Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Author affiliation: Centers for Disease Control and Prevention, Atlanta, Georgia, USA
Elizabethkingia spp. are widely distributed environmental bacteria and opportunistic pathogens that can cause sepsis and meningitis, particularly in neonates (1,2). At least 3 phenotypically similar species (E. anophelis, E. meningoseptica, and E. miricola) are intrinsically resistant to multiple antimicrobial classes and have been implicated in fatal healthcare-associated outbreaks (3). The largest reported United States outbreak was an E. anophelis strain that caused 66 laboratory-confirmed infections in Wisconsin and neighboring states in 2015–2016, for which the infection source was never identified. That outbreak was unusual for primarily consisting of community-acquired infections (4). Plumbing fixtures such as taps and sink drains, which Elizabethkingia bacteria readily colonize in biofilms, are common exposure vehicles in healthcare settings (2,5,6). Transmission from handwashing in contaminated sinks is of particular concern and has been shown to contaminate health worker hands with E. anophelis, even with the use of chlorhexidine soap (5).
Chlorination is the most common disinfection method for public water supplies. When used at the end of the treatment chain, chlorination provides residual disinfection during distribution and storage. Reports of Elizabethkingia bacterial persistence in chlorinated water supplies and plumbing fixtures cleaned with sodium hypochlorite have raised concerns of chlorine tolerance (2,6,7), but no data have been published. We conducted disinfection experiments with a free chlorine residual (FCR) dose of 0.2 mg/L, the minimum disinfectant residual for treated surface water entering distribution systems in the United States, to assess inactivation of 2 Elizabethkingia spp. isolated from clinical and environmental samples (8). We fit inactivation kinetics models to estimate the product of FCR dose (C; mg/L) and contact time (T; minutes) required to reduce Elizabethkingia bacterial concentrations by 99.9% (CT99.9%).
We performed disinfection experiments in triplicate for 6 E. anophelis and 5 E. meningoseptica strains from clinical and environmental sources (Table 1). We prepared bacterial stocks by incubating cultures in tryptic soy broth overnight at 37°C, followed by subculture into tryptic soy broth at 37°C for ≈5 hours (9). We pelleted the log phase cultures, washed with sterile phosphate-buffered saline (PBS), repelleted, and resuspended in 5 ml PBS. We prepared 50-mL glass flasks with 25 mL of sterile oxidant demand–free water buffered at pH 7.5, dosed to 0.2 mg/L FCR with 5.25% sodium hypochlorite, and maintained in a water bath at 25°C. We seeded flasks with 0.1 mL bacterial stock and extracted 10 mL aliquots from 3 flasks after 15, 30, and 60 seconds, immediately quenching the aliquots with 100 µL of 10% sodium thiosulfate (Fisher Scientific, https://www.fishersci.com). We extracted triplicate aliquots from 3 chlorine-free flasks at 60 seconds to examine dieoff with no disinfectant exposure. We also measured FCR in aliquots removed at 30 and 60 seconds from a final seeded flask to assess disinfectant decay. We serially diluted the initial bacterial stocks and experimental aliquots with sterile PBS and enumerated by using membrane filtration plated onto tryptic soy agar containing 5% rabbit blood, incubated at 37°C, and counted after 24–36 hours.
We analyzed inactivation kinetics by modeling the natural log-survival, the ratio of the bacterial concentration at contact time T to the initial concentration, as a function of time and FCR dose governed by an inactivation rate constant k. We estimated k by using the pseudo-first order Chick-Watson model and the nonlinear generalization, the Hom model, assuming first-order disinfectant decay and excluding samples for which the concentration was too low to detect (10). We used R version 4.3.2 (The R Project for Statistical Computing, https://www.R-project.org) to fit inactivation models by nonlinear least squares; estimate disinfectant decay rates, k’, with linear regression; calculate CT values from the fitted kinetic models; and perform z-tests (5% significance level) to compare rate constant estimates between species and between strain sources (11,12). Both inactivation kinetic models provided comparable fits to the experimental data that was indicated by similar values of the Akaike information criterion and root mean square error (Table 2). However, the Hom model was computationally unstable and produced rate constant estimates with larger SEs. Because of the larger SEs, we used only the Chick-Watson model to compare rate constants of different species and between clinical and environmental strains. Sensitivity analyses that accounted for potential correlation between replicates by using the geometric mean concentration to calculate strain-specific log-survival at each time point produced comparable rate constant estimates and CT values.
