Tinnitus is the conscious perception of auditory sensations in the absence of corresponding external stimuli and is mainly divided into subjective tinnitus and objective tinnitus (1). Tinnitus can impair speech comprehension, making it difficult for those who experience it to concentrate, interfering with nighttime rest and increasing anxiety and depression (2). Tinnitus affects more than 740 million adults globally and is perceived as a major problem by more than 120 million people, mostly those aged 65 years or older (3).
The pathogenesis and pathophysiological pathways (4) of tinnitus development are unclear. Multiple forms of tinnitus reflect complex interactions between peripheral and central mechanisms in the auditory pathway (5). Previous studies have suggested that hearing loss leads to reduced upload of auditory impulses, resulting in reduced neuron impulses in the brain region of the corresponding frequency in the auditory cortex of the brain, plasticity changes in the auditory cortex, increased excitability of the auditory cortex surrounding the frequency, and an increased spontaneous discharge rate of neurons, leading to tinnitus (6). Recent studies on rodents and humans have found that emotional and cognitive transmission in the brain such as temporal, parietal, sensorimotor, and limbic cortex are involved in the occurrence of tinnitus (7, 8).
Many treatments are available for tinnitus; however, their efficacy is unsatisfactory due to individual differences, and some treatments are only effective for some patients. According to the tinnitus pathogenesis hypothesis, attempts to restore sound input from the cochlea to the cerebral cortex may reduce tinnitus symptoms, thus, some researchers use hearing aids or tinnitus masking devices to reduce tinnitus symptoms (9). Others have attempted to use oral drugs (10), repetitive transcranial magnetic stimulation (11), transcranial direct current stimulation (12, 13), and vagus nerve stimulation (14) to treat tinnitus; however, the effectiveness of these treatments is not good. The efficacy of cognitive behavioral therapy, a psychological intervention rooted in the cognitive and behavioral traditions of psychology, has been empirically demonstrated in enhancing tinnitus patients’ quality of life and alleviating distress associated with tinnitus (15, 16). However, due to limited accessibility, cognitive behavioral therapy is not widely utilized by medical professionals for the treatment of tinnitus.
Because of the difference in the treatment effect among tinnitus patients, customized treatment of tinnitus may be a key future research direction. Stracke et al. administered compound sound therapy to tinnitus patients for 12 months and found that subjective tinnitus loudness and negative emotion related to tinnitus in patients in the experimental group were significantly reduced, while the corresponding indicators in the placebo group and the blank control group did not change (17). Okamoto et al. found that patients’ tinnitus loudness could be significantly reduced by the combination of sound therapy (18). However, Liu et al. found in a large-sample, single-arm study that the reduction in the Tinnitus Handicap Inventory (THI) score after receiving customized music therapy depended on the severity of the patient’s tinnitus (19).
The effect of compound sound therapy on tinnitus patients is uncertain. Therefore, exploring the clinical characteristics of effective compound sound therapy in tinnitus patients and establishing a model to predict the effectiveness of this therapy according to the clinical characteristics are substantially significant, which will improve its effectiveness in tinnitus patients.
Materials and methods PatientsThis retrospective study was conducted at the Hearing Center of the Department of Otolaryngology at the local hospital. The study included 991 patients with subjective tinnitus who received short-term compound sound therapy at the Department of Otolaryngology at the local hospital from November 2019 to January 2022. All patients completed a questionnaire survey on tinnitus and their psychological status, underwent numerous audiological examinations, and were finally diagnosed with subjective tinnitus. Patients with various other ear diseases such as objective tinnitus, otosclerosis and otitis media were excluded. Patients with serious mental illness or systemic diseases such as cardiovascular and cerebrovascular diseases, those who were unable to complete the scale or cooperate, and those with incomplete medical records were excluded. The investigation was conducted in accordance with the tenets of the Declaration of Helsinki, as depicted in Figure 1, and approved by the Medical Ethics Committee of the local hospital.
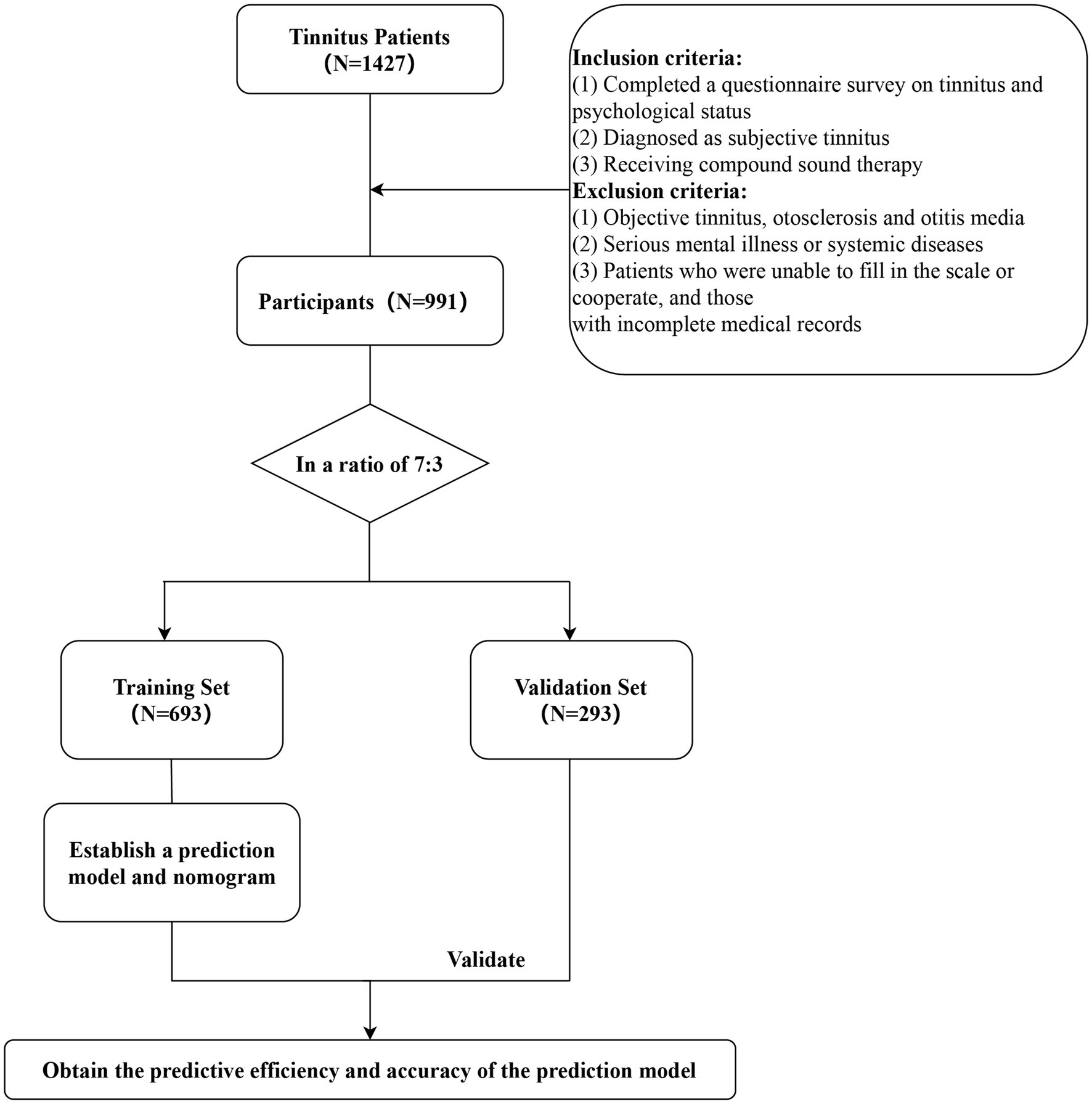
Figure 1. Flow chart.
