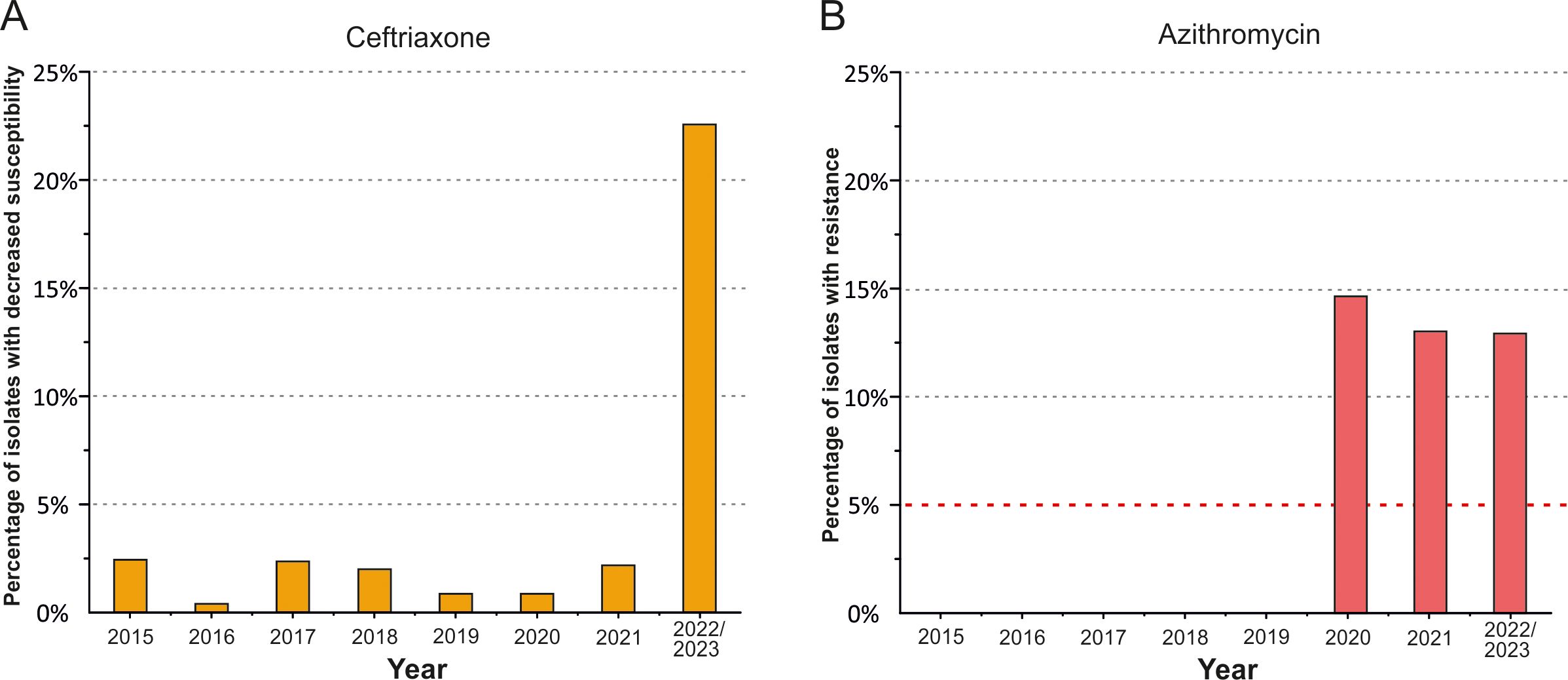
Figure 1. Diagram of the distribution of N. gonorrhoeae isolates in the Russian population (2015-2023). (A) Proportion of isolates with reduced susceptibility to ceftriaxone (MICcro ≥ 0.06 mg/L). (B) Proportion of isolates resistant to azithromycin (MICazi > 1 mg/L). The red dotted line in panel (B) represents the 5% threshold, above which the WHO guidelines no longer recommend azithromycin for the treatment of gonococcal infection.
During the analyzed time period, no ceftriaxone-resistant isolates were detected, confirming the continued suitability of third-generation cephalosporins for the treatment of gonococcal infections in Russia. As shown in Figure 1A, the proportion of isolates with decreased susceptibility to ceftriaxone did not exceed 1–2% per year from 2015 to 2021. However, in 2022–2023, 22.6% of isolates exhibited decreased susceptibility. This indicates a notable increase in the proportion of isolates with reduced susceptibility to ceftriaxone in the Russian population in recent years, which may create conditions for the emergence of resistant isolates in the Russian Federation in the near future.
All isolates collected from 2015 to 2019 were susceptible to azithromycin (Figure 1B). However, in 2020, a sharp increase in azithromycin-resistant isolates was observed, reaching 14.7% in the Russian population. In the subsequent period, from 2021 to 2023, the proportion of azithromycin-resistant isolates remained relatively high, at around 13%, more than double the WHO-recommended threshold of 5% for the use of antibiotic.
Genetic determinants of resistance to ceftriaxone and azithromycin in the Russian population of N. gonorrhoeaeTable 1 presents data on the distribution of genetic determinants of resistance to ceftriaxone and azithromycin in N. gonorrhoeae isolates from the Russian population between 2015 and 2023. As seen in Table 1, the proportion of determinants in the Russian population of N. gonorrhoeae changes from year to year. Since 2015, there has been an increase in the number of isolates with the substitutions Ile312Met, Val316Thr, Ala501Val, Asn512Tyr, Gly542Ser, and Gly545Ser, some of which are associated with an increase in the mosaicism of penA to 11% in 2022-2023 (Supplementary Table S1). Additionally, the determinants Ala501Thr and Pro551Leu are gradually being eliminated and replaced in the population. The observed changes in penA, along with ponA, porB, mtrR, and other unknown mechanisms, may have led to the increase in MIC of ceftriaxone observed in 2022–2023 (Figure 1A).
