Dear Editor,
Ruocco’s concept of the “immunocompromised district” pertains to a specific region of the skin that exhibits an increased susceptibility to certain dermatological diseases. It encompasses various phenomena, including Wolf isotopic response, Koebner’s phenomenon, locus minoris resistentiae, or other related occurrences.1 In 1995, Wolf et al. introduced the term “isotopic response” to describe the emergence of a new skin condition at the site of a previously healed or unrelated skin condition.2 Most cases typically involve a preceding herpes infection, either simplex or zoster, followed by the development of a second dermatological condition. We herein describe a unique case of varicella clustering on co-existing lesions of superficial dermatophytosis.
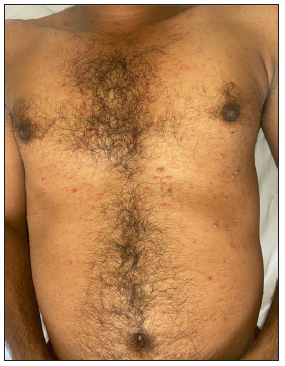
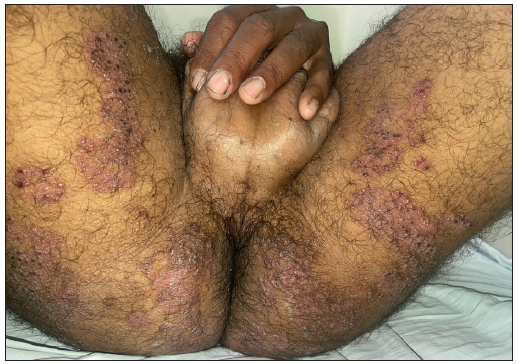
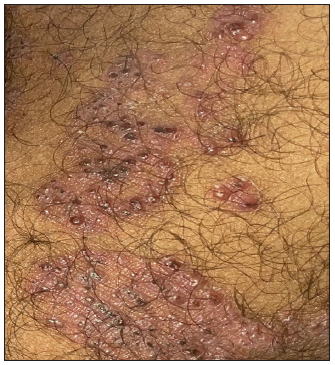
A 24-year-old male patient presented with multiple vesicles all over the body, with a few lesions clustered within annular erythematous scaly lesions over the inner thigh [Figures 1a-1c]. A few of these vesicles were umbilicated with a central necrotic eschar. The patient reported fever and malaise along with intense itching in the affected areas. There was no history of using over-the-counter medications such as topical corticosteroids on the annular erythematous scaly plaques.
Export to PPT
Export to PPT
Export to PPT
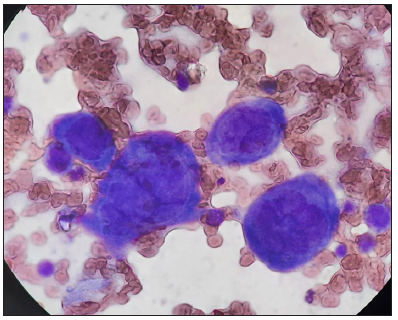
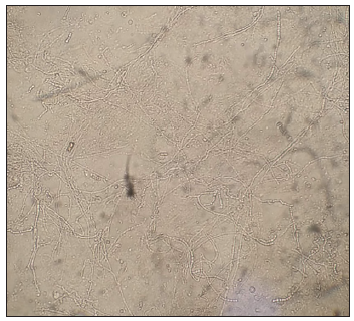
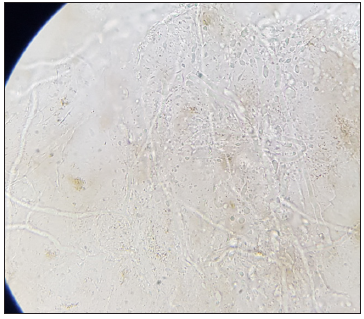
A Tzanck smear was prepared which revealed several multinucleated giant cells and acantholytic cells [Figure 2a]. Further examination through potassium hydroxide (KOH) testing from the periphery detected mycelial elements [Figures 2b and 2c]. The patient received oral acyclovir 800 mg five times a day for seven days, as well as antihistamines and oral itraconazole 100 mg twice a day for four weeks. The varicella lesion eventually healed, though with post-inflammatory hyperpigmentation and atrophic scarring in some areas.
Export to PPT
Export to PPT
Export to PPT
The skin’s primary function is to act as a barrier between the environment and the human body through intercellular fats, cholesterol, and sphingomyelin, which contribute to the barrier function. Additionally, it serves as an immune defence against a diverse array of pathogens through the expression of various innate receptors that detect pathogens and subsequently triggers the production of cytokines and chemokines.3 An immunocompromised district (ICD) is a novel concept that refers to a localised area of the skin with altered immunity, rendering it more susceptible to infections, immune disorders, or skin tumours.4,5
In superficial dermatophytoses, the release of various proteolytic enzymes by dermatophytes lead to the disruption of the epidermal barrier, facilitating their invasion and resulting in alteration to both innate and adaptive immunity. A plausible explanation could be that the downregulation of Th1 and Th17 cells, along with the upregulation of Th2 and regulatory T cells, creates an immunological environment that favours the persistence of fungal infection.6 T-cell exhaustion or unresponsiveness due to chronic exposure to fungal antigens can lead to impaired antiviral response and development of ICD.7 In the present case, the localisation of varicella-zoster virus (VZV) to active dermatophytosis lesions can be ascribed to the migratory routes of cutaneous lymphocyte antigen (CLA+) memory T cells during immune surveillance, which is heightened during active inflammation. Intriguingly, the varicella- zoster virus exhibits an enhanced tropism for these memory T cells that have skin-homing potential, thereby facilitating their accumulation in regions of pre-existing cutaneous inflammation.8 Besides, the upregulation of cellular adhesion molecules on endothelial cells at the active sites of inflammation due to dermatophytosis may contribute as a potential mechanism for enhancing the efficacy of VZV-infected memory T cells trafficking.
In conclusion, locus minoris resistentiae represents an infrequent but intriguing phenomenon that holds significance within the concept of an immunocompromised district. Clinicians should remain vigilant regarding the emergence of atypical presentations of common infections, particularly in individuals with pre-existing skin conditions such as superficial dermatophytosis. Comprehending the underlying pathomechanisms and facilitating timely diagnosis are pivotal in formulating effective therapeutic approaches.
留言 (0)