Precision tinnitus testingThe patients underwent pure tone audiometry in acoustically treated compartments, recording auditory thresholds of 125 Hz, 250 Hz, 500 Hz, 1,000 Hz, 2000 Hz, 4,000 Hz, and 8,000 Hz. The average hearing threshold of all patients was calculated as the average hearing thresholds of 500 Hz, 1,000 Hz, and 2000 Hz. In addition, all tinnitus patients were matched with fine-tuned tinnitus of a 1/12x frequency according to audiogram data, and tinnitus-related data were recorded. These data included the tinnitus type, side, loudness and frequency.
All patients underwent the tone loudness distortion feedback test (FBT) and residual inhibition test (RIT). The FBT is used to provide a specific sound stimulus to the ear with tinnitus according to the loudness of the tinnitus, and after stimulation for a few seconds, the patient is asked whether the tinnitus has changed under the pure tone stimulus. The results were categorized as follows: 1, completely positive (tinnitus was not audible); 2, partial positive (tinnitus became quieter); 3, negative (no change in tinnitus perception); and 4, rebound effect (tinnitus became louder). RIT is a 10 dB narrow band noise stimulus that is administered to the patient over the tinnitus loudness threshold for 1 min, and the patient is asked whether the tinnitus changes after the stimulus sound stops. The results were categorized as follows: 1, completely positive (tinnitus was not audible); 2, partial positive (tinnitus became quieter); 3, negative (no change at tinnitus perception); and 4, rebound effect (tinnitus became louder).
The tinnitus loudness was subtracted from the tinnitus frequency hearing threshold, and this difference was defined as the tinnitus loudness perception level. The tinnitus type, loudness, frequency, FBT and RIT were selected as the characteristics of patients with poor hearing.
Tinnitus severity assessmentPatients completed two questionnaires to measure tinnitus severity. The THI, which includes functional, emotional and severity-related questions, was used to comprehensively assess each patient’s status (20). Each question on the scale had 3 answer options, in which the answer “yes” was worth 4 points, “sometimes” was worth 2 points, and “no” was worth 0 points. According to the difference in the total score, the patients were categorized as Grade 1 (slight, THI total score of 0–16 points), Grade 2 (mild, THI total score of 18–36), Grade 3 (moderate, THI total score 38–56), Grade 4 (severe, THI total score of 58–76), and Grade 5 (catastrophic, THI total score of 78–100). De Ridder and his colleagues proposed the concept of “Tinnitus Disorder” which reflects the auditory component and the associated suffering (21). We combined severe and catastrophic tinnitus severity into one group, where the lives of tinnitus patients were greatly affected by tinnitus. We combined tinnitus patients with slight, mild, and moderate tinnitus severity into one group, where the lives of tinnitus patients were less affected by tinnitus. The visual analog scale (VAS) is a clinical method used to assess the intensity of pain on a scale from 0 to 10, with “0” indicating no pain and “10” representing unendurable and severe pain. Clinically, “0” is “painless, “1–3″ is “mild pain, “4–6″ is “moderate pain, and “7–10″ is severe pain (22).
Treatment protocolThe prescription for compound sound therapy consists of 3 parts. The first part is matched according to the patient’s tinnitus frequency, mainly using narrowband noise. The second part is for different natural sounds, such as rain and bird calls, which can be divided into low-frequency, medium-frequency, high-frequency and multi-frequency sounds, and the masking frequency of natural sounds is selected according to the frequency of the patient’s tinnitus site. Part 3 involves the frequency of music material according to the patient’s personal preference. All three pieces of music must effectively cover the tinnitus sound, and the loudness of the synthesized music file is approximately 10–15 dB above the tinnitus loudness threshold. The duration of sound therapy should be 10–15 min. Sound therapy is considered effective if the patient’s conscious tinnitus lessens or disappears after receiving sound therapy for 10–15 min and ineffective if they experience no change or aggravated tinnitus symptoms.
Statistical analysisSPSS 26.0 (IBM, Armonk, NY, USA) and Graphpad 8.0 (Insightful Science, USA) were used for the statistical analyses and the production of the data. Normally distributed continuous variables are represented as means ± standard deviations, while non-normally distributed data are described as medians (interquartile ranges). Statistical comparisons between groups were performed using analysis of variance or the Mann–Whitney test for continuous variables. Categorical variables were compared using the χ2 test or Fisher’s exact probability test. The demographic characteristics, acoustic characteristics, severity of tinnitus and impact degree of tinnitus in patients with different sound treatment effects were compared.
Patients were randomly divided into a training set and a validation set at a 7:3 ratio. The demographic characteristics, acoustic characteristics and severity of tinnitus were compared between the training set and the validation set. In the training set, the demographic characteristics, acoustic characteristics of tinnitus, tinnitus severity and treatment efficacy were analyzed using univariate logistic regression. Factors with p < 0.05 in the univariate analysis were included in the multivariate logistic regression analysis. The model was established by the forward method in logistic regression, with α in = 0.05 and α out = 0.10. Based on the results of the multiple logistic regression analysis, columns were drawn using the RMS package version R3.0. The nomogram was constructed by scaling each regression coefficient in the multivariate logistic regression from 0 to 100 points. The variable with the highest beta coefficient (absolute value) was scaled to 100 points to represent its impact. These points were added across the independent variables to reach the total point, which was converted into a predicted probability. A corresponding ROC curve was generated to measure the predictive performance of the nomogram through the calibration curve of 1,000 bootstrap samples to reduce overfitting bias. The efficiency of the nomogram was then evaluated using a validation set, and the corresponding ROC curve and calibration curve of 1,000 bootstrap samples were obtained. p < 0.05 was considered to indicate statistical significance.