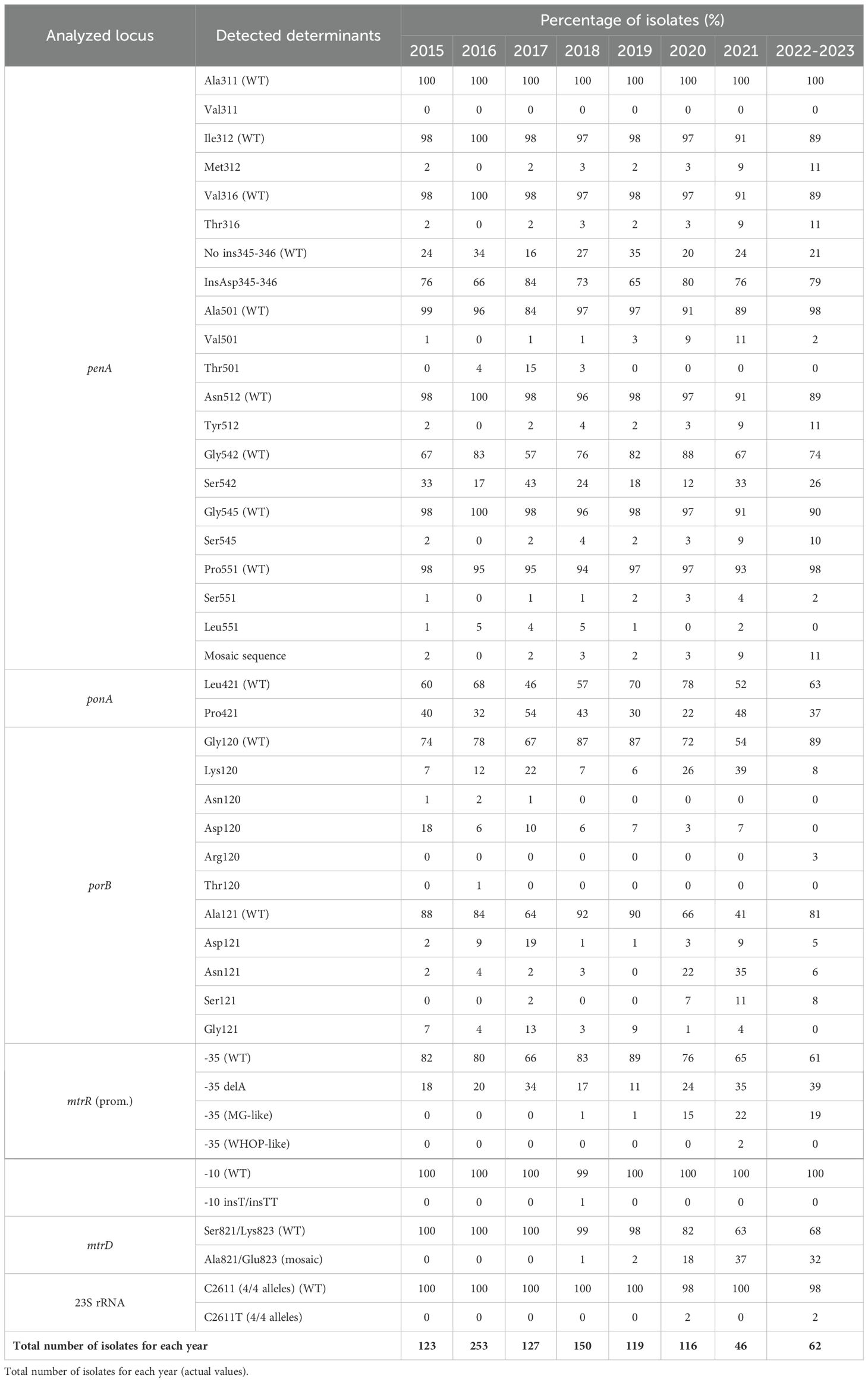
Table 1. Distribution of genetic determinants of N. gonorrhoeae resistance to ceftriaxone and azithromycin in the Russian population (2015-2023).
The increase in the number of azithromycin-resistant isolates in Russia in 2020 is linked to the emergence of mosaic alleles in the mtrR (meningitidis-like promoter) and mtrD genes. From 2021 to 2023, the proportion of isolates with the meningitidis-like promoter in the mtrR gene, as well as the Ala821/Glu823 substitutions in the mtrD gene, remained stable, suggesting that these genetic determinants have become fixed in the population. Consequently, resistance to azithromycin is likely to be sustained. It is also noteworthy that only three isolates with mutations in the 23S rRNA gene (2611 position in all 4 copies) were detected during the entire study period. These isolates, which are associated with resistance to azithromycin (MIC ≥ 4 mg/L), were found sporadically in the population.
During the analyzed period, the proportion of isolates with Pro421 mutations in the ponA gene and with Lys120, Asn120, Thr120, and Asp121 substitutions in the porB gene remained quite stable, with some exceptions. However, the proportion of isolates with Asp120 and Gly121 mutations in the porB gene has markedly decreased, while the percentage of isolates with Arg120, Asn121, and Ser121 mutations has generally increased. This shift is likely due to changes in both porB alleles and the molecular genotypes circulating in Russia.
