Dear Editor,
Gefitinib, an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase, plays a pivotal role in targeted therapy for non-small cell lung cancer (NSCLC). Targeted therapies have been implicated in severe cutaneous adverse reactions (SCARs) like Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug rash with eosinophilia and systemic symptoms (DRESS) and acute generalised exanthematous pustulosis (AGEP). While other EGFR inhibitors such as afatinib have been associated with SJS-TEN, reports of SJS as a result of gefitinib use are comparatively infrequent and uncommon.1 This case report provides an insight into a patient who developed SJS after gefitinib therapy which had clinical manifestations similar to flagellate dermatitis.
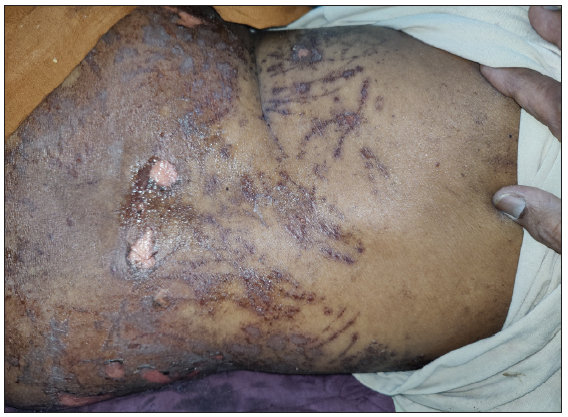
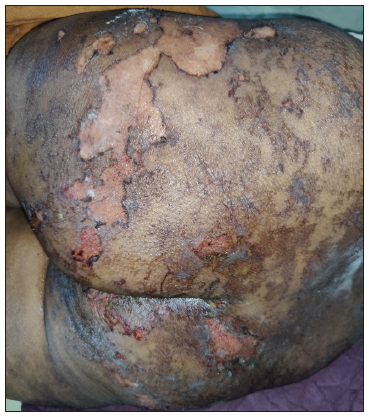
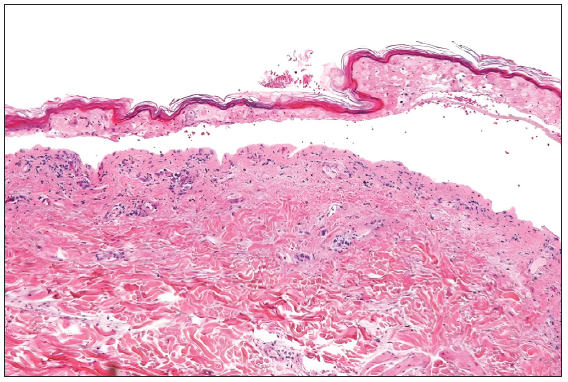
A 64-year-old woman with stage IIIA NSCLC was initiated on oral gefitinib at a standard dose of 250 mg/day as EGFR activating exon 19 mutation was detected in the tumour. Ten days after initiating the gefitinib regimen, she presented with a pruritic erythematous macules spanning her trunk and limbs. Remarkably, the macular eruption assumed a linear and whip-like pattern akin to a flagellate dermatitis over the trunk [Figures 1 and 2]. The evolution was rapid, progressing to blistering, epidermal detachment and oral and genital mucosal involvement and mild conjunctival erythema. The total body surface area involved was 14–15%. Other drugs which the patient was taking were non-steroidal anti-inflammatory drugs and proton pump inhibitors. However, temporal correlation was established only for gefitinib. The patient’s clinical status deteriorated swiftly, necessitating intensive care unit admission with a diagnosis of gefitinib-induced SJS. Histopathological examination of skin from two sites (flagellate dermatitis–like morphology [trunk area] and from the typical SJS-like skin lesions [buttock area]) showed epidermal necrosis with basal cell vacuolisation and numerous apoptotic keratinocytes, thereby corroborating with the clinical presentation [Figure 3]. Serology for recent mycoplasma pneumoniae or herpes simplex virus was non-reactive. Therapeutic interventions encompassed immediate cessation of gefitinib, tapering course of cyclosporine at 5 mg/kg/day for 14 days, meticulous wound care and vigilant fluid management. Over the ensuing weeks, the cutaneous lesions exhibited gradual resolution with post-inflammatory hyperpigmentation as the residual sequelae and the mucosal involvement ameliorated.
Export to PPT
Export to PPT
Export to PPT
SJS is a rare and severe mucocutaneous disorder, often triggered by medications, characterised by epidermal detachment and systemic involvement. In the context of targeted therapies, the emergence of SJS remains a complex and infrequent phenomenon. Our case sheds light on the convergence of gefitinib, an EGFR tyrosine kinase inhibitor used in NSCLC with the distinctive clinical presentation of SJS resembling flagellate dermatitis. Flagellate dermatitis, recognised by its linear, erythematous streaks reminiscent of whipping marks, has been predominantly linked to bleomycin and docetaxel.2 This patient presented with linear streaks which rapidly evolved into blisters, erosions and mucosal involvement – hallmarks of SJS. The flagellate dermatitis–like presentation of SJS can be attributed to the amount of intense pruritus associated with gefitinib therapy.3 EGF fosters keratinocyte proliferation by augmenting Ki67 and filaggrin expression via the mitogen-activated protein kinase kinase.4 In addition, EGFR governs the terminal differentiation of keratinocytes through the phospholipase C-γ1-protein kinase C pathway, upholding continuous epidermal barrier renewal.5 Disruption of the epidermal barriers, both physical and immune, due to EGFR inhibitors, like gefitinib, contributes to skin-related complications such as dryness and rashes. Claudins, vital for tight junction assembly, significantly contribute to maintaining the skin barrier’s integrity. Gefitinib potentially impairs the skin barrier by modulating claudin-1, claudin-4 and claudin-2 expressions in keratinocytes, thereby leading to skin toxicity.6
Other differential diagnosis which can be considered in such a setting of gefitinib therapy is necrolytic migratory erythema (NME). It presents as erythematous scaly plaques which blister centrally forming ulceration and crusting. As the central portion of the ulcer heals, the erythema may expand outward with new vesicles developing over the advancing edges. It mostly involves the intertriginous area such as inguinal creases and popliteal fossa with rare mucosal involvement. The histopathological hallmark of NME is the necrosis of the upper spinous layer of epidermis rather than basal cell vacuolisation.7 Both the clinical and histopathological criteria were absent in our case. Therefore, the possibility of necrolytic migratory erythema was ruled out.
In summary, the convergence of gefitinib-induced SJS with clinical features resembling flagellate dermatitis serves as a compelling reminder of the complexities within the realm of cutaneous adverse events. This case not only broadens our understanding of the potential manifestations of SJS but also underscores the need for heightened vigilance and interdisciplinary collaboration in managing adverse reactions associated with targeted therapies.
留言 (0)