Results Comparison of the characteristics of tinnitus patients with different treatment efficacies of sound therapyThis study included patients with 991 subjective tinnitus treated at the Department of Otolaryngology local hospital from November 2019 to January 2022 who received transient compound sound therapy. Treatment was effective for 752 patients and ineffective for 239 patients. Patients with subjective tinnitus who experienced different therapeutic effects exhibited significant differences in the tinnitus location (p = 0.007), FBT (p = 0.000), RIT (p = 0.000), tinnitus frequency (p = 0.012) and sensation level (p = 0.023) (Table 1). No significant differences in sex (p = 0.737), age (p = 0.126), type of tinnitus (p = 0.585), type of tinnitus sounds (p = 0.680), degree of hearing impairment (p = 0.566), THI score (p = 0.050), VAS score (p = 0.263), impact degree (p = 0.216) or average hearing threshold (p = 0.181) were observed (Table 1).
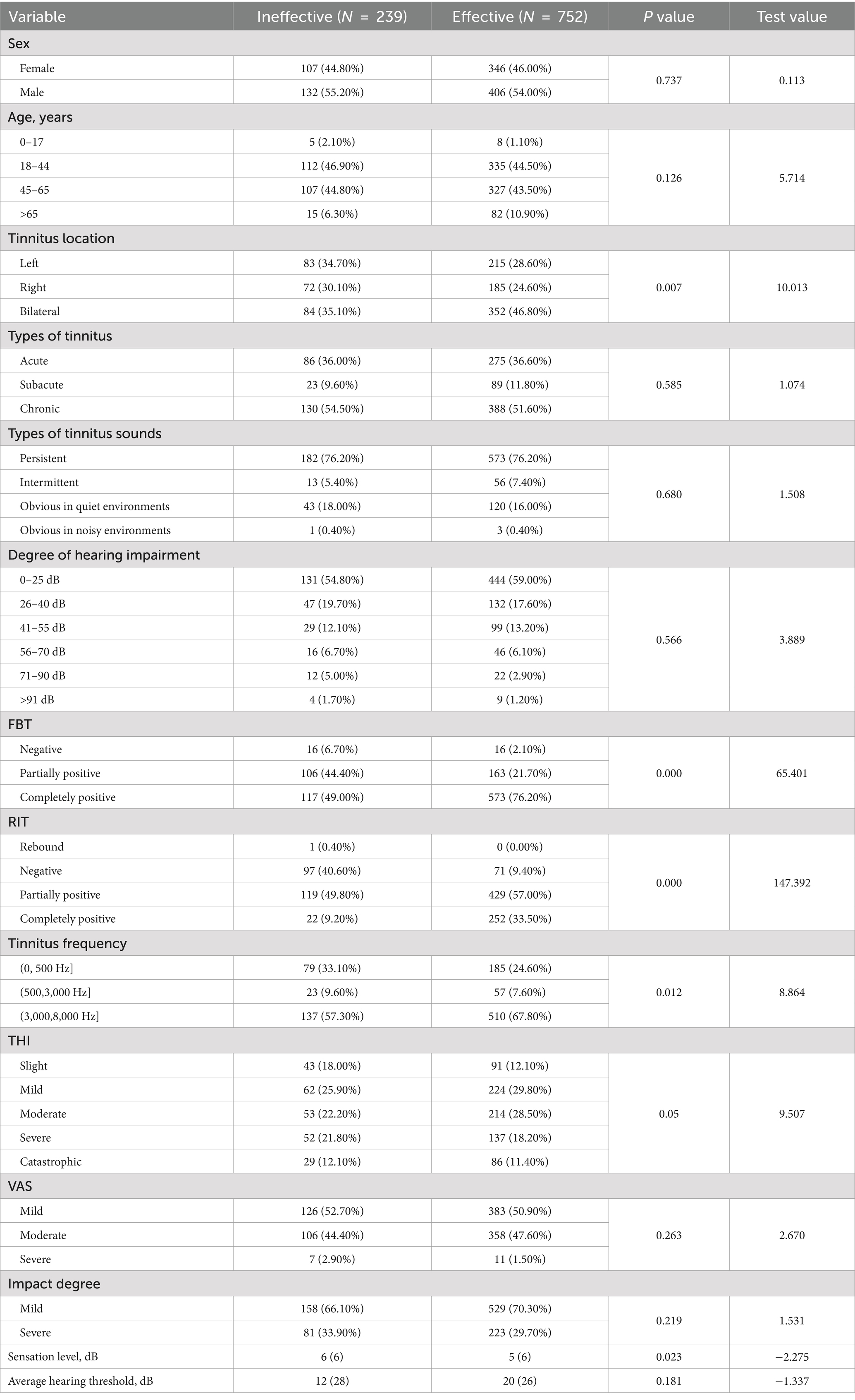
Table 1. Comparison of characteristics of tinnitus patients with different responses to sound therapy.
Univariate and multivariate analysesIn this study, all patients with subjective tinnitus who received compound sound therapy were divided into a training set and a verification set at a ratio of 7:3. The training set included 693 patients with subjective tinnitus and the validation set included 298 patients. Table 2 lists the clinical characteristics of patients with subjective tinnitus in the training and validation sets, which were similar. A total of 526 (75.90%) and 226 (75.80%) patients with subjective tinnitus were included in the two datasets, respectively.