Genotypes of N. gonorrhoeae isolatesBetween 2015 and 2023, 325 different NG-MAST sequencing types and 80 MLST types were identified in the analyzed sample. A complete list of the NG-MAST and MLST types for all samples analyzed is provided in Supplementary Table S1. For NG-MAST, nucleotide distances were calculated, and genetic groups were established. Of the 325 NG-MAST sequencing types, 222 formed 48 distinct genetic groups, while the remaining 103 types were classified as ungrouped STs. The major Russian N. gonorrhoeae genogroups are summarized in Table 2.

Table 2. Distribution of 996 Russian N. gonorrhoeae isolates by the most frequently observed genogroups and linked MLSTs.
MLST genotypes were determined for all isolates within the major NG-MAST genogroups (Table 2). Notably, in most cases, isolates within a single NG-MAST genogroup shared a dominant MLST genotype. As a result, a strong association between NG-MAST genogroups and MLST types was observed, with approximately 80% of isolates in the major genogroups in the Russian population exhibiting this linkage. The exception was genogroup G2212, where 50% of isolates had MLST 1901 and 20% had MLST 1902, accounting for a total of 70% of the genogroup.
The most common genogroup, G807, includes 222 isolates (22% of all samples), representing 34 STs. This genogroup encompasses the three most frequently occurring NG-MASTs in the Russian Federation: ST807, ST228, and ST1544. Among all isolates in genogroup G807, MLST 1594 was detected in 91.9% of isolates. The second most common genogroup in Russia, G1993, consists of 108 isolates (10.8%) belonging to 15 sequence types, with 82.4% of isolates linked to MLST 11177.
The genogroups G2212, linked to MLST 1901/1902 (accounting for 70% of isolates), and G12302, linked to MLST 9363 (96.8% of isolates), pose the greatest threat to the Russian population. Isolates of genogroup G2212 represented 2% of the sample and were most commonly associated with a mosaic structure in the penA gene, leading to decreased susceptibility to ceftriaxone, as previously reported (Kandinov et al., 2020). Genogroup G12302 comprised 3.1% of the sample, and its emergence in Russia in 2020 was linked to the rise of azithromycin-resistant isolates carrying mosaic alleles in the mtrR and mtrD genes (Kandinov et al., 2023b).
A maximum likelihood phylogenetic tree was constructed for the NG-MAST and MLST types of N. gonorrhoeae isolates collected in Russia from 2015 to 2023 (Figure 2). The tree reflects a total of 376 unique NG-MAST + MLST combinations present in the Russian population of N. gonorrhoeae.
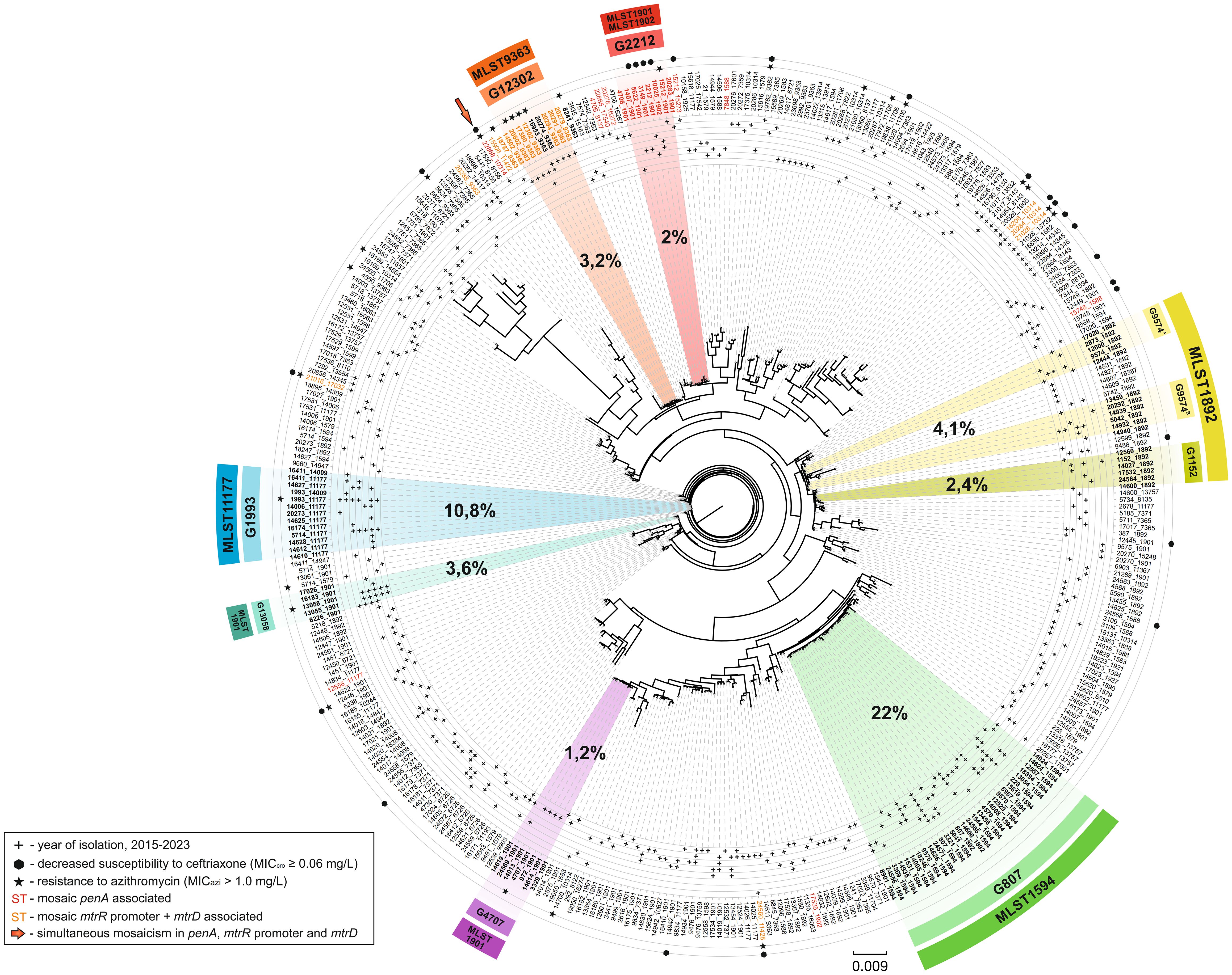