Figure
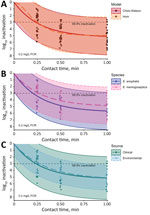
Figure. Observed and model-predicted log10 inactivation in study of chlorine inactivation of Elizabethkingiaspp. in water. Samples from environmental and clinical sources were exposed to chlorine in water...
Both models indicated a free chlorine CT of ≈0.03 milligrams per minute per millilieter (mg·min/L) inactivated 99.9% of Elizabethkingia bacteria (Table 2). In stratified Chick-Watson analyses, both species demonstrated CT99.9%<0.04 mg·min/L and rate constant estimates that were not statistically different (p = 0.92). However, E. anophelis was reduced to undetectable concentrations at 1 minute of exposure in 30% (n = 15) of samples, whereas E. meningoseptica was not detected at 1 minute of exposure in only 11% (n = 6) of samples. Environmental strains displayed less variable log-survival than clinical strains (Figure), but the rate constant estimates were not statistically different (p = 0.37). Both strains produced similar CT99.9% values of 0.04 mg·min/L (environmental) and 0.03 mg·min/L (clinical). All environmental strains were still detectable after 1 minute of exposure, whereas clinical strains were not detected in 18 samples (26%).
The initial FCR dose was reduced by approximately two thirds after 1 minute. The median reduction in Elizabethkingia after 1 minute with no chlorine exposure was 0.13 log10 (26%). In contrast, the Chick-Watson model predicted ≈5 log10 (99.999%) inactivation in 1 minute for a 0.2 mg/L FCR dose (Figure). Model 95% prediction intervals indicated a minimum expected inactivation of ≈2 log10 after 1 minute at the experimental conditions (Figure). Of the 1-minute samples, >3 log10 inactivation was observed for 32 of the 35. The 3 samples below the 3 log10 inactivation threshold were replicates of the same clinical strain and experienced 2.6–2.9 log10 inactivation.
Contrary to the chlorine tolerance hypothesized in the literature, we observed rapid inactivation of Elizabethkingia at typical point-of-use free-chlorine concentrations (≈0.2 mg/L). Across species and sources, Elizabethkingia strains demonstrated greater chlorine susceptibility (CT99.9%; <0.04 mg·min/L) than a reported Escherichia coli reference strain (CT99.9%; 0.09 mg·min/L) that was used to benchmark disinfectant susceptibility of waterborne pathogens (13). However, we also observed cells persisting at detectable concentrations after 1 minute of contact time, particularly among environmental strains. The more persistent subpopulations could seed biofilms, which Elizabethkingia bacteria readily form in plumbing fixtures, and have been shown to rapidly recolonize sink drains within days of seemingly effective disinfection (2,14), possibly accounting for the reported survival of Elizabethkingia spp. after chlorination in healthcare settings. Biofilms can protect embedded organisms from disinfection through multiple mechanisms, including oxidant demand exerted by the extracellular matrix, limited diffusion of the disinfectant to inner layers, and phenotypic adaptations in response to sublethal disinfectant doses and the biofilm environment itself (15). A review of 6 bacteria species reported biofilm-embedded cells required 2–600 times the chlorine dose or contact time for inactivation than their planktonic (free-swimming) counterparts (15). Prevention of Elizabethkingia infections, as with other opportunistic biofilm pathogens, may be most readily accomplished by limiting the environments in which biofilms can form and reducing exposure to potentially contaminated sources (5–7). Building managers should adopt water management programs to limit the growth and transmission of opportunistic pathogens of plumbing.
Dr. Holcomb is a microbiologist in the Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA. His research interests include environmental transmission and control of antimicrobial-resistant pathogens.
留言 (0)