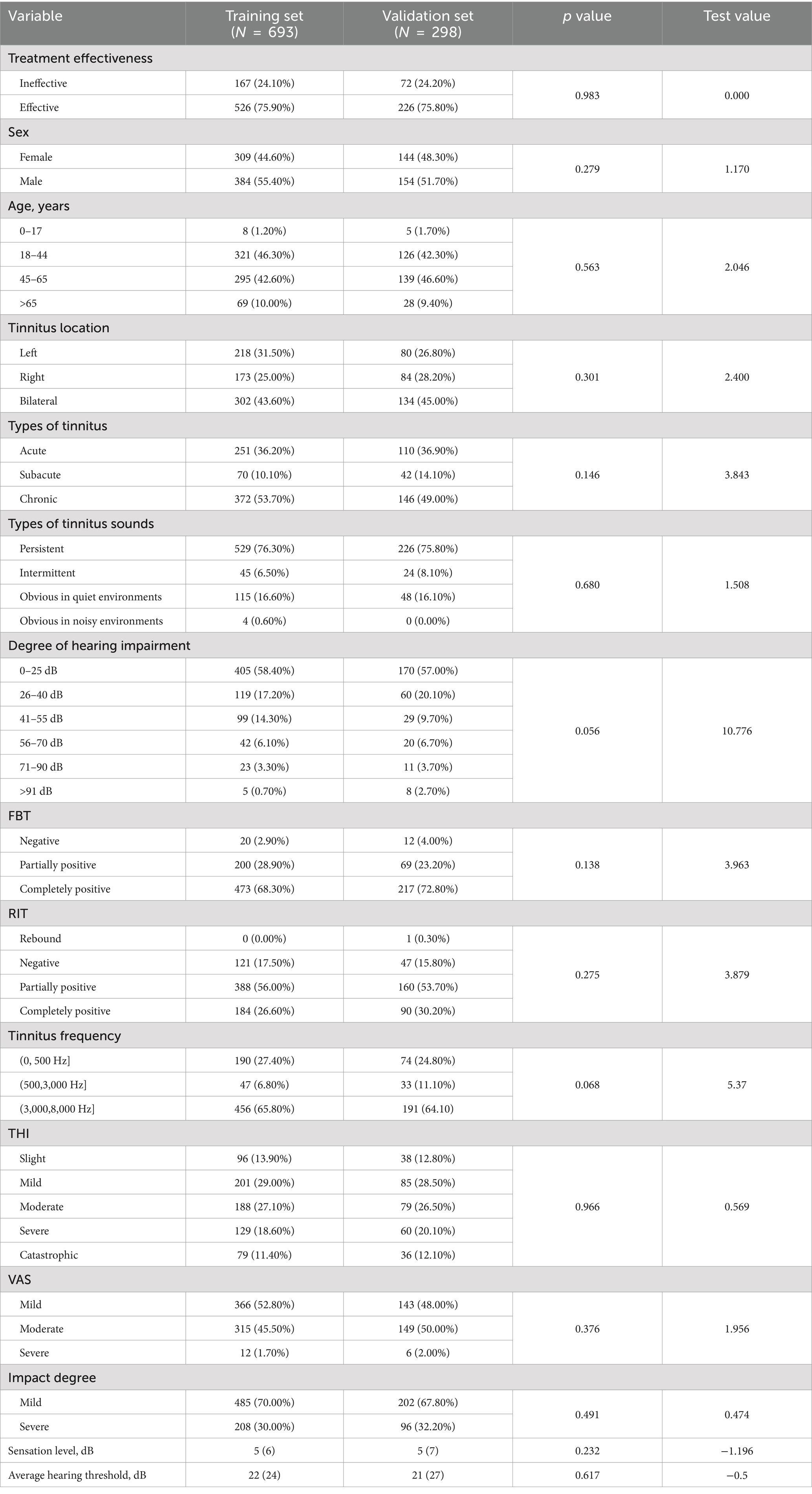
Table 2. Comparison of characteristics of tinnitus patients in different datasets (training set vs. validation set).
The univariate logistic regression results for the training set are shown in Table 3. The tinnitus location (p = 0.037), FBT (p = 0.000), RIT (p = 0.000), tinnitus frequency (p = 0.000), and average hearing threshold (p = 0.039) were included in the multivariate logistic regression analysis to identify independent risk factors affecting treatment efficacy.
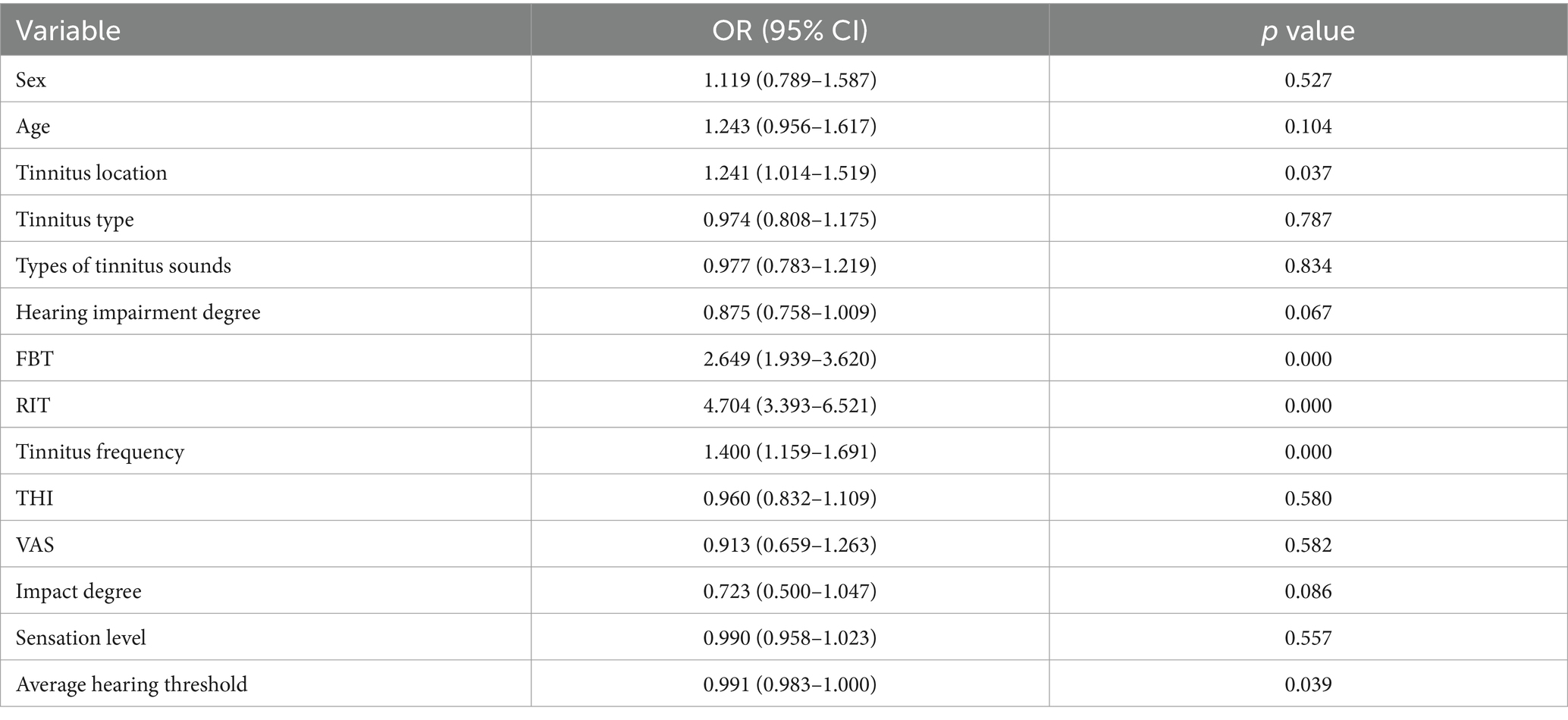
Table 3. Univariate logistic regression analysis of the treatment effectiveness.