Figure 2. Maximum likelihood phylogeny of NG-MAST and MLST types for N. gonorrhoeae isolates collected in Russia (2015-2023). The tree consists of 376 terminal nodes, each representing a unique combination of NG-MAST and MLST types (labeled as node names). The years in which a clade is found are marked by ‘plus’ signs and arranged from 2015 to 2023 in increasing radius order. Clades associated with reduced susceptibility to ceftriaxone and/or resistance to azithromycin are marked by ‘hexagon’ and ‘star’ signs, respectively. Colors on the tree correspond to the main NG-MAST genogroups in the Russian population, along with the MLST types linked to each genogroup. The percentage share of each genogroup in the total sample is indicated in bold black font. Sequence types associated with mosaic penA, as well as mtrR promoter + mtrD, are highlighted in red and orange font, respectively. The sequence type with both mosaic penA and mtrR promoter + mtrD is indicated by an arrow.
The phylogeny of the Russian N. gonorrhoeae population demonstrates a clear linkage between NG-MAST genogroups and MLST types. Notably, some major genogroups are located relatively close to one another on the tree. For example, genogroup G9574 is split into two clusters, G9574A and G9574B, and is positioned near genogroup G1152. The majority of isolates within this genogroup are associated with MLST 1892, suggesting a common ancestral origin for these genogroups. Genogroups G1993 and G13058 are also in close proximity, although they are linked to different MLST types, 11177 and 1901, respectively.
The pandemically significant genogroups G2212 and G12302 are phylogenetically close but linked to distinct MLST types, 1901/1902 and 9363, respectively. Interestingly, both of these genogroups are positioned at a considerable distance from the dominant Russian genogroup, G807.
Association of mosaic variants of penA, mtrR, mtrD genes with molecular genotypesFrom the total sample of isolates collected between 2015 and 2023, we selected those in which mosaic structures in the penA gene (24 isolates) and simultaneous mosaicism in the mtrR (promoter) and mtrD genes (40 isolates) were detected. The distribution of these isolates, based on the year of collection, is shown in Figure 3.
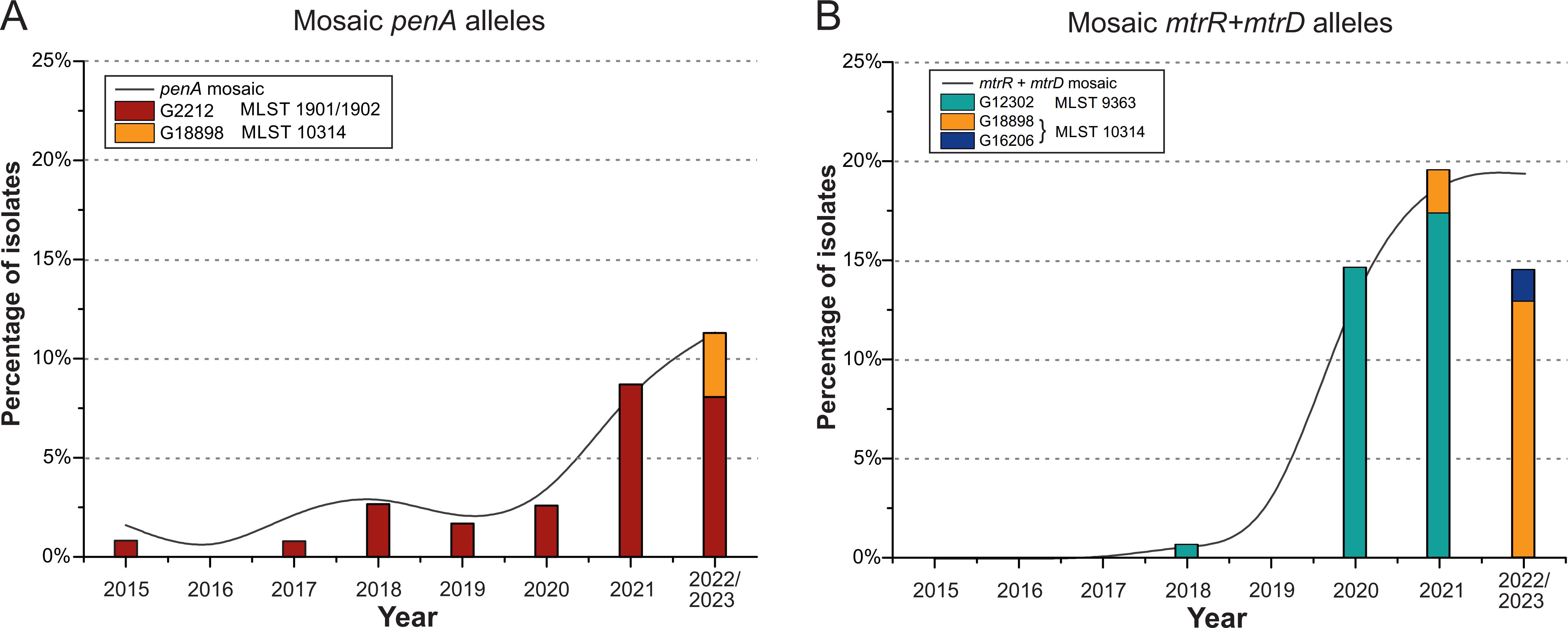
Figure 3. Distribution of the proportion of N. gonorrhoeae isolates by NG-MAST genogroups and MLST in the Russian population (2015-2023). (A) Isolates with mosaic structure of the penA gene. (B) Isolates with mosaic structure of the mtrR (promoter) and mtrD genes. The black line represents the percentage of isolates with the specified mosaic gene structures. The colored bars indicate the number of isolates belonging to different NG-MAST genogroups and MLST types.
As shown in Figure 3A, between 2015 and 2021, penA mosaic alleles were predominantly found in isolates from genogroup G2212. However, in the 2022-2023 sample, penA mosaicism was also detected in isolates from genogroup G18898. Notably, isolates from genogroup G18898 are associated with MLST 10314, in contrast to G2212, which is linked to MLST 1901 and 1902.
In 2020 and 2021, mosaicism in the mtrR (promoter) and mtrD genes was primarily associated with genogroup G12302 and MLST 9363 (Figure 3B). Notably, no isolates from this genogroup were detected in the 2022-2023 sample. Instead, in the 2022-2023 isolates, mosaicism in the mtrR (promoter) and mtrD genes was linked to the novel genogroups G18898 and G16206, both associated with MLST 10314.
For the first time, isolates with simultaneous mosaicism in the penA, mtrR (promoter), and mtrD genes were identified in the Russian population. A total of two isolates exhibiting simultaneous mosaicism in all three genes were found in the 2022–2023 sample. Both isolates had an MICcro of 0.03 mg/L and MICazi of 1 mg/L and 2 mg/L (the second isolate is resistant according to EUCAST criteria). Thus, a new cluster of isolates from genogroup G18898 and MLST 10314 was identified in Russia, which may lead to the development of simultaneous resistance to both ceftriaxone and azithromycin in the near future.
Forecast of the distribution of isolates with mosaic genes penA, mtrR, mtrD in the populationTo better understand the processes within the current Russian population of N. gonorrhoeae and to assess possible future trends, we predicted the distribution of isolates with mosaic penA genes, as well as those with simultaneous mosaicism in the mtrR promoter and the coding region of the mtrD gene. Parameter selection was performed for ARIMA models describing the dynamics of genotypes with mosaic penA and with mosaic mtrR (promoter) and mtrD. In both cases, the best models were of the ARIMA (0,1,0) class, predicting the spread of the corresponding genotypes as a random walk around the trend. The analyzed time series and predictions for the occurrence of penA (A) and mtrR (promoter) and mtrD (B) mosaic genotypes in the Russian population are shown in Figure 4.
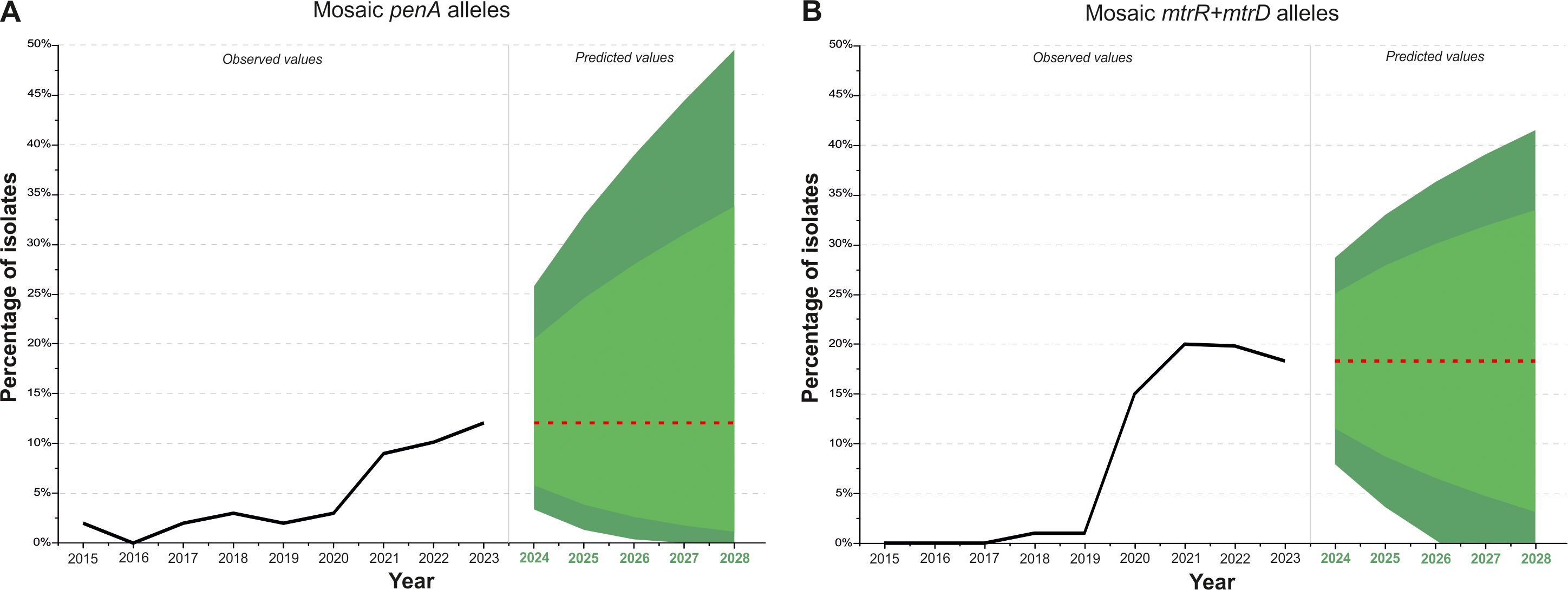
Figure 4. Dynamics of isolates with mosaic penA (A) and mosaic mtrR (promoter) + mtrD (B) in the population, with predictions for the next 5 years (2024-2028). The black line represents the percentage of isolates obtained experimentally between 2015 and 2023. The red line indicates projections for the next 5 years. The light green area represents the 80% confidence interval, while the dark green area represents the 95% confidence interval.