Multivariate logistic regression analysis revealed that FBT (OR = 1.688, p = 0.003), RIT (OR = 3.997, p = 0.000) and tinnitus frequency (OR = 1.263, p = 0.029) were independent risk factors for combined sound therapy.
Development and validation of a therapeutic effectiveness nomogramThe effectiveness of compound sound therapy for patients with subjective tinnitus based on the results of the multiple logistic regression analysis of the training set (Figure 2). Each predictor’s individual scores in the column chart were summed to obtain a total score and used to calculate the probability that the corresponding compound sound treatment would be effective against subjective tinnitus. This approach helps to evaluate the efficacy of compound sound therapy for subjective tinnitus. Internal validation of the model built with the training set using bootstrapping showed that the nomogram had good accuracy in predicting the effectiveness of compound sound therapy for patients with subjective tinnitus. The AUC was 0.766 (95% CI = 0.725–0.807) (Figure 3A). In addition, the calibration curve of the nomogram showed that the actual diagnosis was in good agreement with the predicted probability (Figure 3C).
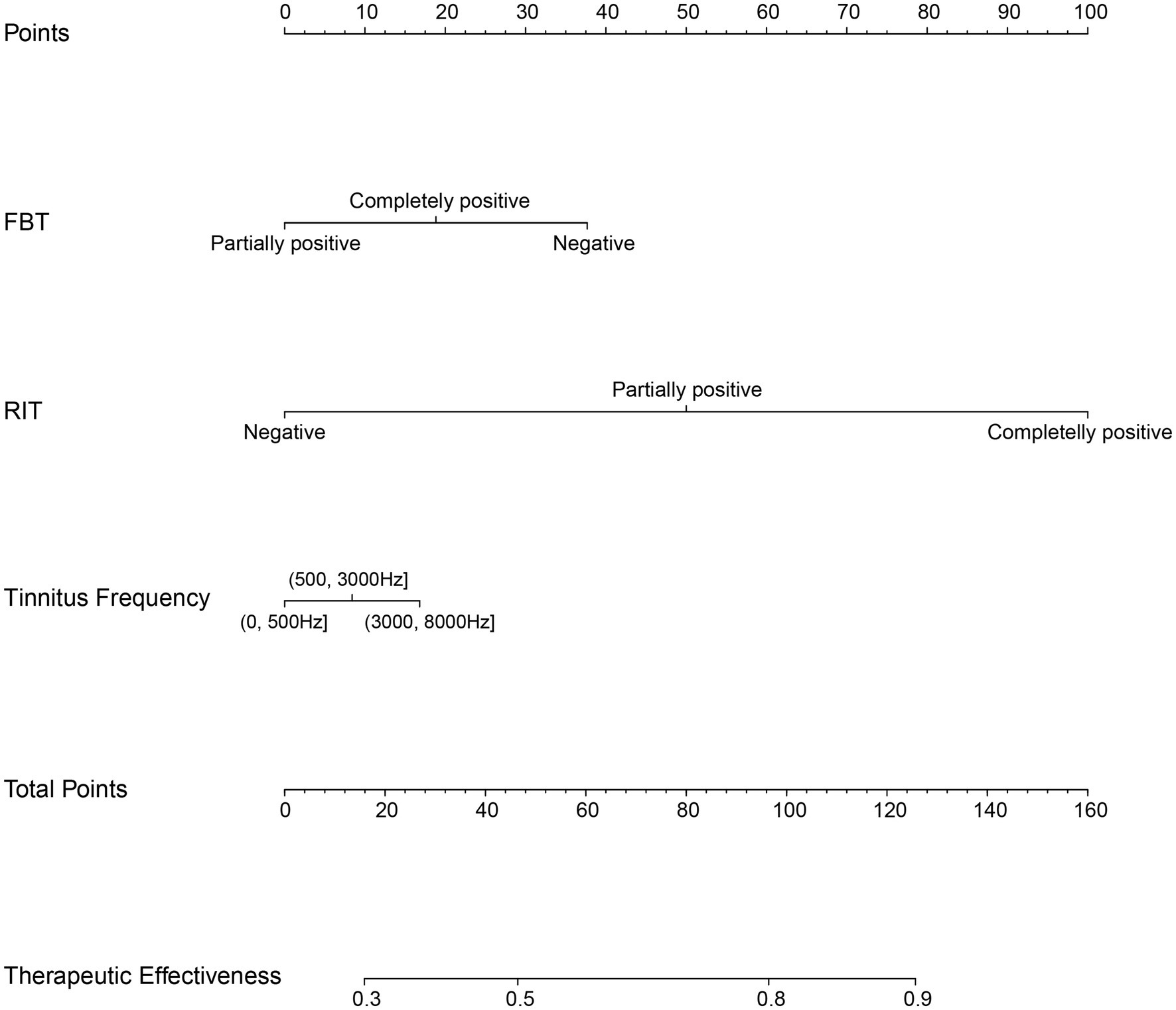
Figure 2. Nomogram for predicting the therapeutic effectiveness of customized compound sound therapy in patients with subjective tinnitus. For example: After examination, a tinnitus patient was found to have a negative FBT and a partially positive RIT, with tinnitus frequency between 3,000–8,000 Hz. The patient’s corresponding total points is 125. The nomogram predicts that the effectiveness of compound sound therapy for treating tinnitus is approximately 90%.
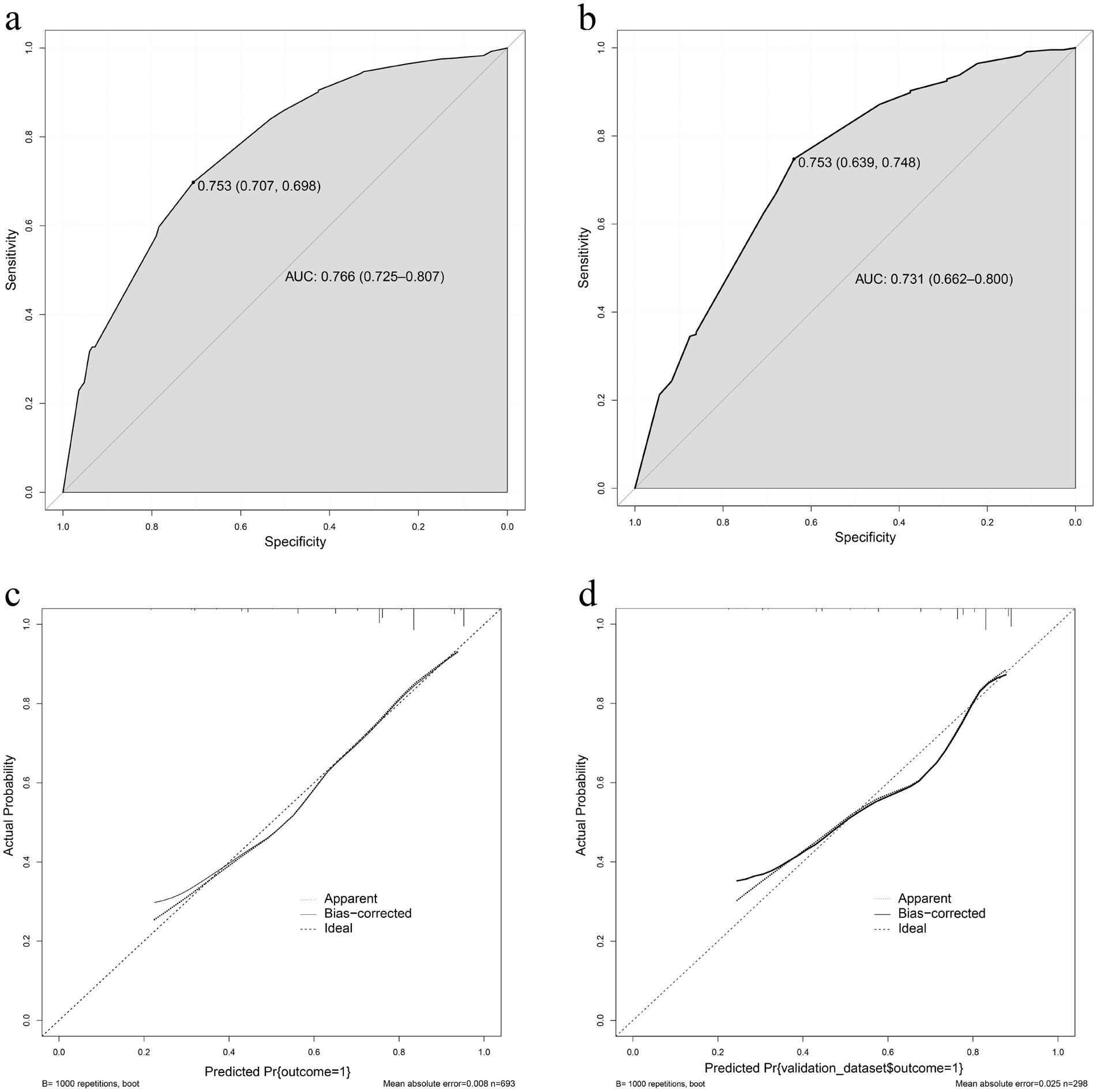
Figure 3. (A) The receiver operating characteristic curve of the nomogram in the training set. (B) The receiver operating characteristic curve of the nomogram in the validation set. (C) The effectiveness of the nomogram in estimating the predictive performance of compound sound therapy on subjective tinnitus in the training set (n = 693). (D) The effectiveness of the nomogram in estimating the predictive performance of compound sound therapy on subjective tinnitus in the validation set (n = 298).
In the validation set, the AUC of the nomogram for predicting the effectiveness of compound sound therapy for subjective tinnitus was 0.731 (95% CI = 0.662 to 0.800) (Figure 3B). A good calibration curve was generated for the risk estimation (Figure 3D).
DiscussionResearchers have tried various methods to treat or even cure tinnitus, however, to date, no single treatment has provided satisfactory results. The effectiveness of sound therapy in altering tinnitus perception has been recognized for centuries, and in recent decades, the use of sound or sound enrichment to mask or suppress tinnitus or disrupt the neural activity that causes tinnitus has become a central part of clinical management (23). At present, the pathogenesis of tinnitus remains unclear (24). Therefore, most treatments focus on alleviating the associated distress caused by tinnitus, and treatments that directly eliminate tinnitus are lacking. Sound therapy is a widely used tinnitus treatment because it is noninvasive, simple and easily accepted by patients, however, its efficacy differs across patients with different tinnitus types.
We designed this study to explore which tinnitus patients are suitable for compound sound therapy and to predict the effectiveness of compound sound therapy for tinnitus patients. We analyzed 991 patients with subjective tinnitus and studied the clinical characteristics associated with compound sound therapy efficacy, such as the tinnitus type, tinnitus location, tinnitus sensation level, tinnitus frequency, and the tinnitus sound type. A predictive clinical nomogram was developed to evaluate the effectiveness of compound sound therapy based on clinical characteristics.
We analyzed the associations of demographic characteristics and tinnitus-related characteristics with the effects of sound therapy. In a study on the treatment of subjective tinnitus with repetitive transcranial magnetic stimulation (rTMS), Wang et al. recruited 289 patients with subjective tinnitus and provided each subject 1,000 1 Hz magnetic stimuli per day for 5 consecutive days per week for 2 weeks and found that age impacted on the efficacy of rTMS (25). In this study, however, we found that age had no effect on the efficacy of compound sound therapy. We believe that this discrepancy may be related to the fact that 52.5% of the subjects in this study had chronic tinnitus, and tinnitus persisted for more than 3 months. Most of the subjects in this study were 18–65 years old. This phenomenon can likely be attributed to several factors. First, the nature of the study was observational, with a focus on patients who presented with tinnitus at the outpatient Department of Otolaryngology. Second, individuals aged 18–65 who experience tinnitus may face heightened daily pressures, including work, study, and personal life stressors, which could contribute to the onset and exacerbation of tinnitus symptoms. Additionally, psychological factors may play a role in the development and progression of tinnitus in this age group. Furthermore, individuals in this demographic group may possess greater financial resources and a stronger motivation to seek treatment for their tinnitus, potentially leading to a higher rate of hospital visits among this population.
Frank et al. recruited 194 tinnitus patients, of whom 130 had bilateral tinnitus patients, 27 had right tinnitus, and 37 had left tinnitus. All patients received rTMS 2,000 times a day for 10 days. The researchers found that patients with left and bilateral tinnitus experienced a significant reduction in tinnitus severity after treatment, but those with right tinnitus did not (26). In this study, we found that patients with tinnitus on different sides had different sensitivities to compound sound therapy. However, at present, we do not know why this phenomenon occurs, and we will continue to explore this phenomenon in future studies. In this study we found that RIT may have an impact on the efficacy of compound sound therapy. Feldmann et al. reported that tinnitus disappeared briefly after sound stimulation (27, 28). The above phenomenon is called residual inhibition and occurs in 60–80% of people with tinnitus (29). Galazyuk et al. suggested that residual inhibition results from a reduction in the spontaneous discharge of central auditory neurons caused by sound stimulation (30). It is hypothesized that patients with a completely positive result on the RIT may exhibit increased sensitivity to sound therapy. Additionally, compound sound therapy has been shown to effectively suppress the self-discharge of central auditory neurons.