Analysis of the frequencies of genotypes with mosaic penA (Figure 4A) shows that, in 2021, there was an increase in the proportion of isolates to 9%, compared to previous years when the frequency of mosaic penA was around 2–3%. The frequency of isolates with the mosaic penA gene remained above 9% after 2020 and is projected to stabilize at approximately 12% between 2024 and 2028, accounting for possible fluctuations. Even under the most favorable conditions, within the 80% confidence interval, gene drift is unlikely to reduce the proportion of mosaic penA isolates to 1% or less in the next few years. In the case of unfavorable developments, with an increase in the proportion of mosaic penA, the 80% confidence interval suggests that the proportion of isolates with mosaic penA will not exceed one-third of all isolates.
Investigation of the frequency of isolates with simultaneous mosaicism in the mtrR (promoter) and mtrD genes (Figure 4B) revealed a sharp increase in the proportion of isolates in 2020, with the frequency remaining consistently high at around 18% between 2020 and 2023. The forecast for 2024–2028 predicts values near 18%. Within the 80% confidence interval, it can be concluded that the proportion of isolates with mosaic mtrR and mtrD genes is unlikely to decrease to 3% or less, considering possible fluctuations. In the case of unfavorable developments, the proportion of genotypes with mosaic mtrR and mtrD genes may increase to one-third of all isolates.
DiscussionIn this study, we investigated the structure of the Russian N. gonorrhoeae population, including susceptibility to ceftriaxone and azithromycin, and the molecular characteristics of 996 isolates collected in Russia between 2015 and 2023. No ceftriaxone-resistant isolates were detected, confirming the continued feasibility of using this antimicrobial in treatment regimens. Among the study samples, the proportion of isolates with reduced susceptibility to ceftriaxone did not exceed 1–2% per year between 2015 and 2021. It should be noted separately that reduced susceptibility to ceftriaxone in the Russian population of N. gonorrhoeae may not always be associated with a single specific determinant and often exhibits a polygenic nature. The combination of mutations in the penA, ponA, porB, and mtrR genes, as well as other unstudied spontaneous mutations in the genome and mechanisms, may be the reason for the demonstrated slow decline in susceptibility to ceftriaxone over the past few years. However, in 2022–2023, 22.6% of isolates showed reduced susceptibility, which may signal a worsening of the epidemiological situation in the near future.
Despite the fact that azithromycin is not recommended for the treatment of gonococcal infections in Russia, approximately 12% of isolates resistant to this antimicrobial agent, according to EUCAST criteria, have been registered annually since 2020. This is higher than the WHO-recommended threshold of 5%. The persistence of a high proportion of azithromycin-resistant isolates in Russia rules out the use of the “ceftriaxone + azithromycin” combination therapy, which is used in some other countries (WHO, 2024b; Golparian et al., 2024). Overall, Russia still has a relatively favorable epidemiological situation compared to Europe, Asia, and the USA, where both multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains are regularly detected (Maubaret et al., 2023; Tang et al., 2023; Ouk et al., 2024).
Oligonucleotide microarrays remain a reliable tool for the identification of genetic determinants of resistance to current antimicrobials, as well as mass genotyping of clinical isolates of N. gonorrhoeae. In this work, using previously developed microarrays (Shaskolskiy et al., 2021a; Kandinov et al., 2023a, 2024), we obtained data on the distribution of genetic determinants of resistance of N. gonorrhoeae to ceftriaxone and azithromycin on the territory of the Russian Federation in 2015-2023.
The dynamics of isolates with mosaic alleles of the penA, mtrR, and mtrD genes are of particular interest in the analyzed sample. Previously, we identified mosaic alleles of the penA gene (type 34.001) in Russian isolates with reduced susceptibility to ceftriaxone (Kandinov et al., 2020). This study shows an increase in the proportion of isolates with mosaic penA to 9% in 2021, and to 11% in 2022–2023, compared to previous years, when the proportion of such isolates did not exceed 2–3%. These findings indicate not only the consolidation of isolates with mosaic penA alleles in Russia, but also a gradual increase in the proportion of such isolates in recent years.