We found that patients with different tinnitus frequencies had different sensitivities to compound sound therapy. We searched the relevant literature and did not find relevant studies on this phenomenon. In the future, we will design relevant experiments to explore the relevant mechanisms of this phenomenon. In this study, the effectiveness of compound sound therapy was influenced by the FBT. The primary purpose of the FBT is to identify the pitch-to-loudness relationship characteristics of tinnitus under specific sound stimulation, validate the precision and controllability of the tinnitus frequency, and establish a foundation for diagnosis. The FBT involves the continuous application of sound stimulation at 1 dB increments above the tinnitus loudness threshold to assess changes in tinnitus perception, thereby providing insights into the patient’s responsiveness to compound sound therapy.
Predictive models are increasingly used in clinical decision-making and can provide personalized or precise medical guidance to patients (31). Ramakers et al. designed a cross-sectional retrospective study of 87 patients with bifocal severe hearing loss and tinnitus who received a unilateral cochlear implant. Tinnitus recovery was predicted by age, sex, the duration of deafness, preoperative hearing performance, duration, severity and location of tinnitus, the duration of follow-up, and the surgical approach. The researchers constructed a model to predict tinnitus recovery after cochlear implantation using variables such as a low preoperative consonant-vowel-consonant score, unilateral tinnitus, and the threshold of hearing deterioration in the operative ear at 250 Hz (32). Niemann et al. retrospectively analyzed a cohort of 4,117 tinnitus patients, analyzed 185 clinically relevant features, and constructed a prediction model comprising 6 clinically relevant features to help select appropriate treatment strategies for patients with chronic tinnitus complicated with or without major depression. Most previous studies have focused on predicting the occurrence of tinnitus, its impact on life and the efficacy of some treatment methods; few have focused on predicting the efficacy of compound sound therapy (33).
In this study, we constructed a prediction model for the efficacy of compound sound therapy on tinnitus, selected 13 variables, and used multivariate binary logistic regression. We found that FBT, RIT and the tinnitus frequency were all independent risk factors for evaluating the effectiveness of compound sound therapy for tinnitus. The AUC of the model constructed in this study was 0.766 (95% CI = 0.725–0.807), indicating a certain predictive ability. The correction curve also shows that the predicted results are in good agreement with the actual results. Through internal verification, we found that the AUC of the constructed model was 0.731 (95% CI = 0.662–0.800), which indicated that the constructed model had certain reliability.
This study had several limitations. First, all the data were retrospective, and numerous confounding factors were present. Second, the prediction model constructed in this study is based on data from a single medical institution and is not supported by data from other medical institutions. Therefore, external verification of the model in future studies is necessary to explore its feasibility. Finally, further determination of the reliability of the nomogram through prospective studies is necessary.
ConclusionAfter fully considering the demographic characteristics and tinnitus characteristics, we used FBT, RIT and the tinnitus frequency as three predictors to establish a multivariate logistic regression model and a nomogram for predicting the efficacy of compound sound therapy. The model can predict the prognosis of tinnitus patients receiving compound sound therapy and help otolaryngologists make the best clinical decisions regarding tinnitus treatment.
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statementThe studies involving humans were approved by the Bioethics Committee of Tangdu Hospital of Air Force Military Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributionsHY: Data curation, Formal analysis, Visualization, Writing – original draft. P-WM: Data curation, Methodology, Writing – original draft. J-WC: Data curation, Methodology, Writing – original draft. W-LW: Data curation, Writing – review & editing. WG: Project administration, Writing – review & editing. P-HL: Visualization, Writing – review & editing. X-RD: Data curation, Writing – review & editing. Y-QL: Data curation, Writing – review & editing. ZW: Data curation, Writing – review & editing. L-JL: Conceptualization, Writing – review & editing, Writing – original draft.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AcknowledgmentsWe would like to thank all participants for their time during this study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statementThe authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes References2. Niemann, U, Boecking, B, Brueggemann, P, Mazurek, B, and Spiliopoulou, M. Gender-specific differences in patients with chronic tinnitus-baseline characteristics and treatment effects. Front Neurosci. (2020) 14:487. doi: 10.3389/fnins.2020.00487
PubMed Abstract | Crossref Full Text | Google Scholar
3. Jarach, CM, Lugo, A, Scala, M, van den Brandt, PA, Cederroth, CR, Odone, A, et al. Global prevalence and incidence of tinnitus: a systematic review and meta-analysis. JAMA Neurol. (2022) 79:888–900. doi: 10.1001/jamaneurol.2022.2189
PubMed Abstract | Crossref Full Text | Google Scholar
4. Czornik, M, Malekshahi, A, Mahmoud, W, Wolpert, S, and Birbaumer, N. Psychophysiological treatment of chronic tinnitus: a review. Clin Psychol Psychother. (2022) 29:1236–53. doi: 10.1002/cpp.2708
PubMed Abstract | Crossref Full Text | Google Scholar
5. Noreña, AJ, and Farley, BJ. Tinnitus-related neural activity: theories of generation, propagation, and centralization. Hear Res. (2013) 295:161–71. doi: 10.1016/j.heares.2012.09.010
PubMed Abstract | Crossref Full Text | Google Scholar
6. Haider, HF, Bojić, T, Ribeiro, SF, Paço, J, Hall, DA, and Szczepek, AJ. Pathophysiology of subjective tinnitus: triggers and maintenance. Front Neurosci. (2018) 12:866. doi: 10.3389/fnins.2018.00866