Isolates with the mtrR mosaic promoter (alleles 485 and 530 on NG-STAR) and the mtrD mosaic promoter (allele 3353 on pubMLST) were first detected in Russia in 2020 (Kandinov et al., 2023b). In the present study, analysis of isolates from 2022–2023 revealed a consistently high frequency of these isolates, indicating their establishment in the population.
Determinants not associated with mosaic alleles in penA, ponA, porB, mtrR, mtrD, and 23S rRNA genes remained relatively stable throughout the study period, with some exceptions. Isolates with C2611T mutations in the 23S rRNA gene and isolates with a WHO-P mosaic promoter in the mtrR gene were sporadically detected. The appearance or disappearance of other determinants in the studied genes appears to be largely associated with changes in molecular genotypes in Russia.
Eight major genetic groups of NG-MAST were detected in Russia. Isolates from genogroups G807 and G1993 were the most common, accounting for 22% and 10.8% of the samples, respectively. Genogroups G9574, G13058, G12302, G1152, G2212, and G4707 were less frequent, with the remaining genogroups comprising 30% of the total sample, and 20% of isolates remained ungrouped. The phylogenetic analysis of all identified NG-MAST and MLST types in Russia revealed a strong association between NG-MAST types within a genogroup and a specific MLST type. For example, all isolates in NG-MAST genogroup G807 were associated with MLST 1594 in 91.9% of cases, isolates from G1993 were linked to MLST 11177 in 82.4%, and so on. Overall, 80% of isolates in the sample showed a clear linkage between NG-MAST genogroups and MLST types.
In 2015, isolates from the pandemically significant genogroup G2212 (known globally as G1407) were detected in Russia for the first time (Kubanov et al., 2016). This genogroup has been detected annually throughout the study period and continues to increase in frequency. It is important to note that isolates from this genogroup exhibit reduced susceptibility to ceftriaxone due to the presence of the mosaic penA 34.001 type. The second genogroup of concern for Russia is G12302, first identified in the Russian Federation in 2020, which is associated with azithromycin resistance due to the presence of mosaic mtrR (promoter) and mtrD genes (Kandinov et al., 2023b). We previously expressed concern that the presence of these two genogroups in Russia could lead to a worsening of the epidemiological situation (Kandinov et al., 2023a). In the present study, we characterized two additional genogroups, G18898 and G16206 (both linked to MLST 10314), in the 2022–2023 sample. These genogroups were found to contain isolates with mosaic penA alleles, either alone or in combination with mosaic mtrR (promoter) and mtrD.
For the first time, two isolates with simultaneous mosaicism of penA, mtrR (promoter), and mtrD were detected. Both isolates belonged to the NG-MAST genogroup G18898 and MLST 10314. MLST 10314 has been previously identified globally, including in China, the UK, and other countries, and isolates with this genotype often carry the penA mosaic type 60.001, which is associated with reduced susceptibility or resistance to third-generation cephalosporins (Maubaret et al., 2023; Tang et al., 2023). Notably, G18898 and MLST 10314 have been present in Russia since 2017 (Shaskolskiy et al., 2020), but earlier isolates from this genogroup did not carry mosaic alleles.
The observed sharp changes in the proportions of isolates with mosaic penA and with mosaic mtrR promoter and mtrD, which occurred in 2021 and 2020 respectively, led to the fixation of the above-mentioned genetic determinants of resistance in the population. The prediction made by the time series analysis suggests that isolates with mosaic alleles will not be eliminated from the population. In case of unfavorable development of events, it is likely that in 5 years every third isolate will have mosaicism of either penA or mtrR and mtrD simultaneously. Successful fixation of G18898 and MLST 10314 in Russia will inevitably lead to deterioration of the epidemiological situation in the country.
In summary, the autochthonous genetic types prevalent in the Russian population of N. gonorrhoeae, which are susceptible to both cephalosporins and macrolides, are gradually being replaced by globally dominant genetic lineages. Over the past three years, genetic determinants of reduced susceptibility to both macrolides and cephalosporins have become firmly established in the population. Ongoing surveillance, including molecular epidemiology methods and the collection of data on genetic markers of antimicrobial resistance, will play a crucial role in curbing the growth and spread of N. gonorrhoeae resistance, preventing further dissemination, and ensuring the continued availability of effective treatment options.
Data availability statementThe original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributionsIK: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. BS: Conceptualization, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. DK: Formal analysis, Methodology, Software, Writing – review & editing. AL: Methodology, Visualization, Writing – review & editing. AK: Conceptualization, Data curation, Formal analysis, Writing – review & editing. MS: Investigation, Methodology, Writing – review & editing. JS: Investigation, Methodology, Validation, Writing – review & editing. NN: Data curation, Formal analysis, Writing – review & editing. DG: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Russian Science Foundation, grant number 24-25-20084 (to the Engelhardt Institute of Molecular Biology, Russian Academy of Sciences) and by Ministry of health of Russian Federation, government assignment No. 056-00003-24-02 (to the State Research Center of Dermatovenerology and Cosmetology, Russian Ministry of Health).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1526859/full#supplementary-material
ReferencesBarbee, L. A., St Cyr, S. B. (2022). Management of Neisseria gonorrhoeae in the United States: summary of evidence from the development of the 2020 gonorrhea treatment recommendations and the 2021 centers for disease control and prevention sexually transmitted infection treatment guidelines. Clin. Infect. diseases: an Off. Publ. Infect. Dis. Soc. America 74, S95–S111. doi: 10.1093/cid/ciac043
Crossref Full Text | Google Scholar
Fifer, H., Doumith, M., Rubinstein, L., Mitchell, L., Wallis, M., Singh, S., et al. (2024). Ceftriaxone-resistant Neisseria gonorrhoeae detected in England 2015-24: an observational analysis. J. antimicrobial chemotherapy. 79: 3332–9. doi: 10.1093/jac/dkae369
Crossref Full Text | Google Scholar
Golparian, D., Cole, M. J., Sánchez-Busó, L., Day, M., Jacobsson, S., Uthayakumaran, T., et al. (2024). Antimicrobial-resistant Neisseria gonorrhoeae in Europe in 2020 compared with in 2013 and 2018: a retrospective genomic surveillance study. Lancet Microbe 5, e478–e488. doi: 10.1016/S2666-5247(23)00370-1
Crossref Full Text | Google Scholar
Gryadunov, D., Shaskolskiy, B., Nasedkina, T., Rubina, A., Zasedatelev, A. (2018). The EIMB hydrogel microarray technology: thirty years later. Acta naturae 10, 4–18. doi: 10.32607/20758251-2018-10-4-4-18
Crossref Full Text | Google Scholar
Harrison, O. B., Cehovin, A., Skett, J., Jolley, K. A., Massari, P., Genco, C. A., et al. (2020). Neisseria gonorrhoeae population genomics: use of the gonococcal core genome to improve surveillance of antimicrobial resistance. J. Infect. Dis. 222, 1816–1825. doi: 10.1093/infdis/jiaa002
Crossref Full Text | Google Scholar
Hyndman, R. J., Athanasopoulos, G. (2018). Forecasting: principles and practice (Melbourne, Australia: OTexts) (Accessed October 23, 2024).
Kandinov, I., Dementieva, E., Filippova, M., Vinokurova, A., Gorshkova, S., Kubanov, A., et al. (2023a). Emergence of azithromycin-resistant neisseria gonorrhoeae isolates belonging to the NG-MAST genogroup 12302 in Russia. Microorganisms 11, 1226. doi: 10.3390/microorganisms11051226
Crossref Full Text | Google Scholar
Kandinov, I., Dementieva, E., Kravtsov, D., Chestkov, A., Kubanov, A., Solomka, V., et al. (2020). Molecular Typing of Neisseria gonorrhoeae Clinical Isolates in Russia 2018-2019: A Link Between penA Alleles and NG-MAST Types. Pathog. (Basel Switzerland) 9, 941. doi: 10.3390/pathogens9110941
PubMed Abstract | Crossref Full Text | Google Scholar
Kandinov, I., Shaskolskiy, B., Kravtsov, D., Filippova, M., Larkin, A., Gryadunov, D. (2024). Mini-multilocus sequence typing scheme for the global population of Neisseria gonorrhoeae. Int. J. Mol. Sci. 25, 5781. doi: 10.3390/ijms25115781
PubMed Abstract | Crossref Full Text | Google Scholar
Kandinov, I., Shaskolskiy, B., Kravtsov, D., Vinokurova, A., Gorshkova, S., Kubanov, A., et al. (2023b). Azithromycin susceptibility testing and molecular investigation of Neisseria gonorrhoeae isolates collected in Russia 2020-2021. Antibiotics (Basel Switzerland) 12, 170. doi: 10.3390/antibiotics12010170
PubMed Abstract | Crossref Full Text | Google Scholar
Kubanov, A., Bogdanova, E. (2024). Dermatovenereology of the Russian federation: results of 2023. Vestnik dermatologii i venerologii 100, 9–24. doi: 10.25208/vdv16795
Crossref Full Text | Google Scholar
Kubanov, A., Solomka, V., Plakhova, X., Chestkov, A., Petrova, N., Shaskolskiy, B., et al. (2019). Summary and trends of the Russian gonococcal antimicrobial surveillance programme 2005 to 2016. J. Clin. Microbiol. 57, e02024–e02018. doi: 10.1128/JCM.02024-18
PubMed Abstract | Crossref Full Text | Google Scholar
Kubanov, A., Vorobyev, D., Chestkov, A., Leinsoo, A., Shaskolskiy, B., Dementieva, E., et al. (2016). Molecular epidemiology of drug-resistant Neisseria gonorrhoeae in Russia (Current Status 2015). BMC Infect. Dis. 16, 389. doi: 10.1186/s12879-016-1688-7
PubMed Abstract | Crossref Full Text | Google Scholar
Maubaret, C., Caméléna, F., Mrimèche, M., Braille, A., Liberge, M., Mainardis, M., et al. (2023). Two cases of extensively drug-resistant (XDR) Neisseria gonorrhoeae infection combining ceftriaxone-resistance and high-level azithromycin resistance, France, November 2022 and May 2023. Euro surveillance 28, 2300456. doi: 10.2807/1560-7917.ES.2023.28.37.2300456
PubMed Abstract | Crossref Full Text | Google Scholar
Ouk, V., Heng, L. S., Virak, M., Deng, S., Lahra, M. M., Frankson, R., et al. (2024). High prevalence of ceftriaxone-resistant and XDR Neisseria gonorrhoeae in several cities of Cambodia 2022-23: WHO Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP). JAC-antimicrobial resistance 6, dlae053. doi: 10.1093/jacamr/dlae053
PubMed Abstract | Crossref Full Text | Google Scholar
Shagabieva, J., Nosov, N., Shpilevaja, M., Deryabin, D., Obraztcova, O., Nikonorova, E., et al. (2023). Analysis of the dynamics of the IPC of antimicrobials against N. gonorrhoeae
留言 (0)