PubMed Abstract | Crossref Full Text | Google Scholar
7. Frank, E, Schecklmann, M, Landgrebe, M, Burger, J, Kreuzer, P, Poeppl, TB, et al. Treatment of chronic tinnitus with repeated sessions of prefrontal transcranial direct current stimulation: outcomes from an open-label pilot study. J Neurol. (2012) 259:327–33. doi: 10.1007/s00415-011-6189-4
PubMed Abstract | Crossref Full Text | Google Scholar
8. Vanneste, S, and De Ridder, D. Bifrontal transcranial direct current stimulation modulates tinnitus intensity and tinnitus-distress-related brain activity. Eur J Neurosci. (2011) 34:605–14. doi: 10.1111/j.1460-9568.2011.07778.x
PubMed Abstract | Crossref Full Text | Google Scholar
10. Cima, RFF, Mazurek, B, Haider, H, Kikidis, D, Lapira, A, Noreña, A, et al. A multidisciplinary European guideline for tinnitus: diagnostics, assessment, and treatment. HNO. (2019) 67:10–42. doi: 10.1007/s00106-019-0633-7
PubMed Abstract | Crossref Full Text | Google Scholar
11. Dong, C, Chen, C, Wang, T, Gao, C, Wang, Y, Guan, X, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of chronic tinnitus: a systematic review and Meta-analysis of randomized controlled trials. Biomed Res Int. (2020) 2020:3141278–9. doi: 10.1155/2020/3141278
PubMed Abstract | Crossref Full Text | Google Scholar
12. Henin, S, Fein, D, Smouha, E, and Parra, LC. The effects of compensatory auditory stimulation and high-definition transcranial direct current stimulation (HD-tDCS) on tinnitus perception - a randomized pilot study. PLoS One. (2016) 11:e0166208. doi: 10.1371/journal.pone.0166208
PubMed Abstract | Crossref Full Text | Google Scholar
13. Pal, N, Maire, R, Stephan, MA, Herrmann, FR, and Benninger, DH. Transcranial direct current stimulation for the treatment of chronic tinnitus: a randomized controlled study. Brain Stimul. (2015) 8:1101–7. doi: 10.1016/j.brs.2015.06.014
Crossref Full Text | Google Scholar
15. Fuller, T, Cima, R, Langguth, B, Mazurek, B, Vlaeyen, JW, and Hoare, DJ. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. (2020) 2020:CD012614. doi: 10.1002/14651858.CD012614.pub2
PubMed Abstract | Crossref Full Text | Google Scholar
16. Rademaker, MM, Stegeman, I, Lieftink, A, Somers, M, Stokroos, R, and Smit, AL. MinT-trial: mindfulness versus cognitive behavioural therapy in tinnitus patients: protocol for a randomised controlled, non-inferiority trial. BMJ Open. (2020) 10:e033210. doi: 10.1136/bmjopen-2019-033210
PubMed Abstract | Crossref Full Text | Google Scholar
18. Okamoto, H, Stracke, H, Stoll, W, and Pantev, C. Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc Natl Acad Sci USA. (2010) 107:1207–10. doi: 10.1073/pnas.0911268107
PubMed Abstract | Crossref Full Text | Google Scholar
19. Liu, Y, Yang, S, Wang, Y, Hu, J, Xie, H, Ni, T, et al. Efficacy and factors influencing outcomes of customized music therapy combined with a follow-up system in chronic tinnitus patients. J Otolaryngol Head Neck Surg. (2023) 52:29. doi: 10.1186/s40463-023-00631-y
PubMed Abstract | Crossref Full Text | Google Scholar
20. Newman, CW, Jacobson, GP, and Spitzer, JB. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg. (1996) 122:143–8. doi: 10.1001/archotol.1996.01890140029007
Crossref Full Text | Google Scholar
21. De Ridder, D, Schlee, W, Vanneste, S, Londero, A, Weisz, N, Kleinjung, T, et al. Tinnitus and tinnitus disorder: theoretical and operational definitions (an international multidisciplinary proposal). Prog Brain Res. (2021) 260:1–25. doi: 10.1016/bs.pbr.2020.12.002
PubMed Abstract | Crossref Full Text | Google Scholar
22. Sung, YT, and Wu, JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behav Res Methods. (2018) 50:1694–715. doi: 10.3758/s13428-018-1041-8
PubMed Abstract | Crossref Full Text | Google Scholar
23. Wang, H, Tang, D, Wu, Y, Zhou, L, and Sun, S. The state of the art of sound therapy for subjective tinnitus in adults. Ther Adv Chronic Dis. (2020) 11:2040622320956426. doi: 10.1177/2040622320956426
PubMed Abstract | Crossref Full Text | Google Scholar
24. Li, Y, Sang, D, Wu, Z, and Cao, X. Systematic evaluation of the efficacy of acupuncture associated with physical and mental intervention when treating idiopathic tinnitus and the improvement of tinnitus symptoms. Comput Math Methods Med. (2022) 2022:6764909–10. doi: 10.1155/2022/6764909
PubMed Abstract | Crossref Full Text | Google Scholar
25. Wang, H, Li, B, Wang, M, Li, M, Yu, D, Shi, H, et al. Factor analysis of low-frequency repetitive transcranial magnetic stimulation to the Temporoparietal junction for tinnitus. Neural Plast. (2016) 2016:1–6. doi: 10.1155/2016/2814056
Crossref Full Text | Google Scholar
26. Frank, G, Kleinjung, T, Landgrebe, M, Vielsmeier, V, Steffenhagen, C, Burger, J, et al. Left temporal low-frequency rTMS for the treatment of tinnitus: clinical predictors of treatment outcome--a retrospective study. Eur J Neurol. (2010) 17:951–6. doi: 10.1111/j.1468-1331.2010.02956.x
Crossref Full Text | Google Scholar
30. Galazyuk, AV, Longenecker, RJ, Voytenko, SV, Kristaponyte, I, and Nelson, GL. Residual inhibition: from the putative mechanisms to potential tinnitus treatment. Hear Res. (2019) 375:1–13. doi: 10.1016/j.heares.2019.01.022
PubMed Abstract | Crossref Full Text | Google Scholar
31. Park, Y, Hu, J, Singh, M, Sylla, I, Dankwa-Mullan, I, Koski, E, et al. Comparison of methods to reduce Bias from clinical prediction models of postpartum depression. JAMA Netw Open. (2021) 4:e213909. doi: 10.1001/jamanetworkopen.2021.3909
PubMed Abstract | Crossref Full Text | Google Scholar
32. Ramakers, GGJ, van Zanten, GA, Thomeer, H, Stokroos, RJ, Heymans, MW, and Stegeman, I. Development and internal validation of a multivariable prediction model for tinnitus recovery following unilateral cochlear implantation: a cross-sectional retrospective study. BMJ Open. (2018) 8:e021068. doi: 10.1136/bmjopen-2017-021068
PubMed Abstract | Crossref Full Text | Google Scholar
33. Niemann, U, Brueggemann, P, Boecking, B, Mazurek, B, and Spiliopoulou, M. Development and internal validation of a depression severity prediction model for tinnitus patients based on questionnaire responses and socio-demographics. Sci Rep. (2020) 10:4664. doi: 10.1038/s41598-020-61593-z
留言 (0)